Abstract
Hepatitis E virus (HEV) is an increasing cause of acute hepatitis in industrialized countries. The aim of this study was to evaluate the presence of HEV in pig manure composting plants located in Spain. For this purpose, a total of 594 samples were taken in 54 sampling sessions from the different stages of composting treatment in these plants as follows: slurry reception ponds, anaerobic ponds, aerobic ponds, fermentation zone and composting final products. HEV was detected by reverse transcription polymerase chain reaction (RT-nested PCR) in four (80%) of five plants studied, mainly in the first stages of the process. HEV was not detected in any final product (compost) sample, destined to be commercialized as a soil fertilizer, suggesting that composting is a suitable method to eliminate HEV and thus, to reduce the transmission of HEV from pigs to humans.
Introduction
Hepatitis E virus (HEV) is the main causative agent of enterically transmitted non-A non-B hepatitis (Purcell and Emerson, 2013; Perez-Gracia and Rodriguez-Iglesias, 2003). Hepatitis E is considered an infectious disease endemic in developing areas such as India, Africa and South-east Asia, because of poor sanitary conditions in drinking water. When it was first reported in developed countries, HEV was related to travel to endemic areas. However, the epidemiology of HEV in industrialized countries like Spain have changed in the last years, with an increasing number of non-travel associated sporadic cases (Perez-Gracia et al., 2004). In industrialized countries, hepatitis E actually represents less than 3% of acute viral hepatitis cases (Purdy and Khudyakov, 2011). However, the overall relevance of HEV infection has been underestimated and the disease may to be considered like a global health problem at the present time. The WHO estimates that more than 3 million individuals suffer symptomatic acute hepatitis E and the disease causes around of 70 000 deaths at year (Pischke and Wedemeyer, 2013). The overall mortality of hepatitis E in the general population is less than 1%; but it can reach up to 28% in infected pregnant women (Khuroo and Kamili, 2003).
The high phylogenetic homology observed between pig and humans strains of genotype 3 in the same geographical area suggests that hepatitis E is a zoonotic disease. This statement has been reinforced by the results of several studies in Japan (Takahashi et al., 2004), Korea (Ahn et al., 2005), the UK (Banks et al., 2004; Ijaz et al., 2005; Tolari et al., 2006) and also in Spain (Pina et al., 2000; Perez-Gracia et al., 2007), where they reported a nucleotide similarity of 94% between human and pig strains.
Besides the high nucleotide homology observed, HEV has been demonstrated to be able of crossing the species barrier, raising concern for the potential ways of zoonotic transmission from swine to humans (Meng, 2011).
The direct application of the slurries in agricultural areas is the more economic and ecological method in the management and recycling of the same ones (Ziemer et al., 2010; Pardo et al., 2011), following the parameters established by the law. Nevertheless, the appearance in the last years of zones with high density of cattle intensive developments generates a large amount of wastes. In this sense, Spain is the second country in the business of pig livestock in European Union (EU), with 25 million of units. The area studied has 170 farms distributed in an approximated surface of 1000 square miles, generating a total amount of 1 million of tones of slurries at year (source: EUROSTAT DATABASE, 2009) of 41 million of tones at year generated in EU. The management of these products turns out to be complicated and inefficient, constituting an environmental and sanitary problem because of zoonotic pathogens like HEV, may be transported to drinking water resources (Bolado-Rodriguez et al., 2010; Hazam et al., 2010; Meng, 2011) and other problems like excessive nitrification of soil and presence of excessive concentration of heavy metals. These problems worry both the livestock sector and Public Health authorities.
In this sense, the pig manure composting plants constitutes an innovative purification system in Spain, and these are the only plants that are currently being used. These procedures performed in these plants are safe alternative methods in the management of the slurries.
The aim of this study was to evaluate the presence of HEV in the different stages of pig manure composting plants.
Results
Two different types of composting plants (type A and type B), based on the type of manure treatment, were studied. Plants performing a total treatment of the slurry (type A plants), include an initial separation of liquid and solid phases of the slurry by centrifugation and posterior purification by fermentation procedures.
The purification process in type A plants (Fig. 1A) starts with an initial centrifuge. Liquid fraction of the slurries is carried to the anaerobic lagoon, where the slurries will remain 100 days. In this time; methanogenics, acetogenics and hydrolysis processes lead to a reduction of the smell, decrease of pathogens and stabilization of organic matter and hence reduction of BOD (biological oxygen demand).
Fig. 1.
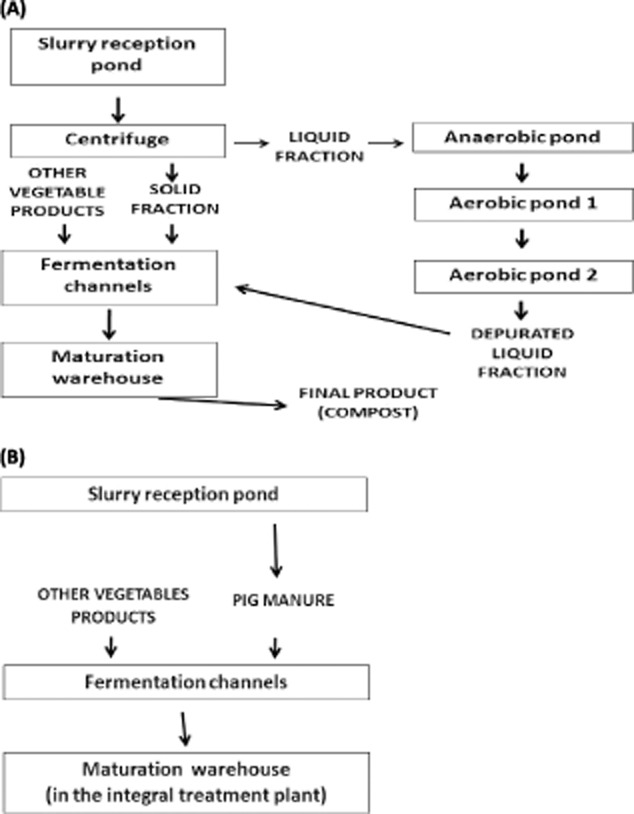
A. Plants type A flow-chart.B. Plants type B flow-chart.
Liquid from the anaerobic pond goes on later to the aerobic ponds 1 and 2, where the slurries will remain 10 days in each one. In this stage, offensive odours decrease and reduction of pathogen continues, conversion of available nitrogen to ammoniacal nitrogen and a decrease of C : N ratio is performed also.
The final product of this step is a depurated liquid fraction that will be mixed with the solid fraction and other vegetables, and carried to the fermentation channels where the composting process is performed. Composting is an aerobic process that requires a continuous supply of air with mechanical mixing during 21 days approximately. The temperature in the pile can rise in the 10 first days to as high as 65°C.
As opposed to type A plants, the type B plants does not make a fractionated treatment of the slurry (Fig. 1B) and directly use it in the composting process.
The final product is the compost, an inoffensive, depurated and a better soil fertilizer than raw slurries.
We have studied two type A plants (A1and A2) and three type B plants (B1, B2 and B3). A total number of four (80%) of five plants studied were positive to the presence of RNA-HEV. According to the type of plant, HEV was detected in two (100%) of the two type A plants and in two (66.66%) of the three type B plants (Table 1).
Table 1.
Nested-PCR results according to the number of sampling sessions in the different stages of treatment in type A and B plants
| Plant | Reception pond | Anaerobic pond | Solid product from centrifuge | Aerobic pond 1 | Aerobic pond 2 | Fermentation channel (Start) | Fermentation channel (End) | Maturation warehouse | Final pellet |
|---|---|---|---|---|---|---|---|---|---|
| A1 | 24/27a (88.89%)b | 18/27 (66.66%) | 3/27 (11.11%) | 3/27 (11.11%) | 0/27 (0%) | 0/27 (0%) | 0/27 (0%) | 0/27 (0%) | 0/27 (0%) |
| A2 | 9/9 (100%) | 6/9 (66.66%) | 6/9 (66.66%) | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) | 0/3 (0%) |
| B1 | 0/3 (0%) | – | – | – | – | 0/3 (0%) | 0/3 (0%) | 0/3 (0%) | 0/3 (0%) |
| B2 | 3/6 (50%) | – | – | – | – | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) | 0/6 (0%) |
| B3 | 3/9 (33.33%) | – | – | – | – | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) | 0/9 (0%) |
Sampling sessions HEV positive/total number of sampling sessions.
Percentage of positive sampling sessions.
According to the different stage of treatment, in plant A1, HEV was detected in 24 (88.89%) of 27 sampling sessions in the reception pond. In 18 (66.67%) of 27 sampling sessions, HEV was detected in the anaerobic pond; which is the first step of purification process. Only in three (11.11%) of the 27 sampling sessions, HEV was detected in the aerobic pond number 1; from this stage forward to the end of the composting process, HEV was not detected (Fig. 2).
Fig. 2.
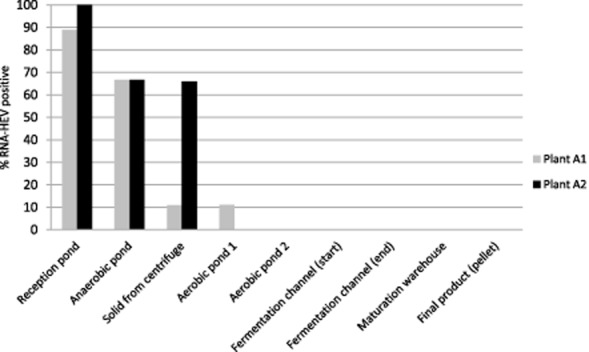
RNA-HEV detection through the different stage of pig manure treatment in type A plants.
In plant A2, HEV was detected in nine (100%) of nine sampling sessions in the reception pond. In six (66.66%) of nine sampling sessions, HEV was detected in the anaerobic pond; after this stage of treatment, HEV was not detected in either solid or liquid samples (Fig. 2).
In type B plants, HEV was not detected in plant B1 and it was detected in three (50%) of six sampling sessions in plant B2, and in three (33.33%) of nine sampling sessions in plant B3 in the respective reception ponds. HEV was not detected in any solid samples in these plants.
Discussion
In recent years, the increase of animal farming has generated an intensive and megascale livestock operations that produce a large amount of animal wastes worldwide (Cole et al., 2000; Spencer and Guan, 2004). A lot of causative agents of many infectious diseases have been identified in slurries, including HEV. The storage and treatment before land application are commonly performed in farms; however, it does not result in a total removal of pathogens from the manure (Ziemer et al., 2010).
Pig manure slurry is an emergent health and environmental problem and a potential source of infectious pathogens like HEV (Kasorndorkbua et al., 2005). If the slurries do not undergo a process of purification, and are spread without control over crop fields, population can be infected by the virus by consuming contaminated water from aquifers or vegetables irrigated with HEV contaminated water. Moreover, veterinarians, farmers and swine workers have been observed to be at a higher risk (Perez-Gracia et al., 2007; Galiana et al., 2008), as well as the contact with pig manure constitutes a factor of higher risk. To reduce the impact of animal waste production several technologies have been developed around the world. One of them has been performed in a high swine production zone in Castellon (Spain), with the installation of five pig manure composting plants (A1, A2, B1, B2, B3) with a pioneer treatment system.
Previous works evaluated the survival of pathogens after the treatment of the sludge (Sobsey et al., 2003; Costantini et al., 2007; Wagner et al., 2008), but this is the first study that evaluates the effectiveness of swine manure composting plants in the elimination of HEV.
The HEV is widely widespread in the zone of action of the plants studied (Fernández-Barredo et al., 2007). In fact, in this study HEV was detected in 80% of the plants. These data coincide with other works in the same geographical zone (Seminati et al., 2008) that reported in some cases 76.19% of HEV prevalence in farms closer to these plants (Fernández-Barredo et al., 2006).
In plants with total treatment of the slurries (type A plants), HEV was not detected in any sample from the liquid fraction after the stage of treatment corresponding to the aerobiosis 1 pond (Fig. 1A), suggesting that a high percentage of HEV had been inactivated to this point in anaerobic and aerobic ponds, and reduced its presence at levels not detectable by PCR.
There are no data reporting the resistance of HEV to anaerobic purification processes; however, a work about the resistance of porcine enteric virus of the Caliciviridae family at different purification treatments of slurry (Costantini et al., 2007) confirmed the reduction of the number of viral particles after subjecting slurry to anaerobic digestion. Additionally, this study confirmed that the aerobic treatment was able to reduce the concentration of more resistant virus, such as rotavirus type A, B and C, which themselves were capable to resist anaerobic processes, to undetectable levels by PCR.
Additionally, the fact of HEV in type A plants was detected in a higher percentage in the reception pond compared with the detection of HEV in solid products from centrifuge, could be due to the lesser resistance of the virus in solid matrices (Pesaro et al., 1995).
HEV was not detected in any solid samples of processed product by fermentation. The high temperatures achieved in the fermentation channels (approximately 65°C), could explain the lack of HEV amplification. In this sense, a study performed by Emerson and colleagues in 2005, conducted with human and swine strains, showed that HEV genotypes 1 and 2, were 50% inactivated at temperatures between 45°C and 50°C, and totally inactivated at 60°C. Only Mex 14 (genotype 2) strain was observed to be 100% resistant to 56°C and 20% to temperatures of 60°C (Emerson et al., 2005). In addition, a recent study (Barnaud et al., 2012), shows an efficient inactivation of the virus when food products from infected by HEV pork livers, were heated at least 20 min to an internal temperature of 71°C.
In this way, the low percentage of HEV positive samples after anaerobic digestion, suggests that large amounts of virus do not exceed this stage of treatment. The lack of detection of HEV in solid samples at the end phases of the composting treatment and in the final product, suggests that composting process is effective to eliminate this virus from the slurries, and thus to reduce the transmission of HEV from pigs to humans.
Experimental procedures
Collection of samples of composting plants
A total number of 594 liquid and solid samples were collected in 54 different sampling sessions from March 2006 to December 2011 in five plants distributed as follows (Table 1): two type A plants (A1 and A2) and three type B plants (B1, B2 and B3). The higher number of samples collected from type A plants, 504 (87.5%) is due to the fact that these plants assemble the majority of purification processes and therefore receive more manure than type B plants. In the same way, the higher number of liquid samples, 360 (60.6%) corresponds to the higher number of purification processes carried out in the liquid phase of the slurry.
The plants are located in Eastern Spain, in a zone endemic for porcine HEV (Fernández-Barredo et al., 2006). The plants were sampled in 54 different sampling sessions, in which a variable number of samples were taken in each stage taking into account the volume of each pond. Thus, in the anaerobic pond a total number of 180 samples were collected in five different points at each sampling. The pond was considered positive to HEV when at least one of the five sampling points was positive. The rest of the ponds were equipped with a homogenization system which was activated 10 min before the sampling, therefore only one sample of a larger volume was taken. Solid samples were homogenized by mechanical processes included in the periodic aeration system performed in the integrated treatment.
It was impossible to take the same number of samples in type B as in type A plants, due to a premature closing of the same ones because of technical and economical problems.
Liquid samples (50 ml), were collected directly from the ponds and kept in sterile falcon tubes until processing. All the samples were transported refrigerated immediately to the laboratory. Solid samples were diluted at 10% (w/v) in sterile phosphate buffer saline (PBS) pH 7.2.
Virus particle concentration, RNA extraction and reverse transcription-nested PCR (RT-nested PCR) with internal control
All the samples collected were initially centrifuged 1 min at 1000 g and 15 ml of the supernatant were centrifuged at 4°C, 1 h at 3000 g to concentrate the virus particles. Remaining supernatant (1.5 ml) was transferred to a sterile eppendorf tube and it was centrifuged at 12 100 g for 10 min, and 1 ml of the supernatant was stored at −80°C.
RNA was extracted from 140 μl of each concentrated sample according to the method described by Fernández-Barredo and colleagues (2006). Two pairs of degenerated oligonucleotide primers based on human and swine HEV sequences, were used to amplify a 348-bp-long fragment from the HEV open reading frame 2 (ORF-2) using a reverse transcription-nested polymerase chain reaction (RT-nested PCR) (Huang et al., 2002). A negative and a positive control from a naturally infected pig (GenBank Accession No. AY323506) were included in each assay. The different stages of the procedure were performed in different places to avoid the possibility of cross-contamination. The faecal samples may contain RT-PCR inhibition substances like phenolic and methabolic compounds, the concentration and presence of these inhibitors is different and heterogeneous from sample to sample (Rutjes et al., 2007). To detect the presence of these substances an internal control was included within RT-PCR reaction. The internal control used is a modified 77 bp PCR product cloned into a plasmid containing a sequence that can be amplified simultaneously with the target using the same primers set in the first PCR performed according to the protocol described by Huang and colleagues in 2002. The 77 bp amplified fragment was detected in the electrophoresis gel. PCR inhibition was not detected. The sensitivity of the PCR was estimated in 31.6 PID50 of infectious swine HEV (Huang et al., 2002).
The PCR products were separated by electrophoresis in a 2% agarose gel and detected by staining with ethidium bromide (0.5 μg ml−1). The samples were considered positive to HEV when a band of 348 bp was seen in the agarose gel. Amplicons from all positive samples were purified and confirmed by sequence analysis. Sequences obtained in this study have been submitted to the GenBank database under Accession No. KC145131–KC145147.
Acknowledgments
We are grateful to Ms Beatriz Suay for the assistance in translating the manuscript.
Conflict of interest
None declared.
References
- Ahn JM, Kang SG, Lee DY, Shin SJ, Yoo HS. Identification of novel human hepatitis E virus (HEV) isolates and determination of the seroprevalence of HEV in Korea. J Clin Microbiol. 2005;43:3042–3048. doi: 10.1128/JCM.43.7.3042-3048.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks M, Heath GS, Grierson SS, King DP, Gresham A, Girones R, et al. Evidence for the presence of hepatitis E virus in pigs in the United Kingdom. Vet Rec. 2004;154:223–227. doi: 10.1136/vr.154.8.223. [DOI] [PubMed] [Google Scholar]
- Barnaud E, Rogee S, Garry P, Rose N, Pavio N. Thermal inactivation of infectious hepatitis E virus in experimentally contaminated food. Appl Environ Microbiol. 2012;78:5153–5159. doi: 10.1128/AEM.00436-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolado-Rodriguez S, Garcia-Sinovas D, Alvarez-Benedi J. Application of pig slurry to soils. Effect of air stripping treatment on nitrogen and TOC leaching. J Environ Manage. 2010;91:2594–2598. doi: 10.1016/j.jenvman.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Cole D, Todd L, Wing S. Concentrated swine feeding operations and public health: a review of occupational and community health effects. Environ Health Perspect. 2000;108:685–699. doi: 10.1289/ehp.00108685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini VP, Azevedo AC, Li X, Williams MC, Michel FC, Jr, Saif LJ. Effects of different animal waste treatment technologies on detection and viability of porcine enteric viruses. Appl Environ Microbiol. 2007;73:5284–5291. doi: 10.1128/AEM.00553-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson SU, Arankalle VA, Purcell RH. Thermal stability of hepatitis E virus. J Infect Dis. 2005;192:930–933. doi: 10.1086/432488. [DOI] [PubMed] [Google Scholar]
- EUROSTAT DATABASE. 2009. Agricultural statistics 2009 edition. Main results — 2007–08. http://epp.eurostat.ec.europa.eu/cache/ITY_OFFPUB/KS-ED-09-001/EN/KS-ED-09-001-EN.PDF.
- Fernández-Barredo S, Galiana C, Garcia A, Vega S, Gomez MT, Perez-Gracia MT. Detection of hepatitis E virus shedding in feces of pigs at different stages of production using reverse transcription-polymerase chain reaction. J Vet Diagn Invest. 2006;18:462–465. doi: 10.1177/104063870601800506. [DOI] [PubMed] [Google Scholar]
- Fernández-Barredo S, Galiana C, Garcia A, Gomez-Munoz MT, Vega S, Rodriguez-Iglesias MA, Perez-Gracia MT. Prevalence and genetic characterization of hepatitis E virus in paired samples of feces and serum from naturally infected pigs. Can J Vet Res. 2007;71:236–240. [PMC free article] [PubMed] [Google Scholar]
- Galiana C, Fernández-Barredo S, Garcia A, Gomez MT, Perez-Gracia MT. Occupational exposure to hepatitis E virus (HEV) in swine workers. Am J Trop Med Hyg. 2008;78:1012–1015. [PubMed] [Google Scholar]
- Hazam RK, Singla R, Kishore J, Singh S, Gupta RK, Kar P. Surveillance of hepatitis E virus in sewage and drinking water in a resettlement colony of Delhi: what has been the experience? Arch Virol. 2010;155:1227–1233. doi: 10.1007/s00705-010-0707-z. [DOI] [PubMed] [Google Scholar]
- Huang FF, Haqshenas G, Guenette DK, Halbur PG, Schommer SK, Pierson FW, et al. Detection by reverse transcription-PCR and genetic characterization of field isolates of swine hepatitis E virus from pigs in different geographic regions of the United States. J Clin Microbiol. 2002;40:1326–1332. doi: 10.1128/JCM.40.4.1326-1332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz S, Arnold E, Banks M, Bendall RP, Cramp ME, Cunningham R, et al. Non-travel-associated hepatitis E in England and Wales: demographic, clinical, and molecular epidemiological characteristics. J Infect Dis. 2005;192:1166–1172. doi: 10.1086/444396. [DOI] [PubMed] [Google Scholar]
- Kasorndorkbua C, Opriessnig T, Huang FF, Guenette DK, Thomas PJ, Meng XJ, Halbur PG. Infectious swine hepatitis E virus is present in pig manure storage facilities on United States farms, but evidence of water contamination is lacking. Appl Environ Microbiol. 2005;71:7831–7837. doi: 10.1128/AEM.71.12.7831-7837.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuroo MS, Kamili S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J Viral Hepat. 2003;10:61–69. doi: 10.1046/j.1365-2893.2003.00398.x. [DOI] [PubMed] [Google Scholar]
- Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo T, Clemente R, Bernal MP. Effects of compost, pig slurry and lime on trace element solubility and toxicity in two soils differently affected by mining activities. Chemosphere. 2011;84:642–650. doi: 10.1016/j.chemosphere.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Perez-Gracia MT, Rodriguez-Iglesias M. [Hepatitis E virus: current status] Med Clin (Barc) 2003;121:787–792. [PubMed] [Google Scholar]
- Perez-Gracia MT, Garcia-Valdivia MS, Galan F, Rodriguez-Iglesias MA. Detection of hepatitis E virus in patients sera in southern Spain. Acta Virol. 2004;48:197–200. [PubMed] [Google Scholar]
- Perez-Gracia MT, Mateos ML, Galiana C, Fernández-Barredo S, Garcia A, Gomez MT, Moreira V. Autochthonous hepatitis E infection in a slaughterhouse worker. Am J Trop Med Hyg. 2007;77:893–896. [PubMed] [Google Scholar]
- Pesaro F, Sorg I, Metzler A. In situ inactivation of animal viruses and a coliphage in nonaerated liquid and semiliquid animal wastes. Appl Environ Microbiol. 1995;61:92–97. doi: 10.1128/aem.61.1.92-97.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pina S, Buti M, Cotrina M, Piella J, Girones R. HEV identified in serum from humans with acute hepatitis and in sewage of animal origin in Spain. J Hepatol. 2000;33:826–833. doi: 10.1016/s0168-8278(00)80316-5. [DOI] [PubMed] [Google Scholar]
- Pischke S, Wedemeyer H. Hepatitis E virus infection: multiple faces of an underestimated problem. J Hepatol. 2013;58:1045–1046. doi: 10.1016/j.jhep.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. Hepatitis E virus infection. Lancet. 2000;355:578. doi: 10.1016/S0140-6736(05)73231-1. [DOI] [PubMed] [Google Scholar]
- Purdy MA, Khudyakov YE. The molecular epidemiology of hepatitis E virus infection. Virus Res. 2011;161:31–39. doi: 10.1016/j.virusres.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Rutjes SA, Lodder WJ, Bouwknegt M, Husman AM. Increased hepatitis E virus prevalence on Dutch pig farms from 33 to 55% by using appropriate internal quality controls for RT-PCR. J Virol Methods. 2007;143:112–116. doi: 10.1016/j.jviromet.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Seminati C, Mateu E, Peralta B, de Deus N, MartIn M. Distribution of hepatitis E virus infection and its prevalence in pigs on commercial farms in Spain. Vet J. 2008;175:130–132. doi: 10.1016/j.tvjl.2006.11.018. [DOI] [PubMed] [Google Scholar]
- Sobsey M, Gwangpyo K, Simmons O, Likirdopulos C, Worley-Davis L. Evaluation of alternative swine waste treatment and management technologies for control of pathogens. In: Havenstein GB, editor. Proceedings North Carolina Animal Waste Management Workshop. Oct. 16–17, 2003, Research Triangle Park, NC. Raleigh, NC, USA: College of Agriculture and Life Sciences, NCSU; 2003. pp. 112–125. [Google Scholar]
- Spencer JL, Guan J. Public health implications related to spread of pathogens in manure from livestock and poultry operations. Methods Mol Biol. 2004;268:503–515. doi: 10.1385/1-59259-766-1:503. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kang JH, Ohnishi S, Hino K, Miyakawa H, Miyakawa Y, et al. Full-length sequences of six hepatitis E virus isolates of genotypes III and IV from patients with sporadic acute or fulminant hepatitis in Japan. Intervirology. 2003;46:308–318. doi: 10.1159/000073210. [DOI] [PubMed] [Google Scholar]
- Tolari F, Chiaro LD, Card R, Mazzei M, Bandecchi P, Banks M. Phylogenetic study of viral isolates of Swine and human hepatitis E virus. Vet Res Commun. 2006;30(Suppl. 1):273–276. [Google Scholar]
- Wagner AO, Gstraunthaler G, Illmer P. Survival of bacterial pathogens during the thermophilic anaerobic digestion of biowaste: laboratory experiments and in situ validation. Anaerobe. 2008;14:181–183. doi: 10.1016/j.anaerobe.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ziemer CJ, Bonner JM, Cole D, Vinje J, Constantini V, Goyal S, et al. Fate and transport of zoonotic, bacterial, viral, and parasitic pathogens during swine manure treatment, storage, and land application. J Anim Sci. 2010;88:E84–E94. doi: 10.2527/jas.2009-2331. [DOI] [PubMed] [Google Scholar]


