Abstract
Objectives
The primary objective of this study was to elucidate mechanisms underlying the link between vitamin D status and cardiovascular disease by exploring the relationship between 25-hydroxyvitamin D (25-OH D), an established marker of vitamin D status, and vascular function in healthy adults.
Background
Mechanisms underlying vitamin D deficiency-mediated increased risk of cardiovascular disease remain unknown. Vitamin D influences endothelial and smooth muscle cell function, mediates inflammation, and modulates the renin-angiotensin-aldosterone axis. We investigated the relationship between vitamin D status and vascular function in humans, with the hypothesis that vitamin D insufficiency will be associated with increased arterial stiffness and abnormal vascular function.
Methods
We measured serum 25-OH D in 554 subjects. Endothelial function was assessed as brachial artery flow-mediated dilation, and microvascular function was assessed as digital reactive hyperemia index. Carotid-femoral pulse wave velocity and radial tonometry-derived central augmentation index and subendocardial viability ratio were measured to assess arterial stiffness.
Results
Mean 25-OH D was 31.8 ± 14 ng/ml. After adjustment for age, sex, race, body mass index, total cholesterol, low-density lipoprotein, triglycerides, C-reactive protein, and medication use, 25-OH D remained independently associated with flow-mediated vasodilation (β = 0.1, p = 0.03), reactive hyperemia index (β = 0.23, p < 0.001), pulse wave velocity (β = −0.09, p = 0.04), augmentation index (β = −0.11, p = 0.03), and subendocardial viability ratio (β = 0.18, p = 0.001). In 42 subjects with vitamin D insufficiency, normalization of 25-OH D at 6 months was associated with increases in reactive hyperemia index (0.38 ± 0.14, p = 0.009) and subendocardial viability ratio (7.7 ± 3.1, p = 0.04), and a decrease in mean arterial pressure (4.6 ± 2.3 mm Hg, p = 0.02).
Conclusions
Vitamin D insufficiency is associated with increased arterial stiffness and endothelial dysfunction in the conductance and resistance blood vessels in humans, irrespective of traditional risk burden. Our findings provide impetus for larger trials to assess the effects of vitamin D therapy in cardiovascular disease.
Keywords: arterial stiffness, endothelial function, vitamin D
Vitamin D deficiency is a highly prevalent condition affecting 30% to 50% of the U.S. population and is associated with hypertension, insulin resistance, and left ventricular hypertrophy (1,2). More recently, vitamin D deficiency has been implicated as a risk factor for cardiovascular disease and overall mortality in the general population (3,4). Although the link between the traditional role of vitamin D in bone and calcium metabolism has been extensively studied, the mechanisms by which vitamin D deficiency confers vascular risk remain unexplained.
Vitamin D receptors and 1-alpha-hydroxylase, which converts vitamin D into the hormonal 1,25-dihydroxyvitamin D form, are present in many tissues including endothelial cells (5). Endothelial cell vitamin D receptors are up-regulated under stress, and its binding with the hormonal vitamin D ligand modulates response elements in the vascular endothelial growth factor promoter (6). Vitamin D also affects the vascular wall by regulating the renin-angiotensin-aldosterone axis and exerts anti-proliferative effects on vascular smooth muscle (7). Moreover, vitamin D plays a crucial role in immune modulation by regulating lymphocyte and monocyte/macrophage differentiation and release of inflammatory cytokines. This in turn might determine monocyte infiltration and cholesterol retention in the vascular wall (8,9).
It is well established that vascular endothelial dysfunction and arterial stiffness precede and contribute to the development of cardiovascular disease and are both predictors of long-term morbidity and mortality (10,11). We investigated the mechanisms underlying the link between vitamin D insufficiency and cardiovascular disease by exploring the relationship between 25-hydroxyvitamin D (25-OH D), an established marker of vitamin D status, and vascular function. Our hypothesis was that vitamin D insufficiency will be associated with increased arterial stiffness and abnormal vascular function.
Methods
Subjects
A total of 554 healthy participants, 20 to 79 years of age, were recruited by advertisement or by invitation to employees of Emory University and Georgia Institute of Technology, as part of the Emory Predictive Health Initiative. Subjects were free of any acute illness and had normal performance status. Hypertension, hypercholesterolemia, and diabetes mellitus were defined according to the Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; Adult Treatment Panel III; and American Diabetes Association criteria, respectively (12–14). Vascular testing and blood draws were performed after an overnight fast. Ninety-one subjects with serum 25-OH D levels <30 ng/ml at baseline testing had repeat measurements performed after 6 months. The study was approved by the Emory University Institutional Review Committee. Informed consent was obtained from all subjects.
Laboratory tests
Blood samples were obtained for a lipid profile, and metabolic panel, urine albumin/creatinine ratio, high-sensitivity C-reactive protein (CRP), and serum 25-OH D levels (liquid chromatography and tandem mass spectrometry) were measured by commercially available assays (Quest Diagnostics, Madison, New Jersey). The detection limit for the 25-OH D was 4 ng/ml, with a previously reported inter-assay variation coefficient of approximately 10% (15).
Arterial stiffness testing
After an initial rest period of 10 min with subjects in a supine position in a quiet, temperature-controlled room, blood pressure was measured 3 times at 5-min intervals by an automatic device (Omron, Kyoto, Japan) from the dominant arm and documented as the mean value of the final 2 measurements.
Pulse wave velocity (PWV), augmentation index (AIX), and subendocardial viability ratio (SEVR) were derived with the Sphygmocor device (Atcor Medical, Sydney, Australia), as previously described (16). Briefly, the setup consists of a handheld tonometer attached to a device and a laptop computer. High-fidelity sequential pressure waveforms are obtained by placing the tonometer on the radial artery of the dominant wrist. The device then applies a transfer function to these peripheral measurements to estimate central (aortic) pressure parameters and the degree of pressure augmentation secondary to reflected waves from the periphery. This permits derivation of an AIX (augmented pressure/total central pulse pressure) and SEVR (area under diastolic phase/systolic phase), which are considered complex and composite markers of wave reflections and arterial stiffening. Due to its sensitivity to heart rate, a standardized value to 75 beats/min is calculated and used for AIX for the purpose of this study.
The PWV was determined by acquiring waveforms at the carotid and femoral arterial sites with electrocardiogram gating. Velocity (distance/time in m/s) was calculated by measuring the time interval between electrocardiogram R-wave and the recorded waveforms at each site, whereas distance between sites was measured manually. Quality control indexes were evaluated at the time of study and nonacceptable readings discarded and repeated. Reproducibility studies in our laboratory on 9 subjects on consecutive days have demonstrated a coefficient of variation of 3.8%, 13.8%, and 20.3% for PWV, SEVR, and AIX, respectively.
Flow-mediated dilation
Endothelium-dependent brachial artery flow-mediated dilation (FMD) was measured to evaluate endothelium-dependent vascular function. The detailed methodology for performing this test has been described previously (17). In our laboratory, the mean difference in FMD between assessments performed in 11 subjects on consecutive days was 1.26 ± 0.76%, with a correlation coefficient of 0.75. The mean difference in the FMD between 2 readings of the same 11 measurements was 0.82 ± 0.48% (r = 0.97).
Pulsatile arterial tonometry
During the FMD testing described in the preceding text, digital vascular function was also assessed simultaneously as fingertip reactive hyperemia (Endo-PAT2000, Itamar Medical, Caesarea, Israel) as described previously (18). Briefly, pneumatic plethysmographs apply uniform pressure at both index fingers that allows for measurement of minute, pulsatile volume changes, generating a pulse wave tracing. Reactive hyperemia index (RHI) is defined as the ratio of post-deflation to baseline pulse amplitude in the hyperemic finger divided by that in the contra-lateral finger.
Statistical considerations
Study variables are described as the mean ± SD (unless otherwise specified) for continuous variables or as counts or proportions for categorical variables. Age, body mass index, 25-OH D level, CRP, lipid profile, FMD, RHI, PWV, AIX, and SEVR values were treated as continuous variables. Diagnosed hypertension, hypercholesterolemia, diabetes mellitus, smoking, sex, medication use (anti-hypertensive drugs, lipid or glucose lowering agents), and vitamin D supplementation (≥10 μg/day) were categorical variables. Continuous variables were tested for normality with the Kolmogorov-Smirnov criterion. Skewed variables were log transformed and tested again for normality before any parametric analysis.
Univariate correlations between serum 25-OH D concentrations and measured parameters were performed with Pearson's correlation. Multivariate linear regression models were constructed to determine relationships between vitamin D status and vascular function parameters before and after adjustment for age, sex, race, body mass index, total cholesterol, low-density lipoprotein, triglycerides, CRP, and medication use. Group differences were evaluated by Student t tests or 1-way analysis of variance.
For follow-up analysis, participants with vitamin D insufficiency at baseline (n = 91) were divided into those who normalized their 25-OH D ≥30 ng/ml levels and those who remained insufficient (25-OH <30 ng/ml) after 6 months. We compared average changes in mean arterial pressure, FMD, RHI, PWV, AIX, and SEVR (baseline – 6-month value) between groups with unpaired samples t tests. Paired samples t tests were performed for comparison of within-group differences. Data are presented as mean ± SD in the text and as specified in the legends of Figures 1, 2, and 3. Statistical significance was based on 2-tailed tests, and p values ≤0.05 were considered significant. Analyses were performed with SPSS (version 17.0, SPSS, Inc., Chicago, Illinois).
Figure 1. Relationship Between Serum 25-OH D, Arterial Stiffness, and Vascular Function.
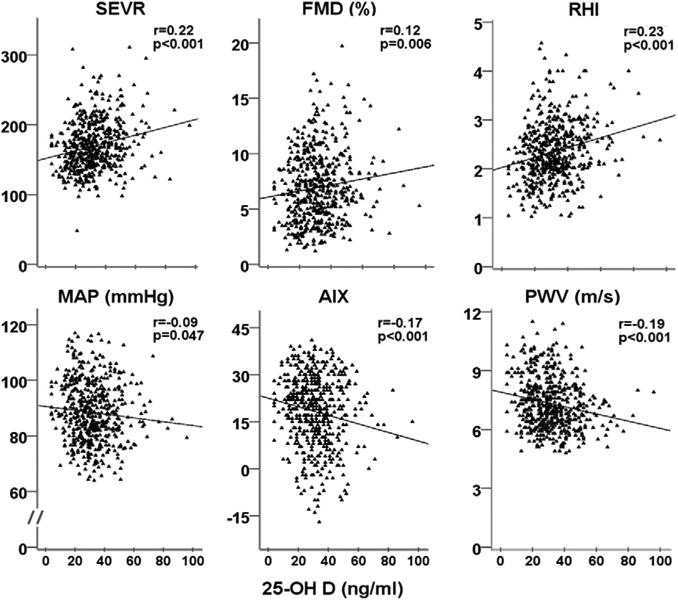
25-OH D = 25-hydroxyvitamin D (ng/ml); AIX = augmentation index; FMD = brachial artery flow mediated dilation (%); MAP = mean arterial pressure (mm Hg); PWV = pulse wave velocity (m/s); RHI = reactive hyperemia index; SEVR = subendocardial viability ratio. p values are for 2-tailed test of significance.
Figure 2. Cardiovascular Risk Burden and Levels of Serum 25-OH D.
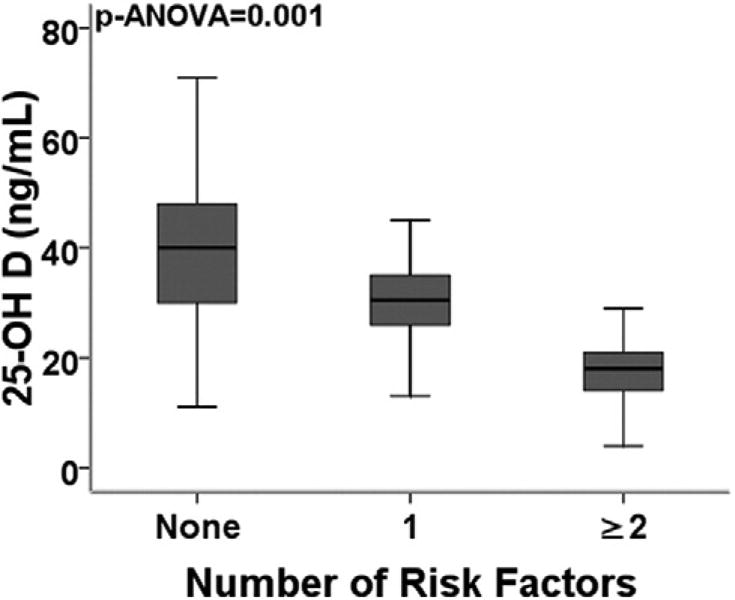
Risk factors included: age (men older than 55 or women older than 65 years), presence of diabetes mellitus, hypertension, hypercholesterolemia, or smoking history. Box plots: the middle band represents median, bottom and top of the box represent lower and upper quartiles, and end of whiskers represent highest and lowest values that are not outliers. p value for 1-way analysis of variance. n = 266, 128, and 150 for groups with 0, 1, or >2 risk factors, respectively. 25-OH D = 25-hydroxyvitamin D.
Figure 3. Follow-Up Measurements in Subjects With Vitamin D Insufficiency.
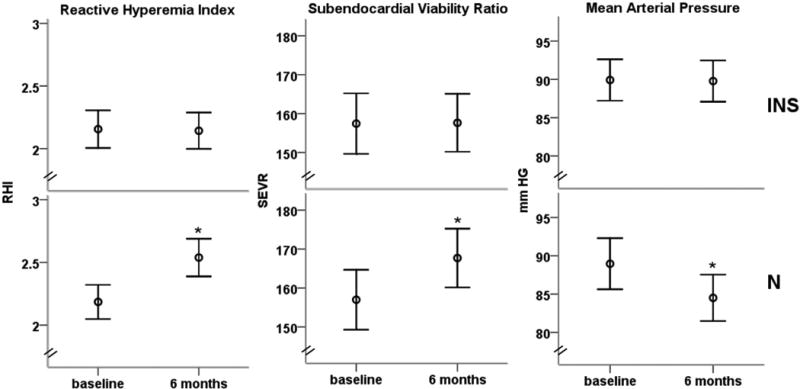
Mean arterial pressure, reactive hyperemia index, and subendocardial viability ratio at baseline and after 6 months in 49 subjects who remained insufficient (25-OH D <30 ng/ml) after 6 months (top panels), and those in whom vitamin D levels normalized after 6 months (n = 42, bottom panels). Error bars represent standard error. *Significant between and within-group differences.
Results
Baseline characteristics of the 554 subjects are shown in Table 1. Mean 25-OH D level was 31.8 ng/ml (range 4 to 96 ng/ml), and 47% of subjects had insufficient 25-OH D levels (<30 ng/ml).
Table 1. Demographic Characteristics.
| Age (yrs) | 47 ± 13 |
| Men | 45 |
| Caucasian | 71 |
| African American | 22 |
| Hispanic | 2 |
| Body mass index (kg/m2) | 26 ± 5 |
| Diabetes | 5 |
| Hypertension | 19 |
| Hypercholesterolemia | 25 |
| Smoking | 3 |
| 25-hydroxyvitamin D (ng/ml) | 32 ± 14 |
| Calcium (mg/dl) | 9.4 ± 0.3 |
| Cholesterol/high-density lipoprotein | 3.1 ± 0.9 |
| Triglycerides (mg/dl) | 94 ± 46 |
| hs-CRP (mg/dl) | 0.6 ± 0.8 |
| Statin use | 25 |
| Vitamin D supplements | 32 |
| Framingham Risk Score | 2.7 ± 6.2 |
Values are mean ± SD or %.
CRP = C-reactive protein.
Relationship between vitamin D status and subject characteristics
Serum 25-OH D correlated with body mass index (r = −0.23, p < 0.001) and high-density lipoprotein levels (r = 0.2, p < 0.001). Among the categorical variables, 25-OH D was significantly lower in women, African Americans, Hispanics, and those with hypertension or diabetes (Table 2). Subjects taking regular vitamin D supplementation had higher 25-OH D levels (38 ng/ml vs. 29 ng/ml, p < 0.001) and were significantly older (54 years vs. 46 years, p = 0.001). When the 544 subjects were divided on the basis of risk factor burden into groups with none, 1, or 2 or more risk factors, a stepwise decline in mean 25-OH D levels was observed with increasing risk factor burden (p analysis of variance = 0.012) (Fig. 2).
Table 2. Relationship Between Vitamin D Status and Subject Characteristics.
| ng/ml | p Value | |
|---|---|---|
| Sex | ||
| Men | 34 ± 14 | 0.03 |
| Women | 31 ± 13 | |
|
| ||
| Race | ||
| Caucasian | 35 ± 14 | <0.001 |
| African American | 23 ± 12 | |
|
| ||
| Diabetes | ||
| Yes | 22 ± 8 | 0.001 |
| No | 32 ± 14 | |
|
| ||
| Hypertension | ||
| Yes | 26 ± 15 | 0.006 |
| No | 34 ± 13 | |
|
| ||
| Hyperlipidemia | ||
| Yes | 32 ± 14 | NS |
| No | 32 ± 14 | |
|
| ||
| Vitamin D supplement | ||
| Yes | 38 ± 12 | <0.001 |
| No | 29 ± 14 | |
Values are mean ± SD serum 25-hydroxyvitamin D concentration. p values were derived from the Student independent samples t test.
Relationship between vitamin D status and arterial stiffness
Lower levels of 25-OH D were associated with abnormalities in the indexes of arterial stiffness with higher AIX and PWV and lower SEVR (Fig. 1). After multivariate adjustment for age, sex, race, body mass index, total cholesterol, low-density lipoprotein, triglycerides, C-reactive protein, and medication use, 25-OH D remained independently associated with PWV (adjusted R2 = 0.32, β = −0.09, p = 0.04), AIX (adjusted R2 = 0.42, β = −0.11, p = 0.03), and SEVR (adjusted R2 = 0.16, β = 0.18, p = 0.001).
Relationship between vitamin D status and vascular function
Lower levels of 25-OH D were associated with abnormalities in the indexes of vascular function with lower FMD and RHI (Fig. 1). Moreover, 25-OH D levels had an independent association with FMD (adjusted R2 = 0.11, β = 0.1, p = 0.03) and RHI (adjusted R2 = 0.09, β = 0.23, p < 0.001) in multivariate analysis adjusting for the aforementioned variables.
In age-, sex-, and race-adjusted analyses, no significant correlation existed between log CRP and 25-OH D.
Subgroup analysis in subjects free of cardiovascular risk factors
A secondary analysis was performed in a subgroup of 233 “healthy” subjects with absence of traditional cardiovascular risk factors, to explore the association between levels of 25-OH D and vascular function independent of the confounding effects of risk factors and vitamin D intake. After multivariate adjustment for age, sex, race, body mass index, systolic arterial pressure, and total cholesterol/high-density lipoprotein ratio, 25-OH D levels remained independently associated with AIX (adjusted R2 = 0.44, β = −0.02, p < 0.001), PWV (adjusted R2 = 0.2, β = 0.02, p = 0.005), FMD (adjusted R2 = 0.12, β = −0.01, p = 0.04), and RHI (adjusted R2 = 0.07, β = 0.02, p = 0.003) in this cohort but accounted for small changes in variability.
Follow-up
Of the 91 participants with vitamin D insufficiency, 42 had normalized vitamin D status (25-OH D ≥30 ng/ml) and 49 subjects remained in insufficient status after 6 months. Subjects who normalized their 25-OH D levels had significant increases in RHI (0.38 ± 0.29, p = 0.009) and SEVR (7.7 ± 11.4, p = 0.04) and a decrease in mean arterial pressure (4.6 ± 5.6 mm Hg, p = 0.02). These differences were significantly greater when compared with subjects where 25-OH D levels remained in the insufficient range (0.38 ± 0.29 vs. −0.07 ± 0.33, p = 0.01; 7.7 ± 11.4 vs. −1.1 ± 9.5, p = 0.03; −4.6 ± 5.6 mm Hg vs. −0.9 ± 4.7 mm Hg, p = 0.02, for RHI, SEVR, and mean arterial pressure, respectively) (Fig. 3). Thus, subjects with corrected vitamin D insufficiency had improved microvascular vasodilation and arterial stiffness.
Discussion
In a study that comprehensively assesses vascular function in a community-based asymptomatic population, we demonstrated an association between vitamin D status and several indexes of vascular health, including arterial stiffness and endothelial function. We studied a large multiethnic population over a wide age range but with a relatively low risk factor burden, and we employed multiple established modalities to assess subclinical vascular dysfunction. Increased arterial stiffness—assessed by measurements of PWV, AIX, and SEVR—was associated with lower 25-OH D levels. In addition, lower 25-OH D levels were associated with worse vascular endothelial function, assessed in the conductance vessels with FMD and in the microvasculature with pulsatile digital arterial tonometry. Moreover, subgroup analysis performed in selected individuals free of all vascular risk factors in order to exclude the confounding effects of risk factors on vascular function also revealed an independent association between vitamin D status and vascular function. These observations are consistent with the well-established associations between vitamin D deficiency and a broad range of cardiovascular disorders and risk factors (1,3).
25-hydroxyvitamin D (25-OH D) is considered to be the best indicator of vitamin D status in those with normal kidney function and reflects the level of circulating substrate for the tightly regulated hydroxylation into the active, hormonal form of vitamin D (1,25-OH2 D) (1). Alternatively, measurement of 1,25-OH2 D is performed in chronic kidney disease, as decreases in renal 1-α-hydroxylase activity often result in low 1,25-OH2 D levels and hypocalcemia.
Previous small studies have also shown increased AIX and lower FMD in hemodialysis patients with lower levels of 1,25-OH2 D, but our findings in a community-based population are novel (19,20). In hypertensive patients, levels of 25-OH D were inversely associated with plethysmography-derived calf vascular resistance (21), and the third National Health and Nutrition Examination Survey found increased pulse pressure, a nonspecific marker of arterial compliance, with vitamin D deficiency (22). Finally, few well-designed interventional trials have explored whether vascular function can be improved by vitamin D supplementation (23,24).
In experimental studies vitamin D modulates endothelial cell function by decreasing expression of adhesion molecules, providing protection against advanced glycation products, by reduction in endothelium-dependent contractions and modulation of calcium influx (25–27). Vitamin D deficiency also activates the renin-angiotensin-aldosterone system, causes proliferation of vascular smooth muscle cells, activates macrophage invasion of the vascular wall, promotes calcification, and increases parathyroid hormone release, effects that are ameliorated by vitamin D supplementation (7,25,28). Finally, vitamin D might modulate adaptive immunity by reducing inflammatory cytokine gene expression, alternating circulating subsets of T-cells, and reducing inflammation (29,30). In clinical studies, an increased incidence of adverse cardiovascular events has been found in the Framingham cohort with decreased serum 25-OH D, and community-based studies have observed associations between vitamin D deficiency and cardiovascular disease and its risk factors, as in our population (3,4).
Our findings of increased arterial stiffness and endothelial dysfunction with low 25-OH D provide mechanistic explanation of how depressed vitamin D status, by precipitating vascular dysfunction, might predispose individuals to a higher risk for the development of cardiovascular disease and adverse events. Brachial artery FMD, a measure of endothelial function and nitric oxide bioavailability, is also an independent predictor of future adverse cardiovascular events (31). Persistence of endothelial dysfunction despite therapeutic interventions is associated with worse prognosis, and normalization of FMD leads to improved outcomes (32). Similarly, RHI has been negatively associated with cardiovascular risk factors, and low RHI seems to be associated with worse long-term outcome (33). Similarly, arterial stiffness indexes including AIX and SEVR are independent predictors of adverse outcomes (16,34).
Increased arterial stiffness and endothelial dysfunction are also strong predictors for future development of hypertension (35). In this context, the majority of large cross-sectional studies have demonstrated an inverse relationship between 25-OH D levels and blood pressure, and the risk of future hypertension seems to be greater in those with vitamin D deficiency (36,37). Although interventional trials with vitamin D supplementation in hypertension are sparse, a study in post-menopausal women reported a 7.8-mm Hg reduction in systolic blood pressure with vitamin D replacement (38).
Our findings of higher serum 25-OH D in men compared with women, Caucasians compared with African Americans and Hispanics, and in participants taking vitamin D have been previously reported (39). Similarly, higher body mass index, lower high-density lipoprotein, and presence of diabetes or hypertension were associated with decreased 25-OH D levels (1) (Table 2).
It is noteworthy that our subjects were asymptomatic and had normal serum calcium and urinary albumin/creatinine ratio (Table 1). In addition, all subjects had an estimated glomerular filtration rate >60 ml/min/1.73 m2, and none reported use of high-dose vitamin D supplements (>2,000 IU daily) at baseline. Although the highest 25-OH D level was 96 ng/ml in our population, levels >240 ng/ml have been associated with displacement of bound 1,25-OH2 D and subsequent hypercalcemia (40). Although vitamin D toxicity can be serious, vitamin D supplementation normalizes 25-OH D levels and exerts antirachitic effects. Finally, novel vitamin D analogs with less calciphylactic effects need to be investigated in cardiovascular disease.
Study limitations
The cross-sectional, observational design of our current study precludes definitive conclusions regarding the causal relationship between vascular dysfunction and hypovitaminosis D. Also, the magnitude of contribution of hypovitaminosis D to vascular dysfunction might be variable and modest. However, the improvements we observed in some of these subclinical markers of vascular health with normalization of vitamin D insufficiency help confirm the link between vitamin D status and vascular health and also highlight a potential role for vitamin D therapy in reducing cardiovascular risk. We did not find significant improvements in all measures of vascular dysfunction in the follow-up study in those who normalized vitamin D levels. This might be because we were underpowered to observe such a change, the study was observational, and the supplementation was variable. However, vitamin D supplementation seems to significantly improve vascular function (23,24).
It is also possible that other subject characteristics such as physical activity might account for the associations we observed. Increased physical activity is known to improve vascular function and might also improve vitamin D status by increasing sunlight exposure. We previously demonstrated an independent correlation between vitamin D status and cardiovascular fitness, measured by cardiopulmonary exercise testing (41).
Conclusions
Vitamin D insufficiency is associated with increased arterial stiffness and endothelial dysfunction in the conductance and resistance blood vessels in humans, irrespective of traditional risk factor burden. Normalization of vitamin D status in insufficient individuals is associated with improvements in several parameters of vascular function. Our findings provide impetus for larger trials to assess the effect of vitamin D therapy on prevention of cardiovascular disease in individuals with vitamin D insufficiency.
Supplementary Material
Acknowledgments
This study was supported by the American Heart Association, The Emory Predictive Health Institute, Woodruff Fund, and in part by National Institutes of Health Grants UL1RR025008 from the Clinical and Translational Science Award program and M01RR0039.
Abbreviations and Acronyms
- 25-OH D
25-hydroxyvitamin D
- AIX
augmentation index
- CRP
C-reactive protein
- FMD
(brachial artery) flow-mediated dilation
- PWV
pulse wave velocity
- RHI
reactive hyperemia index
- SEVR
subendocardial viability ratio
Footnotes
The authors have reported that they have no relationships to disclose.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Judd SE, Nanes MS, Ziegler TR, Wilson PW, Tangpricha V. Optimal vitamin D status attenuates the age-associated increase in systolic blood pressure in white Americans: results from the third National Health and Nutrition Examination Survey. Am J Clin Nutr. 2008;87:136–41. doi: 10.1093/ajcn/87.1.136. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–37. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1,25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13:621–9. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 6.Raymond MA, Desormeaux A, Labelle A, et al. Endothelial stress induces the release of vitamin D-binding protein, a novel growth factor. Biochem Biophys Res Commun. 2005;338:1374–82. doi: 10.1016/j.bbrc.2005.10.105. [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kreindler JL, Steele C, Nguyen N, et al. Vitamin D3 attenuates Th2 responses to Aspergillus fumigatus mounted by CD4+ T cells from cystic fibrosis patients with allergic bronchopulmonary aspergillosis. J Clin Invest. 2010;120:3242–54. doi: 10.1172/JCI42388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oh J, Weng S, Felton SK, et al. 1,25(OH)2 vitamin d inhibits foam cell formation and suppresses macrophage cholesterol uptake in patients with type 2 diabetes mellitus. Circulation. 2009;120:687–98. doi: 10.1161/CIRCULATIONAHA.109.856070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quyyumi AA. Prognostic value of endothelial function. Am J Cardiol. 2003;91:19H–24H. doi: 10.1016/s0002-9149(03)00430-2. [DOI] [PubMed] [Google Scholar]
- 11.Weber T, Auer J, O'Rourke MF, et al. Arterial stiffness, wave reflections, and the risk of coronary artery disease. Circulation. 2004;109:184–9. doi: 10.1161/01.CIR.0000105767.94169.E3. [DOI] [PubMed] [Google Scholar]
- 12.Lenfant C, Chobanian AV, Jones DW, Roccella EJ, Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure Seventh Report of the Joint National Committee on the Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7): resetting the hypertension sails. Hypertension. 2003;41:1178–9. doi: 10.1161/01.HYP.0000075790.33892.AE. [DOI] [PubMed] [Google Scholar]
- 13.Executive Summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 14.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF, Biancuzzo RM, Chen TC, et al. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab. 2008;93:677–81. doi: 10.1210/jc.2007-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 18.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–74. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade J, Er L, Ignaszewski A, Levin A. Exploration of association of 1,25-OH2D3 with augmentation index, a composite measure of arterial stiffness. Clin J Am Soc Nephrol. 2008;3:1800–6. doi: 10.2215/CJN.00900208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.London GM, Guerin AP, Verbeke FH, et al. Mineral metabolism and arterial functions in end-stage renal disease: potential role of 25-hydroxyvitamin D deficiency. J Am Soc Nephrol. 2007;18:613–20. doi: 10.1681/ASN.2006060573. [DOI] [PubMed] [Google Scholar]
- 21.Duprez D, de Buyzere M, de Backer T, Clement D. Relationship between vitamin D3 and the peripheral circulation in moderate arterial primary hypertension. Blood Press. 1994;3:389–93. doi: 10.3109/08037059409102292. [DOI] [PubMed] [Google Scholar]
- 22.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 23.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with Type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 24.Tarcin O, Yavuz DG, Ozben B, et al. Effect of vitamin D deficiency and replacement on endothelial function in asymptomatic subjects. J Clin Endocrinol Metab. 2009;94:4023–30. doi: 10.1210/jc.2008-1212. [DOI] [PubMed] [Google Scholar]
- 25.Martinesi M, Bruni S, Stio M, Treves C. 1,25-Dihydroxyvitamin D3 inhibits tumor necrosis factor-alpha-induced adhesion molecule expression in endothelial cells. Cell Biol Int. 2006;30:365–75. doi: 10.1016/j.cellbi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 26.Talmor Y, Golan E, Benchetrit S, et al. Calcitriol blunts the deleterious impact of advanced glycation end products on endothelial cells. Am J Physiol Renal Physiol. 2008;294:F1059–64. doi: 10.1152/ajprenal.00051.2008. [DOI] [PubMed] [Google Scholar]
- 27.Wong MS, Delansorne R, Man RY, Vanhoutte PM. Vitamin D derivatives acutely reduce endothelium-dependent contractions in the aorta of the spontaneously hypertensive rat. Am J Physiol Heart Circ Physiol. 2008;295:H289–96. doi: 10.1152/ajpheart.00116.2008. [DOI] [PubMed] [Google Scholar]
- 28.Helming L, Bose J, Ehrchen J, et al. 1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation. Blood. 2005;106:4351–8. doi: 10.1182/blood-2005-03-1029. [DOI] [PubMed] [Google Scholar]
- 29.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834–9. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92:2017–29. doi: 10.1210/jc.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin EJ, Larson MG, Keyes MJ, et al. Clinical correlates and heritability of flow-mediated dilation in the community: the Framingham Heart Study. Circulation. 2004;109:613–9. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 32.Kitta Y, Obata JE, Nakamura T, et al. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–30. doi: 10.1016/j.jacc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 33.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31:1142–8. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 34.Sutton-Tyrrell K, Najjar SS, Boudreau RM, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111:3384–90. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 35.Quyyumi AA, Patel RS. Endothelial dysfunction and hypertension: cause or effect? Hypertension. 2010;55:1092–4. doi: 10.1161/HYPERTENSIONAHA.109.148957. [DOI] [PubMed] [Google Scholar]
- 36.Pilz S, Tomaschitz A, Ritz E, Pieber TR. Vitamin D status and arterial hypertension: a systematic review. Nat Rev Cardiol. 2009;6:621–30. doi: 10.1038/nrcardio.2009.135. [DOI] [PubMed] [Google Scholar]
- 37.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 38.Pfeifer M, Begerow B, Minne HW, Nachtigall D, Hansen C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J Clin Endocrinol Metab. 2001;86:1633–7. doi: 10.1210/jcem.86.4.7393. [DOI] [PubMed] [Google Scholar]
- 39.Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am J Hypertens. 2007;20:713–9. doi: 10.1016/j.amjhyper.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 40.Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22(Suppl 2):V64–8. doi: 10.1359/jbmr.07s221. [DOI] [PubMed] [Google Scholar]
- 41.Al Mheid, Ramadan Ronnie, Kavtaradze Nino, et al. Vitamin D levels are associated with exercise capacity and measures of endothelial function in healthy humans (abstr) Circulation. 2009;120:s551. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


