Abstract
Background
In 2006, the U.S. Food and Drug Administration (FDA) investigated cardiac and psychiatric risks associated with attention deficit/hyperactivity disorder (ADHD) medication use.
Aims of the Study
To examine how disclosure of safety risks affected pediatric ADHD use, and to assess news media coverage of the issue to better understand trends in treatment patterns.
Methods
We used the AHRQ’s Medical Expenditure Panel Survey (MEPS), a nationally representative household panel survey, to calculate unadjusted rates of pediatric ADHD use from 2002 to 2008 overall and by parents’ education. We examined whether children (ages 0 to 20) filled a prescription for any ADHD medication during the calendar year. Next, we used content analysis methods to analyze news coverage of the issue in 10 high-circulation newspapers, the 3 major television networks and a major cable news network in the U.S. We examined 6 measures capturing information conveyed on risk and benefits of ADHD medication use.
Results
No declines in medication use following FDA safety warnings overall or by parental education level were observed. News media coverage was relatively balanced in its portrayal of the risks and benefits of ADHD medication use by children.
Discussion
ADHD risk warnings were not associated with large declines in medication use, and balanced news coverage may have contributed to the treatment patterns observed. Self-reported surveys like the MEPS rely on the recall of respondents and may be subject to reporting bias. However, the validity of these data is supported by their consistency with other data on drug use from other sources.
Implications for Health Care Provision and Use
These findings are in direct contrast to the substantial declines in use observed after pediatric antidepressant risk warnings in the context of a news media environment that emphasized risks over benefits.
Implications for Health Policies
Our findings are relevant to the ongoing discussion about improving the FDA’s ability to monitor drug safety. Safety warnings occur amid ongoing concern that the agency has insufficient authority and resources to fulfill its mission to protect the public’s health. Efforts to bolster the FDA’s postmarketing surveillance system have the potential to incorporate more data in decision making to allow for earlier detection of health risks.
Implications for Further Research
Further research is needed to assess whether other treatment changes occurred following risk warnings. For example, it is important to determine whether an increase in cardiac screening prior to medication initiation occurred. Likewise, the FDA advises that children experiencing hallucinations or other psychiatric responses to medication be discontinued from drug treatment. If it is determined that instead of being discontinued from medication treatment, children experiencing hallucinations are put on additional medication (e.g., antipsychotics), additional efforts by the FDA to better inform the public are warranted.
Four and one-half million school age children had a diagnosis of attention deficit/hyperactivity disorder (ADHD) in 2006.1 The primary symptoms of ADHD are inattention, hyperactivity and impulsivity, and the condition can lead to difficulties in school, challenges in relationships with peers and family members, and self-esteem problems.2 There are several effective and clinically proven options for treating children with ADHD including medication treatment.3 As of 2007, $2.3 billion was spent on prescription medication to treat the condition.4 Research indicates that use of ADHD medications rose steeply from 1987 to 1996,5 but has leveled off in recent years.6
Recently, concerns have arisen related to cardiac and psychiatric risks associated with ADHD medication use. In February 2005, Health Canada suspended sales of Adderall due to reports of 20 sudden unexplained deaths in children taking the medication. At this time, the FDA issued a statement that there was no conclusive evidence linking stimulant use with cardiac risks. In August 2005, Health Canada returned Adderall to the market with revised warning labels noting that the medication should not be used in patients with structural cardiac abnormalities. In February 2006, the FDA convened its Drug Safety and Risk Management Advisory Committee to make recommendations to the agency on how to research the possible association between ADHD medications and cardiac problems. Controlled trials had failed to show a correlation between these medications and serious heart problems, but there was concern that trials did not include enough patients with an underlying heart issue and did not last long enough to uncover evidence of a connection. The FDA advisory committee voted unanimously to recommend new medication guides and, in an unexpected move, voted 8 to 7 in favor of adding a black box warning to ADHD medications. The next month, in March 2006, a second FDA panel, the FDA Pediatric Advisory Committee, reviewed evidence of a slight increase in risk (1 per 1,000) of psychiatric events including hallucinations and hearing voices in patients with no previous psychiatric problems and recommended new warning labels but not a black box warning.
In February 2007, the FDA declined to follow the drug safety advisory committee’s recommendation to issue a black box warning on ADHD medications related to cardiac risks. However, the agency directed manufacturers to develop medication guides to alert patients and providers to both cardiac risks and psychiatric symptoms associated with ADHD medication use.
In this study, we considered three research questions.
First, did rates of ADHD medication use change after the FDA investigation of cardiac and psychiatric safety risks? Safety concerns about ADHD medications followed on the heels of the much-publicized controversy related to possible links between pediatric antidepressant use and suicidality. Sharp declines in pediatric antidepressant use occurred following the FDA’s release of safety information. Estimates of the magnitudes of these declines ranged from about 20 to 30 percent.7–12
Second, did responses to risk information differ among children with more compared with less educated parents? College-educated parents may have greater access to scientific risk information, and may be more likely to make treatment decisions on the basis of this information compared to other parents. Conversely, more educated parents, with a heightened awareness of the long-term effects of untreated ADHD, may be more reticent to forgo medication for their child. Prior work suggested that, in the case of pediatric antidepressant use, children of college educated parents had more pronounced declines in medication use compared with other parents.13
Finally, in reporting on FDA actions, did the news media emphasize the risks of pediatric ADHD medication use over the benefits? A majority of Americans describe the news media as a critical source of health information,14 and how the news media frames the problem will likely affect how parents and providers respond to safety information.15 One prior study identified a high volume of news coverage regarding FDA disclosure of antidepressant risks,16 and these news stories could have played a role in the drop in antidepressant use. Our interest is in better understanding how news media coverage may have shaped how parents and providers understood the risks associated with ADHD medications.
Data and Methods
Survey Data
To examine ADHD medication use, we used the Medical Expenditure Panel Survey (MEPS), an ongoing nationally representative panel survey of the U.S. civilian non-institutionalized population, with approximately 17,000 individuals in each cohort. A household remains in the panel for two years, and is surveyed five times over that period. During each round, household respondents are asked to record all health care utilization, including prescribed medication, since the prior round. For families reporting prescription drug use, pharmacies identified by the household were contacted to identify the date a prescription was filled, national drug code (NDC), medication name, strength (amount and unit), and quantity (package size and amount dispensed). We limited our sample to children, adolescents and young adults ages 0 to 20, excluding individuals without information for all survey rounds or not in their household for the entire calendar year. We created a dichotomous variable indicating whether the subject filled a prescription for any ADHD medication during the calendar year. ADHD medication use was identified using NDC codes and brand and generic drug names included in the MEPS prescription drug files. (See list of medications in Appendix, Table Al). We included all ADHD medications listed on the FDA safety warning.17 We created a dichotomous variable indicating whether either the child’s mother or father had a bachelor’s degree or higher at the time of the survey.
News Media Data
To examine news media coverage, we collected all news stories on pediatric ADHD use and cardiac or hallucination-related safety warnings published in a convenience sample of 10 of the highest circulation print newspapers in the U.S., the three major television networks (ABC, NBC, and CBS) and a major cable news network (CNN) over the two-year period from June 2005 to May 2007. The news outlets included in our study are USA Today, Wall Street Journal, New York Times, Los Angeles Times, Washington Post, Chicago Tribune, New York Daily News, Philadelphia Inquirer, Denver Post/Rocky Mountain News, and Houston Chronicle. We used data from the Audit Bureau of Circulation to identify daily U.S. newspaper circulation rates and Nielsen Media Research to identify television viewership. We chose a convenience sample of news outlets with high circulation or viewership to analyze news coverage transmitted to a large subset of the American news-viewing public.
We used Lexis-Nexis, Factiva, and newspaper online archives to collect newspaper articles and Lexis-Nexis to collect television transcripts using the terms ADHD and FDA or close variants with specific mention of either cardiac or hallucination-related pediatric health risks. We excluded stories coded as editorials, corrections, book reviews, letters to the editor, business/stock information, Q&A format, duplicate wire stories, index, or obituaries. We also excluded stories where risk warnings were not the primary focus and stories in which the primary focus was not children or adolescents. Exclusions were determined by the study authors (CB, SB).
The methods for collecting news media data on ADHD medication safety warnings were identical to a previously published study of news media coverage of FDA safety warnings related to suicidality and pediatric antidepressant use.16 News media coverage of pediatric antidepressant use was also collected over a two-year period, January 2003-December 2004, before and after the release of safety warnings. Identical news sources and the same data collection approach (including inclusion and exclusion criteria) were used. We adopted an analogous approach to allow us to compare the content of news coverage in these two cases.
Data Analytic Procedures
We examined unadjusted rates of use of ADHD medications over a seven-year study period (2002–2008) overall and by parents’ education using MEPS. In calculating rates of use, we use survey weights, allowing us to make nationally representative estimates of ADHD use over time. To analyze the content of each news story, we used a six-item coding instrument (See Appendix, Table A2). The instrument was pilot tested by the authors, and minor adjustments were made to clarify question wording and item response coding. Each article was independently coded by two authors (CB, SB). To resolve coding disputes on specific items, the authors discussed and reached agreement on final coding. There were no items for which agreement could not be reached. Inter-rater reliability for all items measured using kappa statistics was high. Raw agreement ranged from 91 to 100% (range from κ=.80 to κ=1.0). (See Appendix, Table A2).
Results
Table 1 shows descriptive statistics on the proportion of the MEPS sample using ADHD medications over the study period. We find that 3.3 percent of children used ADHD medications, and children ages 10 to 13 were the most likely age group to use these medications (6.7 percent). As expected, boys were more likely than girls, and white children were slightly more likely than children in other racial groups to use ADHD medications. We did not identify substantial differences by income, insurance type, or parents’ education level.
Table 1.
Sample Characteristics for Children Using Stimulants Ages 0 – 20, MEPS 2002–2008
| Full Sample, 2002 – 2008 | ||
|---|---|---|
|
| ||
| Variable | N | % (weighted) |
| Total (N, %) | 2185 | 3.3 |
| Age (N, %) | ||
| Age 0–9 | 585 | 1.7 |
| Age 10–13 | 894 | 6.7 |
| Age 14–20 | 706 | 3.4 |
| Income (N, %) | ||
| Low income (<200 % FPL) | 1175 | 3.3 |
| Non-low income (=200 % FPL) | 1010 | 3.3 |
| Male (N, %) | 1600 | 4.6 |
| Female (N, %) | 585 | 1.9 |
| Insurance status (N, %) | ||
| Private | 1140 | 3.3 |
| Public | 987 | 3.8 |
| Race (N, %) | ||
| Black | 388 | 2.5 |
| White | 1649 | 3.5 |
| Other | 148 | 2.4 |
| Education (N, %) | ||
| Parents have a high school degree or less | 1298 | 3.2 |
| At least one parent has more than high school | 713 | 3.3 |
Note: MEPS = Medical Expenditure Panel Survey; FPL = Federal Poverty Guidelines
All estimates are for the civilian non-institutionalized population ages 0–20 and are person weighted using MEPS sampling weights.
We detected no declines in pediatric ADHD medication use over the study period (see Figure 1). (Results were qualitatively similar for adult ADHD medication users and are available upon request from authors). In 2002, 2.8 percent of children used ADHD medications, and use increased to 3.5 percent by 2008. Figure 2 indicates that, for all seven years, we found few differences in ADHD medication use between children of parents with more than or less than a high school degree.
Figure 1.
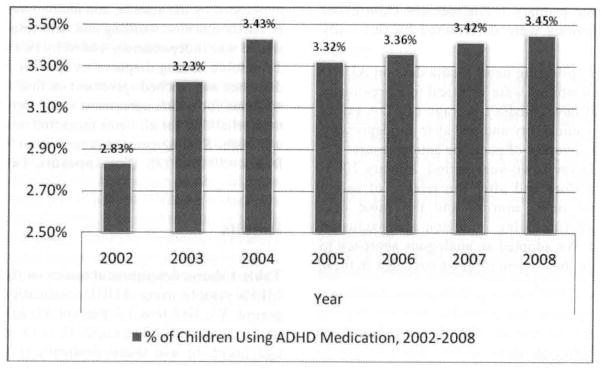
ADHD Medication Use for Children Ages 0 – 20, MEPS 2002 to 2008
Note: All estimates are for the civilian non-institutionalized population ages 0–20 and are person weighted using MEPS sampling weights.
Figure 2.

ADHD Medication Use for Children Ages 0 – 20 by Parental Education Level, MEPS 2002 to 2008
Note: All estimates are for the civilian non-institutionalized population ages 0–20 and are person weighted using MEPS sampling weights.
Table 2, column 1 presents data on news media coverage of possible risks of pediatric ADHD medication use. Over the two-year period studied, 69 news stories focused on these risks. Overall, these stories were neutral in their portrayal of the risks and benefits of ADHD medication use. The overwhelming majority of these studies (97 percent) did not emphasize risks over benefits or benefits over risks. Fifty-seven percent of news stories mentioned that children may benefit from taking ADHD medications. Often, news reporting included anecdotes about individual children who are harmed or hurt or expert quotes about risks and benefits to dramatize a news story. In this case, however, few stories included either anecdotes or expert source quotes. Among the news stories that did, benefits were emphasized over risks. Nineteen percent noted an individual child that was helped by ADHD medication compared with only 9 percent noting an individual child that was harmed. Journalists were also more likely to quote an expert source who suggested that benefits outweighed risks (17 percent), compared to a source that suggested risks outweighed benefits (9 percent).
Table 2.
Comparison of Content of News Media Coverage on Pediatric ADHD and Antidepressant Risk Warnings by the Food and Drug Administration
| Characteristic | ADHD News Stories June 2005–May2006 no. (%) | Antidepressant News Stories January 2003–December 2004 no. (%) |
|---|---|---|
| Total news stories | 69 (100.0) | 167(100.0) |
| Print news stories | 48 (69.6) | 126(75.5) |
| Television news stories | 21 (30.4) | 41 (24.5) |
| Story length (mean words) | 674 | 844 |
| Print news stories | 777 | 922 |
| Television news stories | 627 | 606 |
| Does news story leave the overall impression that: | ||
| Risks of medication use outweigh benefits (%) | 1(1.5) | 58 (34.7) |
| Benefits of medication use outweigh risks (%) | 1 (1.5) | 6(3.6) |
| Neither (%) | 67 (97.0) | 103(61.7) |
| Does the news story mention that children may benefit from medication use | 39 (56.5) | 116(69.5) |
| Does the news story reference an individual child/adolescent who it is claimed was: | ||
| Harmed by medication use? | 6 (8.7) | 59(35.3) |
| Helped by medication use? | 11 (15.9) | 24 (14.4) |
| Either harmed or helped by medication use? | 13(18.8) | 66(39.5) |
| Does new story include at least one quote from an expert source suggesting that: | ||
| Risks may outweigh benefits? (%) | 6(8.7) | 34 (20.4) |
| Benefits may outweigh risks? (%) | 12(17.4) | 54 (32.3) |
| Either risks may outweigh benefits or benefits may outweigh risks (%) | 14 (20.3) | 73 (43.7) |
Note: Expert sources do not include family members or medical correspondents.
To help interpret these results, we compared news media coverage of the ADHD risk disclosure to an earlier published study of antidepressant risk warnings by the FDA (reproduced in Table 2, column 2). As noted above, news coverage of FDA safety warnings related to suicidality and pediatric antidepressant use over a similar two-year period (January 2003-December 2004) was collected and analyzed using an analogous approach. Substantially fewer print and television news stories were focused on ADHD medication risk warnings (N=69) compared with antidepressant risk warnings (N=167). There were also important differences in how the two cases were covered. Compared with news stories about pediatric antidepressant risks, the ADHD-focused news articles were less likely (1.5 percent vs. 34.7 percent) to convey the overall impression that the risks of medication use outweighed the benefits. News stories about FDA action on ADHD medications were less likely to include anecdotes about specific children harmed or helped by the drugs, or expert quotes touting the drug’s risks and benefits.
Discussion
The purpose of this study was to examine treatment patterns following the release of new safety information related to pediatric ADHD medication use. A strength of the MEPS data was that they allowed us to calculate national estimates of changes in medication use. We did not observe declines in use following FDA safety warnings overall or by parents’ education level. This is in direct contrast to the case of pediatric antidepressants, where new safety information led to steep declines in use. Using the same MEPS data, a prior published study found that antidepressant use declined from 2.91% before safety warnings to 2.16 percent after the warnings, to 26% reduction.16 News media coverage was relatively balanced in its portrayal of the risks and benefits of ADHD medication use in contrast with news coverage of antidepressant safety warnings. These differences in news coverage may help to explain the different treatment patterns observed.
There are several important differences between the cases of stimulants and antidepressants that may explain the different responses to risk information observed.
First, the FDA opted not to follow its drug safety advisory committee’s recommendation to issue a black box warning in contrast to the pediatric antidepressant case. This decision transmitted a different message to both parents and providers about the relative safety of these medication classes.
A second important difference relates to the comparative efficacy of the two classes of medications in child populations. Clinical trial data provides clear evidence of the efficacy of ADHD medications in treating children. In contrast, evidence of the efficacy of a wide range of antidepressants in treating children was much less strong, particularly in 2003 when concerns related to pediatric antidepressant use first arose, and only fluoxetine had been approved for treatment of depression in children at that time.
A third distinction is that the risk per user was estimated to be much greater in the case of antidepressants. Clinical trial data presented at that time suggested a 2 percentage point increase in the risk of suicidality, compared to stimulant use where case reports suggested the estimate of the risk of hallucination was 0.10 percent. Nonetheless, the contrasting patterns are surprising give that the main concern with depression had been under-treatment prior to risk disclosure, whereas the main concern with ADHD medication has been with overtreatment.
There are several important limitations to this study. First, self-reported surveys like the MEPS rely on the recall of respondents and may be subject to reporting bias. However, the validity of these data is supported by their consistency with other data on drug use from other sources.18,19 A second important limitation is that it is possible there were small changes in use that are not captured by these data. Compared to administrative claims data, the MEPS is relatively small and can only capture relatively large changes in use; it is possible this study has failed to capture small changes in use that may have occurred. Third, our findings indicated that the release of safety information did not affect the probability that a child filled a prescription for an ADHD medication during the calendar year. However, our analysis does not allow us to determine whether the warnings affected intensity of use, conditional on obtaining at least one prescription. This issue is particularly relevant in the case of ADHD medication because discretionary use of ADHD drugs has been show to be greatest during summer vacation months when children are out of school.20 Thus, we might be more likely to see declines in utilization in response to warnings during certain periods of the calendar year. Finally, it is important to note that both in the case of ADHD and antidepressant safety warnings, analyses are pre-post comparisons only due to the lack of appropriate comparison populations. Therefore, it is possible that other factors might be offsetting declines in ADHD use due to safety warnings and media coverage.
Our findings are relevant to the ongoing discussion about improving the FDA’s ability to monitor drug safety. Safety warnings occur amid ongoing concern that the agency has insufficient authority and resources to fulfill its mission to protect the public’s health. Efforts to bolster the FDA’s postmarketing surveillance system have the potential bring more data to bear to allow for earlier detection of health risks.
Further research is needed to assess whether other treatment changes occurred following risk warnings. For example, it is important to determine whether an increase in cardiac screening prior to medication initiation occurred. Likewise, the FDA advises that children experiencing hallucinations or other psychiatric responses to medication be discontinued from drug treatment. If it is determined that instead of being discontinued from medication treatment, children experiencing hallucinations are put on additional medication (e.g., antipsychotics) to address these adverse effects, additional efforts by the FDA to better inform the public are warranted. Finally, as consumers assume a larger role in health care decision making, it will be important to assess the extent to which the news media conveys increasingly complex health risk information to the public in an accurate and interpretable manner.
Acknowledgments
Source of Funding: This study was supported by a grant from the National Institute of Mental Health (R01 MH 080883). The authors gratefully acknowledge expert research assistance by Rachael Sorg.
Appendix
Table A1.
Generic and Branded ADHD Drugs Included
| Adderall | Focalin XR |
| Adderall XR | Metadate CD |
| Amphetamine | Methylin |
| Amphetamine salt | Methylin ER |
| Concerta | Methylphenidate hydrochloride |
| Daytrana | Ritalin |
| Dexmethylphenidate | Ritalin SR |
| Dexmethylphenidate hydrochloride | Ritalin LA |
| Dextroamphetamine sulfate | Strattera |
| Focalin |
Table A2.
Content Analysis Instrument and Kappa Statistics
| Item | Raw agreement | Kappa |
|---|---|---|
| Does the news story leave the overall impression that (choose one): | 100.0% | 1.0 |
| (1) Risks of ADHD use outweigh benefits of ADHD use | ||
| (2) Benefits of ADHD use outweigh risks of ADHD use | ||
| (3) Neither | ||
| Does the news story mention that children may benefit from ADHD use | 91.3% | 0.83 |
| Does the news story reference an individual child/adolescent who it is claimed was harmed by ADHD use? | 100.0% | 1.0 |
| Does the news story reference an individual child/adolescent who it is claimed was helped by ADHD use? | 100.0% | 1.0 |
| Does the new story include at least one quote from an expert source (not family member or medical correspondent) suggesting that risks of ADHD use may use outweigh benefits of ADHD use? | 97.1% | 0.82 |
| Does the new story include at least one quote from an expert source (not family member or medical correspondent) suggesting that benefits of ADHD use may outweigh risks of ADHD use? | 94.2% | 0.80 |
References
- 1.Bloom B, Cohen RA. Summary Health Statistics for U.S. Children: National Health Interview Survey, 2006. National Center for Health Statistics. Vital Health Stat. 2007;10(234) [PubMed] [Google Scholar]
- 2.Strine TW, Lesesne CA, Okoro CA, McGuire LC, Chapman DP, Balluz LS, Mokdad AH. Emotional and Behavioral Difficulties and Impairments in Everyday Functioning Among Children With a History of Attention-Deficit/Hyperactivity Disorder. Prev Chronic Dis. 2006;3(2):A52. [PMC free article] [PubMed] [Google Scholar]
- 3.The MTA Cooperative Group. A 14-Month Randomized Clinical Trial of Treatment Strategies for Attention-Deficit/Hyperactivity Disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- 4.Sonni A. Statistical Brief: Attention-Deficit Hyperactivity Disorder (ADHD) in Children, Ages 5–17: Use and Expenditures. Medical Expenditure Panel Survey, Agency for Healthcare Research and Quality; 2009. [Google Scholar]
- 5.Olfson M, Marcus SC, Weissman MM, Mensen PS. National Trends in the Use of Psychotropic Medications by Children. J Am Acad Child Adolesc Psychiatry. 2002;41(5):514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Zuvekas SH, Vitiello B, Norquist GS. Recent Trends in Stimulant Medication Use Among U.S. Children. Am J Psychiatry. 2006;163(4):579–585. doi: 10.1176/ajp.2006.163.4.579. [DOI] [PubMed] [Google Scholar]
- 7.Busch SH, Frank RG, Leslie DL, Martin A, Rosenheck RA, Martin EG, Barry CL. Antidepressants and Suicide Risk: How Did Specific Information in FDA Safety Warnings Affect Treatment Patterns? Psychiatr Serv. 2009;61(1):11–16. doi: 10.1176/appi.ps.61.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Herings RM, Mann JJ. Early evidence on the effects of regulators’ suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry. 2007;164(9):1356–1363. doi: 10.1176/appi.ajp.2007.07030454. [DOI] [PubMed] [Google Scholar]
- 9.Libby AM, Brent EH, Morrato EH, Orton HD, Allen R, Valuck RJ. Decline in treatment of pediatric depression after FDA advisory on risk of suicidality with SSRIs. Am J Psychiatry. 2007;164(6):884–891. doi: 10.1176/ajp.2007.164.6.884. [DOI] [PubMed] [Google Scholar]
- 10.Nemeroff CB, Kalali A, Keller MB, Charney DS, Lenderts SE, Cascade EF, Stephenson H, Schatzberg AF. Impact of publicity concerning pediatric suicidality data on physician practice patterns in the United States. Arch Gen Psychiatry. 2007;64(4):466–472. doi: 10.1001/archpsyc.64.4.466. [DOI] [PubMed] [Google Scholar]
- 11.Olfson M, Marcus SC, Druss BG. Effects of Food and Drug Administration warnings on antidepressant use in a national sample. Arch Gen Psychiatry. 2008;65(1):94–101. doi: 10.1001/archgenpsychiatry.2007.5. [DOI] [PubMed] [Google Scholar]
- 12.Valluri S, Zito JM, Safer DJ, Zuckerman IH, Mullins CD, Korelitz JJ. Impact of the 2004 Food and Drug Administration Pediatric Suicidality Warning on Antidepressant and Psychotherapy Treatment for New-Onset Depression. Med Care. 2010;48(11):947–954. doi: 10.1097/MLR.0b013e3181ef9d2b. [DOI] [PubMed] [Google Scholar]
- 13.Busch SH, Martin A, Frank RG, Barry CL. Characterizing declines in pediatric antidepressant use after new risk disclosures. Med Care Res Rev. 2011;61(1):96–111. doi: 10.1177/1077558710374197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaiser Family Foundation/Harvard School of Public Health. September/October 2001 Health News Index. Menlo Park, CA: Kaiser Family Foundation; 2001. [Google Scholar]
- 15.Dentzer S. Communicating Medical News: Pitfalls of Health Care Journalism. N Engl J Med. 2009 Jan 1;360(1):1–3. doi: 10.1056/NEJMp0805753. [DOI] [PubMed] [Google Scholar]
- 16.Barry CL, Busch SH. News Media Coverage of FDA Warnings on Pediatric Antidepressant Use and Suicidality. Pediatrics. 2010;125(1):88–95. doi: 10.1542/peds.2009-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. [accessed on November 7, 2010]; http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2007/ucml08849.htm.
- 18.Selden TM, Levit KR, Cohen JW, Zuvekas SH, Moeller JF, McKusick D, Arnett RH., 3rd Reconciling medical expenditure estimates from the MEPS and the NHA, 1996. Health Care Financ Rev. 2001;23( 1):161–178. [PMC free article] [PubMed] [Google Scholar]
- 19.Zuvekas SH. Prescription Drugs and the Changing Patterns of Treatment for Mental Disorders, 1996–2001. Health Aff. 2005;24(1):195–205. doi: 10.1377/hlthaff.24.1.195. [DOI] [PubMed] [Google Scholar]
- 20.Cascade E, Kalali AH, Weisler RH, Lenderts S. Seasonality and the Changing Adult/Child Prescription Ratios in ADHD Therapy. Psychiatry. 2008;5(1):23–25. [PMC free article] [PubMed] [Google Scholar]


