Abstract
Obesity is a major cause of type 2 diabetes, clinically evidenced as hyperglycemia. The altered glucose homeostasis is caused by faulty signal transduction via the insulin signaling proteins, which results in decreased glucose uptake by the muscle, altered lipogenesis, and increased glucose output by the liver. The etiology of this derangement in insulin signaling is related to a chronic inflammatory state, leading to the induction of inducible nitric oxide synthase and release of high levels of nitric oxide and reactive nitrogen species, which together cause post-translational modifications in the signaling proteins. There are substantial differences in the molecular mechanisms of insulin resistance in muscle versus liver. Hormones and cytokines from adipocytes can enhance or inhibit both glycemic sensing and insulin signaling. The role of the central nervous system in glucose homeostasis has also been established. Multi-pronged therapies aimed at rectifying obesity-induced anomalies in both central nervous system and peripheral tissues may prove to be beneficial.
Introduction
Type-2 diabetes is a polygenic disease. Obesity has been identified as a major causative factor for the insulin resistance and hyperglycemia associated with diabetes.1 Developed and developing countries, alike, are experiencing a sharp rise in the incidence of obesity-linked type-2 diabetes.2 Consequently, obesity-induced diabetes is emerging as a global health-care problem threatening to reach pandemic levels by 2030, when the incidence is projected to more than double in a period of only thirty years (from 171 million in 2000 to 366 million in 2030).3 The problem is not limited to adults. There has also been a marked increase in obesity (defined as a body mass index of greater than 30 kg/m2) among children.4 In the early stages of type-2 diabetes, insulin resistance is countered by a state of hyperinsulinemia, brought about by stepped up insulin production in the pancreas. Euglycemia is therefore maintained. Overt hyperglycemia does not develop until later stages, when pancreatic β cells can no longer compensate for the high levels of insulin resistance in peripheral tissues. Along with diabetes, there has been a concomitant increase of the incidence of metabolic syndrome, an obesity-linked condition characterized by clinical features of insulin resistance, dyslipidemia, and hypertension.5,6 Ultimately, it is the compounding effects of these cardiovascular, renal, cerebral, and thrombogenic anomalies that give rise to the increased morbidity and mortality associated with obesity, type-2 diabetes, and metabolic syndrome.6
Malfunctions in energy homeostasis resulting from genetic predisposition can lead to obesity. However, the recent increase in obesity is not thought to be due to specific congenital or hereditary defects in lipid metabolism, but to the inability of the body to cope with high-energy food intake, along with a sedentary life style and lack of exercise and/or mechanized work playing a contributing role.2-4 Adipocyte tissue, previously considered to be no more than an energy-storing depot, has also become a focus of intense scientific interest and is now thought to integrate a wide array of pathophysiologic processes, including nutrient homeostasis, immune response, blood pressure control, hemostasis, bone mass, and thyroid and reproductive function, in both physiological and pathophysiological states.7,8 Some of these effects are brought about by adipogenic hormones including leptin, adiponectin, and other adipokines (cytokines from fat). This review begins with a brief presentation of the molecular aspects of normal signal transduction via the insulin signaling network, followed by in depth discussion of the molecular mechanisms specific to obesity that induce derangements in insulin signaling (insulin resistance) and hyperglycemia. Metabolic syndrome and its effects on perioperative care have been reviewed recently in ANESTHESIOLOGY9 and will not be discussed.
Physiologic Actions of Insulin and the Insulin-Signaling Network
Insulin is the principal hormone of glucose homeostasis; it stimulates glucose influx into muscle, glycogen synthesis in the liver and muscle, and fat deposition in adipocytes.10 Other important actions of insulin include the enhancement of protein synthesis, cell survival and growth, prevention of protein catabolism, and anti-inflammatory effects.11-14 Insulin-like growth factor (IGF-1) can behave like insulin, producing the same beneficial effects.13,14 Obesity-associated type 2 diabetes is evidenced by increased glucose levels in the blood, which result from elevated glucose production in the liver (gluconeogenesis and glycogenolysis) and decreased glucose uptake by muscle.1,10 In obesity-induced diabetes, or any pathologic condition associated with decreased insulin function, hyperglycemia together with the attenuated anabolic and anti-inflammatory effects leads to muscle protein loss (muscle wasting), increased tendency to infection, and enhanced inflammatory response.
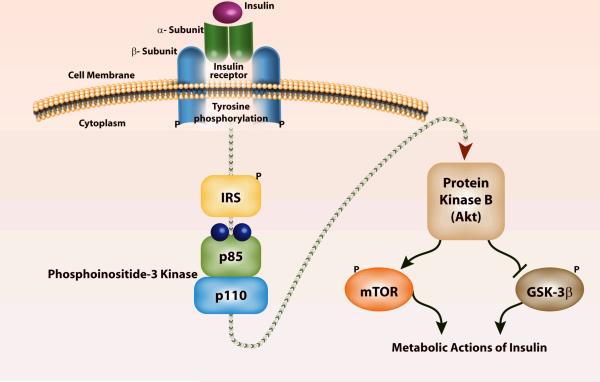
Similar to the G-protein coupled adrenoceptor, the paradigm of eukaryotic signal transduction,15 insulin, too, relies on a series of intracellular downstream signals to produce its physiologic effects.10 The binding of insulin to the α-subunit of the insulin receptor (IR) molecule induces rapid autophosphorylation (addition of a PO4 - - group from ATP) of the β-subunit, which turns on its tyrosine kinase activity (Figure 1).10 This gives IR the ability to phosphorylate various tyrosine residues of other cytosolic moieties, including insulin receptor substrates -1 and -2 (IRS-1, IRS-2). Phosphorylation of the tyrosine residues that reside on these substrates is pivotal for the biologic actions of insulin.16,17 The tyrosine phosphorylation of IRS proteins leads to the second intracellular step of insulin action, the association of phosphorylated IRS-1 or IRS-2 with the enzyme, phosphoinositide-3-kinase (PI3K). The association of IRS-1 with PI3K occurs through the phosphorylation of YMXM or YXXM motifs on IRS-1 and SH2 domains on the 85-kDa subunit of PI3K.18 Absence of PI3K function due to disease or treatment with wortmannin (a potent PI3K inhibitor) or with dominant negative p85, which inactivates endogenous PI3K activity, abolishes glucose uptake in muscle and adipose tissue.18,19
Figure 1. Signal transduction via insulin receptor (IR) and its downstream signaling proteins.
The IR is a kinase, an enzyme that catalyzes the transfer of phosphate from adenosine triphosphate (ATP) to another substrate. When insulin binds to the IR it undergoes autophosphorylation and catalyzes the tyrosine phosphorylation of insulin receptor substrates-1 and -2 (IRS). These IRS proteins interact with diverse signaling molecules including phosphoinositide-3 kinase (PI3K), which in turn activates protein kinase B (a.k.a. Akt). The downstream proteins controlled by Akt/PKB include mammalian target of rapamycin (mTOR) and glycogen synthase kinase-3β (GSK-3β). The metabolic and potent anabolic actions of insulin include glucose metabolism, glycogen/lipid/protein synthesis, cell growth and survival, and anti-inflammation. These pleiotropic effects of insulin are mediated by specific gene expression, translation of proteins, and enhanced mitochondrial function.
The IRS-activated PI3K in turn affects several downstream signaling pathways through the generation of a lipid second messenger, phosphatidyl-inositol-3, 4, 5-triphosphosphate. A critical target for PI3K is Akt/PKB (protein kinase B).20,21 A serine/threonine kinase, Akt/PKB is the major effector of the IR-IRS-1-PI3K pathway and is activated by the phosphorylation of its threonine 308 and serine 473 residues. Akt/PKB drives the metabolic actions of insulin including glucose transport, glycogen synthesis, fat deposition, and protein synthesis. It also drives cell growth and cell survival via pathways both dependent and independent of activation of the rapamycin-sensitive kinase known as mTOR (mammalian target of rapamycin) whose downstream targets are p70 S6 kinase and 4E-BP1.13,14,22 For example, loss of trophic signaling via Akt/PKB and downstream proteins not only leads to loss of glucose homeostasis,19 but also to loss of muscle mass and increased cellular loss by apoptosis.23-25 Some of the cell survival or cell growth effects of Akt/PKB are mediated by its actions on the mitochondria. Parenthetically, because the Akt/PKB-PI3-K-mTOR pathway plays a pivotal role in cell growth and survival, these proteins have become the target of novel signal transduction inhibitors that are used to produce cell death in the area of cancer research.
Another target of Akt/PKB is glycogen synthase kinase-3β (GSK-3β). GSK-3β is a negative regulator of glycogen synthase, the enzyme that catalyzes the synthesis of glycogen. When the serine 9 on GSK-3β is phosphorylated and inactivated by Akt/PKB, glycogen and protein synthesis is enhanced.26,27 It has also been proposed that the Akt/PKB-GSK-3β pathway mediates some of the anti-inflammatory effects of insulin.26,27 In addition, GSK-3β operates not only as a downstream component of insulin signaling, but also as a negative regulator of upstream insulin signaling. GSK-3β phosphorylates IRS-1 at serine 332, which in turn attenuates the insulin response by inhibiting IR-mediated tyrosine phosphorylation of IRS-1.28 Thus, whether occurring dependently or independently of Akt/PKB activity, increased GSK-3β activity decreases glycogen synthesis and can lead to impaired upstream insulin signaling as well as enhanced inflammation.26,27
Pathogenesis of Insulin Resistance and Hyperglycemia in Obesity
Despite several years of intense investigation, the pathogenesis of obesity-induced insulin resistance has not been fully elucidated. Recently, however, some of the derangements in insulin signaling have been clarified, along with the etiological factors that lead to obesity-associated hyperglycemia.
Adipocytes decrease glucose uptake in peripheral tissues by the release of free fatty acids.29 In humans, even brief periods of lipid infusion (e.g., four hours) will decrease insulin-stimulated glucose uptake in muscle as well as PI3K activity.30 Although the classic features of acute inflammation, i.e., swelling, redness, pain, and fever (tumor, rubor, dolor, calor), are absent, signs of chronic inflammation are observed with the release of inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-6, and interleukin-1β (IL-1β). These cytokines have been implicated in the pathogenesis of insulin resistance.28,31 In a published report, however, when antibodies to TNF-α were infused to diabetics, they did not consistently effect an improvement in insulin signaling.32 Nevertheless, contradictory evidence has been observed in rheumatoid arthritis, where patients treated with anti-TNF-α antibody experienced a secondary benefit of enhanced insulin sensitivity.33 Similarly, IL-1β antagonist , a suppressor of Il-1β cytokine, has been shown to improve glycemia and β-cell secretory function in obese humans.34
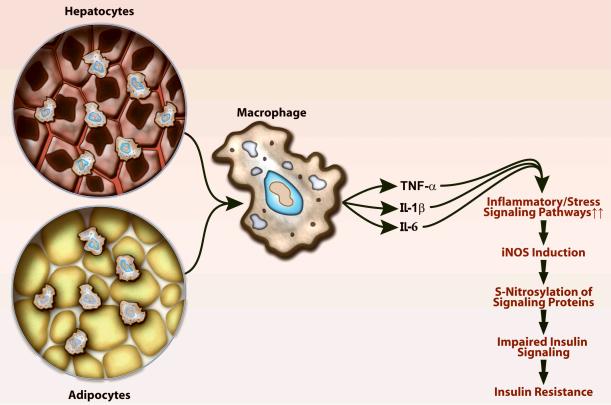
Obesity-associated adipocyte apoptosis (cell death) appears to be the primary event underlying insulin insensitivity. The subsequent cell death-associated infiltration of macrophages appears to explain the presence of chronic inflammation.35 The release of macrophage chemoattractant protein-1 (MCP-1) by the adipocyte plays a role in the recruitment of macrophages.7 The infiltrating macrophages are implicated in cytokine production (Figure 2). These adipogenic cytokines appear to function in a paracrine fashion, since circulating cytokines are not consistently elevated. The concomitant release of reactive oxygen species exaggerate or play a causal role in cytokine-related insulin resistance.36,37 The intracellular mediators of this inflammatory response include nuclear factor-κB (NF-κB), c-Jun amino-terminal kinase/stress activated protein kinase (JNK/SAPK), and induction of the suppressor-of-cytokine-signaling-3 (SOCS3).5,28,38-40 Macrophage infiltration occurs not only in the adipocyte but also in the liver.28 The associated activation of the JNK/SAPK pathway also promotes the development of hepatic inflammation leading to hepatic steatosis (fat deposition), lipid peroxidation, and hepatic apoptosis, all of which are seen with obesity-induced diabetes.38 It has been suggested that these inflammatory mediators also lead to serine phosphorylation of IRS-1.39,40 Serine phosphorylation, as opposed to tyrosine phosphorylation, inhibits insulin signaling.28 Thus, high-dose aspirin or salycilate has been used as an anti-inflammatory agent to treat fat-induced insulin resistance in humans with good success.41 The anti-inflammatory effect of salycilates has been attributed to the inhibition of inhibitor of nuclear factor kappa β kinase (IKKβ) and NFκB, which has lead to the speculation that the glucose lowering and insulin sensitization might be due to NFκB inhibition.
Figure 2. Obesity leads to an inflammatory response in the liver and in adipocyte tissues.
Obesity-induced inflammation results in infiltration of macrophages and release of cytokines, tumor necrosis-factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β). The downstream effector of cytokine-induced inflammation is induction of inducible nitric oxide synthase (iNOS). The extremely high levels of nitric oxide that are released, together with reactive oxygen species, generate reactive nitrogen species including peroxynitrite, which leads to S-nitrosylation and tyrosine nitration (postranslational modifications) of proteins. This calcium-independent process alters the function of many proteins including those involved in insulin signaling. Gene manipulation of iNOS or treatment with iNOS inhibitors ameliorates the deranged insulin signaling as shown in Figure 3.
Other etiological factors have been proposed in the pathogenesis of obesity-induced insulin resistance. These factors include oxidative stress, mitochondrial dysfunction, intracellular lipid accumulation in skeletal muscle and liver, and decreased β-oxidation. Importantly, however, it remains unclear how these factors induce and/or exacerbate insulin resistance, how they interrelate and by what specific mechanisms. Recent studies have begun to answer some of these questions, by elucidating that these pathological and etiological factors converge to activate inflammatory/stress signaling pathways. 8-10,36-41
Obesity-induced Insulin Resistance in Skeletal Muscle
Skeletal muscle is the major site of glucose dispostion in the body. The glucose that is transported into muscle is used or stored as glycogen. In skeletal muscle, lipid accumulation has been implicated in the induction of obesity-related insulin resistance. In humans, intramyocellular lipid content correlates quite well with insulin resistance.42 Increased lipid accumulation results in activation of protein kinase C theta (PKCθ) and the JNK/SAPK pathway, in part through the elevated production of ceramide, which mediates impaired insulin signal transduction in muscle.43,44 JNK2 deficiency ameliorated impaired insulin signaling in skeletal muscle of obese, diabetic (ob/ob) mice.45 In contrast, unlike the liver, inhibition of the IKK-NF-κB pathway46 or attenuation of endoplasmic reticulum (ER) stress (see section titled Endoplasmic Reticulum Stress).47 did not improve insulin resistance in skeletal muscle in mice. These observations indicate that, in skeletal muscle, the JNK/SAPK pathway plays an important role in obesity-induced insulin resistance, whereas the IKK-NF-κB pathway and ER stress are not involved. However, the molecular bases that would explain these differences in obesity-induced insulin resistance in skeletal muscle versus liver remain largely unknown.
Insulin Resistance and Glucose Homeostasis in the Liver with Obesity
Circulating glucose levels reflect a balance between glucose production by the liver and glucose uptake by the muscle.10 The liver plays a key role in obesity-induced hyperglycemia and affects multiple pathways.5,48 Insulin promotes the synthesis of glycogen, while repressing glucose release in the liver. This effect is enhanced and coordinated through multiple genes that control glycolysis, fatty acid synthesis, gluconeogenesis (synthesis of glucose from proteins) and glycogenolysis (breakdown of glycogen).10 Insulin inhibits transcription of the gene encoding for phosphoenolpyruvate carboxykinase, the rate limiting enzyme that controls gluconeogenesis.49 Insulin also increases transcription of glycolytic enzymes (glucokinase and pyruvate kinase) and lipogenic enzymes (fatty acid synthase, acetyl-CoA carboxylase). These effects may be mediated by a host of transcription factors and co-factors including forkhead (FOXOs) and peroxisome proliferators that activate receptors α and γ (PPARα and PPARβ) and its coactivators.50,51 Akt/PKB modulates these transcription factors and coactivators.10,13,17 Recent studies indicate that the effect of insulin on lipid metabolism in the liver is also mediated by changes in sterol regulatory element-binding protein-1c (SREBP). Increase in SREBP expression can increase expression of gluconeogenic and lipogenic genes and vice versa.52,53 Human studies confirm the importance of SREBP in hepatic insulin resistance and hepatic steatosis, particularly in obesity.54,55 The presence of inflammation (macrophage infiltration), hepatocellular swelling, and steatosis constitute an operative risk, particularly in the liver, and increase the peri-operative morbidity and mortality.56,57
Evidence for the Role of Inducible Nitric Oxide in Obesity-Induced Insulin Resistance
Although inflammation leading to activation of the JNK/SAPK and IKK-NFκβ pathways has been implicated in the pathogenesis of obesity-related insulin resistance, little has been published about the downstream effectors of these pathways. Our recent studies in rodents indicate that inducible nitric oxide synthase (iNOS) is a pivotal downstream effector of insulin resistance in many pathologic states including obesity. Three isoforms of nitric oxide synthase are expressed in mammalian tissues. Endothelial and neuronal nitric oxide are constitutively expressed and generate small amounts of nitric oxide, which produces physiologic action by elevating cyclic guanosine monophosphate (cGMP) in a calcium-dependent manner.58 In contrast, inflammation results in sustained high output of nitric oxide (up to thousand fold).59 These extremely high concentrations of nitric oxide, particularly in the presence of reactive oxygen species, form highly reactive nitric oxide-related species, including peroxynitrite, which lead to cyclic guanosine monophosphate-independent and calcium-independent post-translational modifications of proteins such as thiol nitrosylation (S-nitrosylation of cysteine residues) and tyrosine nitration.60
Initially, we tested the beneficial effects of iNOS inhibition on inflammation and hyperglycemia induced by lipopolysaccharide.61 In these studies, using the euglycemic insulin clamp, we documented that lipopolysaccharide-induced hyperglycemia and hepatic glucose output can be completely suppressed by iNOS inhibitor, aminoguanidine.61 Subsequent studies from our laboratory have more clearly demonstrated the molecular mechanisms by which iNOS induces insulin resistance.62-64 Diabetic mice had increased expression of S-nitrosylated proteins. S-nitrosylation of tyrosine residues leads to decreased signaling via these proteins.62-64 Increased levels of nitric oxide inactivated insulin-signaling proteins including IRS and Akt/PKB in a concentration dependent manner.62-64 The concomitant presence of oxidative stress accelerated the S-nitrosylation and inactivation and/or breakdown of insulin signaling proteins.63,64 Denitrosylation with a reducing agent reactivated the nitrosylation-mediated inactivation of Akt/PKB.62
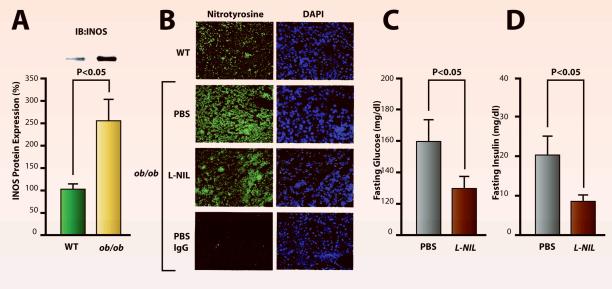
In the liver, obese (ob/ob) mice have a 2.5-fold increase in iNOS protein expression compared to wild type mice (Figure 3A). The imunoreactivity for nitrotyrosine, a marker for nitrosative stress, is elevated in the liver of these obese mice. Treatment with the iNOS inhibitor, L-NIL, reverses elevated nitrotyrosine immunoreactivity in the liver (Fig. 3B). In hepatic insulin resistance, there is decreased expression of IRS proteins.16 The treatment of obese mice with iNOS inhibitor, L-NIL, increased protein expression of IRS-1 in a proteosome-dependent manner, while IRS-2 expression was increased by L-NIL at the mRNA level.64 The increased expression of IRS proteins in the liver was associated with improved insulin signaling via IRSs-PI3K together with better glycemic control and insulin sensitivity. The improved insulin sensitivity was evidenced as lower fasting insulin and glucose levels in L-NIL treated animals (Figure 3 C&D).63 All these effects with iNOS inhibitor were not associated with changes in cyclic guanosine monophosphate in the liver, indicating that endothelial or neuronal NOS does not play a role in insulin resistance. It is also interesting to note that the expression of SREBPs, which is usually increased in hepatic insulin resistance, was also reduced after treatment with L-NIL.63 In addition, inhibition of iNOS by gene disruption also reversed the decreased IRS-1 expression and thereby improved IRS-1-mediated insulin signaling in skeletal muscle of obese, diabetic (ob/ob) mice.64 Some of these findings are consistent with studies by others which have shown that iNOS expression, S-nitrosylated proteins, and tyrosine nitration are elevated in patients with type-2 diabetes,65-67 and that genetic disruption of iNOS improves insulin-signaling in obese mice.68 On the basis of these observations, iNOS inhibitors may prove to be a useful tool for treating obesity-induced diabetes.
Figure 3. Expression of inducible nitric oxide synthase (iNOS) and tyrosine nitration in ob/ob mice and its reversal with iNOS inhibitor, L-NIL.
A: Immunoblot analyses (IB) revealed that iNOS expression was increased in the liver of ob/ob mice compared with wild-type (WT) mice. B: Nitrotyrosine immunoreactivity was elevated in the liver of ob/ob mice treated with phosphate buffered saline (PBS) compared with WT mice. L-NIL reduced nitrotyrosine immunoreactivity in ob/ob mice. Magnifcation: X400. C&D: The decrease in nitrotyrosine immunoreactivity observed during treatment of ob/ob mice with L-NIL was associated with improved glycemic control, evidenced as normalization of fasting blood glucose level with reduced plasma insulin concentration.
Endoplasmic Reticulum Stress, Hyperglycemia, and Insulin Resistance
As indicated previously, chronic low-grade inflammation with activation of the IKK-NFκB and JNK/SAPK pathways has been implicated as a causative factor in hepatic insulin resistance.38-40 In fact, obesity-related insulin resistance is associated with elevated circulating levels of C-reactive protein, a clinically measurable indicator and marker of acute-phase protein-response to inflammation by the liver.69,70 An alternative explanation has been proposed implicating ER stress as a key factor in obesity-linked chronic inflammation and the associated insulin resistance in liver.28,47,71
From an evolutionary standpoint, the ER stress response is a cellular mechanism that evolved in eukaryotes as a coping mechanism for glucose/nutrition deprivation. The ER stress response in mammals was first characterized as the transcriptional activation of glucose-regulated proteins (e.g., Grp78) in response to glucose deprivation.72 Of note, absence of this ER stress response to glucose deprivation in genetically mutated mice leads to hypoglycemia and death shortly after birth,73 and further supports the notion that ER stress response plays a critical role in glucose homeostasis, particularly in the liver. A critical role for ER stress in obesity-induced hepatic insulin resistance has been demonstrated by the following observations:28,47,71 Expression of glucose-regulated protein78 (Grp78) and phosphorylation of PKR-like kinase (PERK), both indicators of ER stress response, was increased in the liver of ob/ob mice and in mice fed a high-fat diet.47,71 Mice deficient in X-box protein (XBP-1) developed insulin resistance concomitant with elevated ER stress response.47 Increased expression in the liver of oxygen-regulated protein150 (ORP150), which is a resident ER chaperone, protects cells from ER stress73,74 and attenuates obesity-induced diabetes in rodents.71 Conversely, decreased expression of ORP150 causes ER stress and insulin resistance in the liver.71 ER stress mediates activation of IKK-NF-κB and JNK/SAPK in cells75,76 in association with derangements in lipid metabolism.77,78 Induction of iNOS and/or high levels of nitric oxide can also induce IKK-NF-κB and JNK/SAPK with induction of ER stress.79-81 The interaction between iNOS and the IKKNF-κB-JNK/SAPK pathway, along with the potentiation of effects caused by this interaction, leads to a vicious cycle of insulin resistance. The clinical significance of ER stress in obesity, steatosis, and insulin resistance in humans is unclear, but pharmacologic agents are being developed to counteract these effects and have proved successful in rodents.75
Recently, inflammatory/stress signaling pathways have been highlighted as a major culprit in obesity-induced insulin resistance.28 iNOS has been proposed as an important component of feed-forward mechanisms that lead to insulin resistance, in which it functions as both a downstream effector and an upstream enhancer of inflammation. Contrary to findings in liver, however, published studies do not find increased ER stress in skeletal muscle of obese, diabetic mice,71,75 and although it is conceivable that ER stress may contribute to apoptosis in adipocytes, this has not been investigated.
Central Nervous System Control of Glucose Homeostasis
A close connection between output from the central nervous system (CNS) and glucose homeostasis is now well established.82 Adipose tissue, previously thought to be just an energy storage site, controls many physiologic functions by the release of adipokines.5 Leptin (Greek:leptos=thin) was the first adipokine released by adipocytes to be identified. In addition to exerting insulin-enhancing effects in peripheral tissues, leptin also affects the CNS by controlling food intake through its actions on the mediobasal hypothalamic area (arcuate nucleus), which contains high concentrations of leptin receptor (Figure 4).83 Mutations in the leptin receptor (which cause decreased sensitivity to leptin) or low levels of leptin can lead to increased appetite and obesity in rodents and humans.83,84 Some obese subjects have an increase in appetite despite high levels of leptin, which may be related to an obesity-induced attenuated response to leptin. Thus, the actions of leptin on energy and food intake are independent of its peripheral (endocrine) effects, where, among other physiologic functions, it also enhances insulin sensitivity and bone density.83,84 The dysregulation of this sensing pathway may lead to obesity and diabetes.
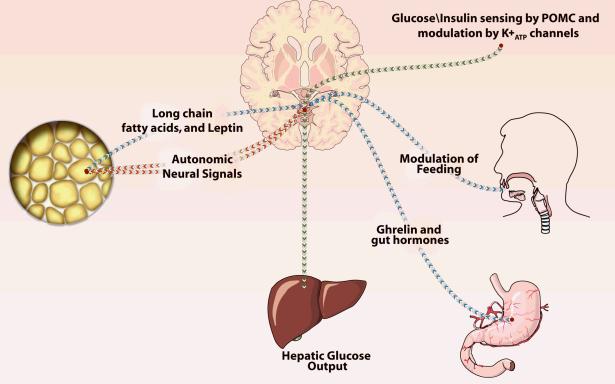
Figure 4. Central nervous system control of glucose homeostasis.
Leptin and long-chain fatty acids released from adipocytes influence food intake via the hypothalamus (depicted in blue). Ghrelin and other hormones from the gut also influence food intake and satiety (blue). Afferent and efferent autonomic signals from and to the fat pad, via the sympathetic and parasympathetic nerves to and from the hypothalamus influence fat synthesis and breakdown of fat (depicted in red). Insulin and glucose levels influence potassium adenosine triphosphate (K+ATP) channels in the medial pro-opiomelanocortin (POMC) neurons on the arcuate nucleus and control neural output to modulate hepatic glucose output (depicted in green). In obesity, the ability to sense these afferent inputs to the hypothalamus is impaired resulting in increased or exigenic signals (decreased satiety) and increased nerve-mediated glucose output by the liver.
Afferent nerve fibers from fat modulate sensitivity of the CNS to leptin and this effect can be abrogated by denervation.85 Long-chain fatty acids (released from the adipocyte) have been added to the growing list of metabolic signals that exert physiologic actions on the hypothalamus.83,84 Gut hormones, notably ghrelin, can activate circuits in the hypothalamus; for example, increased levels of ghrelin augment feeding. Several other gut hormones also control food intake, and these humoral circuits from fat and gut are dysfunctional in obesity.83-86 In particular, it has been suggested that evolutionally conserved fuel sensors, such as adenosine monophosphate-activated protein kinase and mTOR, may integrate sensory input from nutrients, including those derived from food intake and adipose tissue. These sensors then may use this input to regulate efferent pathways responsible for fuel intake and utilization.87
Centrally mediated neural autonomic (sympathetic and parasympathetic) actions can modulate adipocyte functions. It is well known that fat pads are well innervated by the sympathetic nervous system, and sympathetic nerve-mediated-lipolysis via the adrenoceptor can lead to alterations in lipolysis.5,88 There is also evidence that parasympathetic innervation is involved in the control of glucose homeostasis, particularly with respect to abdominal fat tissue.89 Using microsurgery, transneuronal retrograde tracing, and hyperinsulinemic euglycemic clamp, Krier et al., demonstrated that parasympathetic innervation of fat tissue stimulates fat growth. They also substantiated preexisting evidence that the brain controls fat growth by a selective group of neurons.90 These investigators, in fact, hypothesize that obesity and metabolic syndrome are diseases more of the brain than of any other organ or system.91 Other investigators have questioned the validity of the role of the parasympathetic nervous system.92,93 It is of interest to note that the autonomic nervous system and the immune system (e.g., macrophages) cross-talk during inflammation via the sympathetic and parasympathetic pathways.94,95 Details of these interactions and cross-talk and how these mechanisms are modified in obesity are unknown.
Insulin and blood glucose levels, known to target liver glucose output via glycogenolysis and gluconeogenesis, have now been shown to act on the CNS to control glucose output by the liver. The studies of Pocai et al., show that over and above its direct effects on the liver, insulin also acts on specialized ion channels called potassium adenosine triphosphate (K+ATP) channels located in the pro-opiomelanocortin (POMC) neuron on the arcuate nucleus of the hypothalamus to control hepatic glucose production.96 By opening and closing these K+ATP channels, the hypothalamus controls output to the liver via the vagus, since isolating the hepatic branches of the vagus obviates this response. The physiologic significance of this finding is that increases in insulin concentration in response to feeding in turn decreases the hepatic glucose output. It is also noteworthy that the ability to sense increased concentrations of glucose or insulin (e.g., due to feeding) by the POMC neurons in the hypothalamus is defective in obesity.97 The CNS-mediated hepatic glucose output continues in obese subjects despite high glucose and high insulin levels and for this reason may have a pathogenic role in maintaining the hyperglycemia of obesity.97 The mechanism for obesity-induced loss of glucose sensing by POMC involves uncoupling protein-2, since pharmacological inhibition of uncoupling protein-2 by genipin reverses the loss of glucose sensing. Sulphonylureas acting on the K+ATP channels in the hypothalamus decrease neural signal output from the hypothalamus and decrease hepatic glucose output.82 The physiological significance in humans of CNS control of glucose homeostasis in the liver has not been ascertained.
Therapeutic Choices for Treatment of Obesity-Induced Insulin Resistance
It is now clear that aggressive control of hyperglycemia in patients with diabetes can attenuate the long-term cardiovascular and renal complications of this disease.98,99 Exercise, life style modification, and weight reduction should go pari passu with pharmacotherapy.100 Exercise seems to increase glucose transport in cells independent of Akt/PKB, most likely through the adenosine monophosphate (AMP) kinase pathway.101 Although weight reduction by liposuction in obesity has no effect on insulin action or risk factors for coronary artery disease,102 reduction in body weight with gastric bypass surgery not only improves the diabetes but also morbidity and mortality.103-105 The complications related to gastric bypass surgery, however, are high in this population.103-104
Metformin is a biguanide. It has a well established, beneficial effect on peripheral tissues and mitochondrial function, where it enhances the oxidation of fat and glucose presumably by activating adenosine monophosphate kinase.106 The thiazolidinediones are a newer class of insulin-sensitizing drugs that not only increase peripheral utilization of glucose and suppress glucose production, but also exert pleiotropic effects on fat and inflammation.5,107 The thiazolidinediones function as PPAR-γ agonists. In severe obesity, the adipose tissue macrophage is switched from its alternative activated form to its pro-inflammatory form.108 The PPAR-γ agonists prime the adipose tissue, and the macrophages revert to the alternative activated form,109 with improvement of insulin resistance.110 These new findings are consistent with the theory that obesity-induced adipose tissue inflammation is a pivotal mediator of insulin resistance (Figure 2) and provide additional scientific basis for therapy with PPAR-γ agonists. Thiazolidinediones, however, are associated with a high incidence of adverse cardiovascular events including congestive heart failure and myocardial infarction and therefore caution should be exercised when using these drugs.111 Additional approaches that could be used to treat obesity and its effects on hyperglycemia include drugs that attenuate appetite and enhance energy expenditure. There are several reports of success with such approaches.7,97,112
GSK-3β inhibitors, which have multipronged effects on insulin signaling and inflammation as well as glycogen synthesis, could also prove beneficial. Experimental evidence on GSK-3β inhibitors exists,26,27 but clinical studies in obesity are lacking. Although salycilates have proved useful,41 the use of these derivatives in surgical patients needs careful evaluation. Complications associated with salycilates, particularly in the high doses recommended for treatment of diabetes, include gastric ulceration, increased bleeding, and renal dysfunction. Future therapeutic regimens probably will include the use of iNOS inhibitors, which are currently in development for human use. The rationale for this line of treatment has already been discussed.
Potential hyperglycemia-independent impact of insulin resistance in critical illness
Obesity is associated with worse prognosis in patients with trauma,113 although conflicting results have also been reported. The deleterious impact of obesity has been postulated to be attributable, at least in part, to derangements in metabolic function. Insulin resistance is a major denominator and a central player of obesity-related metabolic derangements. Attenuated actions of insulin result in hyperglycemia, decreased protein synthesis, increased protein degradation, increased susceptibility to infection, reduced anti-apoptotic (prosurvival) and anti-inflammatory actions of insulin,10-14 all of which contribute to exacerbation of critical illness. For critically ill patients with hyperglycemia, therefore, intensive insulin therapy has been employed in intensive care units to achieve tight glycemic control.9 Although most of the detrimental effects of insulin resistance may be substantially reversed by exogenous insulin administration, it remains an open question whether intensive insulin therapy can fully reverse the detrimental effects of insulin resistance in critical illness, even if tight glycemic control is achieved. Hyperinsulinemia is an independent risk factor for the development of atherosclerosis, a major complication in type 2 diabetes. It is conceivable, therefore, that even if tight glycemic control without hypoglycemia is achieved by insulin therapy, the combination of hyperinsulinemia and insulin resistance might elicit some deleterious outcomes in critically ill patients.
Hyperglycemia is usually a good indicator of insulin insufficiency. However, there are some exceptions in the intensive care unit. Rarely, some patients may exhibit euglycemia or even hypoglycemia despite serious insulin resistance with normal or decreased plasma insulin concentration. Critical illness, such as sepsis and trauma, induces impaired hepatic glucose production independent of insulin action, leading to hypoglycemia,114,115 despite the co-existing attenuation of insulin action caused by insulin resistance. In these cases, although hyperglycemia is not observed, other beneficial actions of insulin, including anabolic, anti-inflammatory and anti-apoptotic functions, are impaired owing to insulin resistance.
Control of excessive inflammation and subsequent apoptotic cell death has been proposed to be of essential importance to prevent and/or reverse tissue organ failure. Improvement in insulin sensitivity is accompanied by decreased inflammation and vice versa. This suggests that insulin-sensitizing drugs may be candidates for reducing the mortality of critically ill patients by their ability to improve insulin sensitivity and reverse excessive inflammation. Unfortunately, thiazolidinediones and metformin, the two clinically approved insulin sensitizers, cannot be used for critically ill patients because of adverse side effects (e.g., edema and heart failure by thiazolidinediones,116 lactic acidosis by metformin117). Therefore, novel types of insulin-sensitizing drugs, which do not elicit such adverse side effects, would need to be developed to help improve the mortality of critically ill patients by reversal of inflammation and insulin resistance.
As discussed in this review, obesity-induced insulin resistance is closely linked to the inflammatory response/stress signaling pathways. The implication of hyperglycemia in critical illness has been an intense area of investigation for a number of years.118-120 In contrast, the glycemic control-independent influence of insulin resistance in morbidity and mortality of critically ill patients is poorly understood, although previous studies have documented the glucose level-independent beneficial effects of insulin in rodent models of critical illness. Of interest, a recent study121 showed that the severity of insulin resistance is associated with the severity of critical illness and body mass index, although no significant association was found between insulin resistance and basal blood glucose level. Thus, a potential hyperglycemia-independent impact of insulin resistance on prognosis during and after critical illness is worthy of further investigation.
Conclusion
For individuals born in the United States in 2000, the lifetime probability of being diagnosed with obesity and diabetes is substantial.122 Obesity is an independent risk factor for cardiovascular disease,1-3 which increases the risk of peri-operative complications. Interventions to reduce body weight have beneficial effects on decreasing cardiac complications in the perioperative period. Hyperglycemia and/or insulin resistance is associated with increased morbidity. Correction of hyperglycemia in critically ill and/or surgical patients decreases intensive care unit stay and improves hospital mortality, an effect particularly evident in surgical patients.122,123 This beneficial effect is probably related to the pleiotropic effects of insulin on glucose, protein, and inflammation.10-14 Thus, insulin resistance in the absence of obesity is also a major risk factor for surgical patients.119-125 The compounding effect of obesity further aggravates peri-operative morbidity and leads to systemic diseases that affect vital organs including brain, kidney, heart, and liver.1,6,12
Obesity-induced diabetes is related to decreased insulin-signaling via IR and its downstream proteins. A chronic inflammatory state seems to play a pivotal role in the insulin resistance. Differences do exist in the mechanisms of insulin resistance between liver, muscle, and adipocyte. The role of the CNS in insulin sensors sensing and glucose homeostasis is now well established. Understanding the impairments of insulin-signaling related to obesity-induced diabetes may lead to better pharmacological methods not only to treat but also to prevent obesity and type-2 diabetes. In this regard, in addition to behavior modifications that modify food (calorie) intake or alter the composition of foods ingested, development of drugs to decrease appetite and increase metabolism may also prove useful. Recent studies do confirm that multimodal, multifactorial interventions including behavior modifications and surgery have sustained beneficial effects on insulin sensitivity, cardiovascular complications, and mortality.9,99,100
Acknowledgments
Supported by National Institute of Health, Bethesda, MD, grant #'s GM2500-Project 4, GM055082, and GM 31569 (J.A.J.M.), DK 58127 (M.K.) and Shriners Hospitals Philanthropy, Tampa, FL (J.A.J.M., M.K., and S.Y.)
The authors acknowledge the invaluable assistance of Don Poulsen, Senior Medical Illustrator Shriners Hospital for Children, Boston, MA for the art-work presented in the text.
References
- 1.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world--a growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Rocchini AP. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346:854–5. doi: 10.1056/NEJM200203143461112. [DOI] [PubMed] [Google Scholar]
- 5.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 6.Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29:109–17. doi: 10.1385/ENDO:29:1:109. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trayhurn P. Endocrine and signalling role of adipose tissue: new perspectives on fat. Acta Physiol Scand. 2005;184:285–93. doi: 10.1111/j.1365-201X.2005.01468.x. [DOI] [PubMed] [Google Scholar]
- 9.Bagry HS, Raghavendran S, Carli F. Metabolic syndrome and insulin resistance. Anesthesiology. 2008;108:506–23. doi: 10.1097/ALN.0b013e3181649314. [DOI] [PubMed] [Google Scholar]
- 10.Saltiel AR, Kahn CR. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 11.Hansen TK, Thiel S, Wouters PJ, Christiansen JS, Van den Berghe G. Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose-binding lectin levels. J Clin Endocrinol Metab. 2003;88:1082–8. doi: 10.1210/jc.2002-021478. [DOI] [PubMed] [Google Scholar]
- 12.Turina M, Fry DE, Polk HC., Jr. Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med. 2005;33:1624–33. doi: 10.1097/01.ccm.0000170106.61978.d8. [DOI] [PubMed] [Google Scholar]
- 13.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 14.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–9. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 15.Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res. 2006;99:570–82. doi: 10.1161/01.RES.0000242563.47507.ce. [DOI] [PubMed] [Google Scholar]
- 16.White MF. IRS proteins and the common path to diabetes. Am J Physiol Endocrinol Metab. 2002;283:E413–22. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- 18.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994;269:3568–73. [PubMed] [Google Scholar]
- 19.Ikezu T, Okamoto T, Yonezawa K, Tompinks RG, Martyn JAJ. Analysis of thermal injury-induced insulin resistance in rodents: Implication of post-receptor mechanism. J Biol Chem. 1997;272:25289–95. doi: 10.1074/jbc.272.40.25289. [DOI] [PubMed] [Google Scholar]
- 20.Sugita H, Kaneki M, Sugita M, Yasukawa T, Martyn JAJ. Burn injury impairs insulin-stimulated Akt/PKB activation in skeletal muscle. American Journal of Physiology Endo Metab. 2005;288:E585–91. doi: 10.1152/ajpendo.00321.2004. [DOI] [PubMed] [Google Scholar]
- 21.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–8. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 22.Datta SR, Brunet A, Greenberg ME. Cellular survival. a play in three Akts. Genes Dev. 1999;13:2905–27. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 23.Yasuhara S, Perez ME, Kanakubo E, Yasuhara Y, Shin YS, Kaneki M, Fujita T, Martyn JA. Skeletal muscle apoptosis after burns is associated with activation of proapoptotic signals. Am J Physiol Endocrinol Metab. 2000;279:E1114–21. doi: 10.1152/ajpendo.2000.279.5.E1114. [DOI] [PubMed] [Google Scholar]
- 24.Yasuhara S, Kanakubo E, Perez ME, Kaneki M, Fujita T, Okamoto T, Martyn JAJ. The 1999 Moyer award. Burn injury induces skeletal muscle apoptosis and the activation of caspase pathways in rats. J Burn Care Rehabil. 1999;20:462–70. doi: 10.1097/00004630-199920060-00007. [DOI] [PubMed] [Google Scholar]
- 25.Hirose M, Kaneki M, Sugita H, Yasuhara S, Martyn JAJ. Immobilization depresses insulin signaling in skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279:E1235–41. doi: 10.1152/ajpendo.2000.279.6.E1235. [DOI] [PubMed] [Google Scholar]
- 26.Dugo L, Collin M, Thiemermann C. Glycogen synthase kinase 3beta as a target for the therapy of shock and inflammation. Shock. 2007;27:113–23. doi: 10.1097/01.shk.0000238059.23837.68. [DOI] [PubMed] [Google Scholar]
- 27.Dugo L, Collin M, Allen DA, Murch O, Foster SJ, Yaqoob MM, Thiemermann C. Insulin reduces the multiple organ injury and dysfunction caused by coadministration of lipopolysaccharide and peptidoglycan independently of blood glucose: role of glycogen synthase kinase-3beta inhibition. Crit Care Med. 2006;34:1489–96. doi: 10.1097/01.CCM.0000215457.83953.E3. [DOI] [PubMed] [Google Scholar]
- 28.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 29.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 30.Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, Slezak LA, Andersen DK, Hundal RS, Rothman DL, Petersen KF, Shulman GI. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–9. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shoelson SE, Herrero L, Naaz A. Obesity, Inflammation and Insulin Resistance. Gastroenterology. 2007;132:2169–80. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 32.Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–5. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–6. [PubMed] [Google Scholar]
- 34.Larsen CM, Faulenbach M, Vaag A, Volund A, Ehses JA, Seifert B, Mandrup-Poulsen T, Donath MY. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–26. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 35.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–55. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 36.Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–8. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- 37.Yasukawa T, Tokunaga E, Ota H, Sugita H, Martyn JAJ, Kaneki M. S-nitrosylation-dependent inactivation of Akt/PKB in insulin resistance. J Biol Chem. 2005;280:7511–18. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 38.Schattenberg JM, Singh R, Wang Y, Lefkowitch JH, Rigoli RM, Scherer PE, Czaja MJ. JNK1 but not JNK2 promotes the development of steatohepatitis in mice. Hepatology. 2006;43:163–72. doi: 10.1002/hep.20999. [DOI] [PubMed] [Google Scholar]
- 39.Aguirre V, Uchida T, Yenush L, Davis R, White MF. The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307) J Biol Chem. 2000;275:9047–54. doi: 10.1074/jbc.275.12.9047. [DOI] [PubMed] [Google Scholar]
- 40.Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–9. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- 41.Hundal RS, Petersen KF, Mayerson AB, Randhawa PS, Inzucchi S, Shoelson SE, Shulman GI. Mechanism by which high-dose aspirin improves glucose metabolism in type 2 diabetes. J Clin Invest. 2002;109:1321–6. doi: 10.1172/JCI14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phillips DI, Caddy S, Ilic V, Fielding BA, Frayn KN, Borthwick AC, Taylor R. Intramuscular triglyceride and muscle insulin sensitivity: evidence for a relationship in nondiabetic subjects. Metabolism. 1996;45:947–50. doi: 10.1016/s0026-0495(96)90260-7. [DOI] [PubMed] [Google Scholar]
- 43.Kim JK, Fillmore JJ, Sunshine MJ, Albrecht B, Higashimori T, Kim DW, Liu ZX, Soos TJ, Cline GW, O’Brien WR, Littman DR, Shulman GI. PKC-theta Knockout mice are protected from fat-induced insulin resistance. J Clin Invest. 2004 Sep;114:823–7. doi: 10.1172/JCI22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prada PO, Zecchin HG, Gasparetti AL, Torsoni MA, Ueno M, Hirata AE, Corezola do Amaral ME, Höer NF, Boschero AC, Saad MJ. Western diet modulates insulin signaling, c-Jun N-terminal kinase activity, and insulin receptor substrate-1ser307 phosphorylation in a tissue-specific fashion. Endocrinology, 2005;146:1576–87. doi: 10.1210/en.2004-0767. [DOI] [PubMed] [Google Scholar]
- 45.Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- 46.Röhl M, Pasparakis M, Baudler S, Baumgartl J, Gautam D, Huth M, De Lorenzi R, Krone W, Rajewsky K, Bruning JC. Conditional disruption of IkappaB kinase 2 fails to prevent obesity-induced insulin resistance. J Clin Invest. 2004;113:474–81. doi: 10.1172/JCI18712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–61. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 48.Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu Rev Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- 49.Sutherland C, O'Brien RM, Granner DK. New connections in the regulation of PEPCK gene expression by insulin. Philos Trans R Soc Lond B Biol Sci. 1996;351:191–9. doi: 10.1098/rstb.1996.0016. [DOI] [PubMed] [Google Scholar]
- 50.Li X, Monks B, Ge Q, Birnbaum MJ. Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature. 2007;447:1012–6. doi: 10.1038/nature05861. [DOI] [PubMed] [Google Scholar]
- 51.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–5. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 52.Ide T, Shimano H, Yahagi N, Matsuzaka T, Nakakuki M, Yamamoto T, Nakagawa Y, Takahashi A, Suzuki H, Sone H, Toyoshima H, Fukamizu A, Yamada N. SREBPs suppress IRS-2-mediated insulin signalling in the liver. Nat Cell Biol. 2004;6:351–7. doi: 10.1038/ncb1111. [DOI] [PubMed] [Google Scholar]
- 53.Foretz M, Pacot C, Dugail I, Lemarchand P, Guichard C, Le Liepvre X, Berthelier-Lubrano C, Spiegelman B, Kim JB, Ferre P, Foufelle F. ADD1/SREBP-1c is required in the activation of hepatic lipogenic gene expression by glucose. Mol Cell Biol. 1999;19:3760–8. doi: 10.1128/mcb.19.5.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ferre P, Foufelle F. SREBP-1c transcription factor and lipid homeostasis: clinical perspective. Horm Res. 2007;68:72–82. doi: 10.1159/000100426. [DOI] [PubMed] [Google Scholar]
- 55.Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R, Landaker EJ, Goldfine AB, Mun E, DeFronzo R, Finlayson J, Kahn CR, Mandarino LJ. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100:8466–71. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 57.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–59. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 58.Su Y, Kondrikov D, Block ER. Cytoskeletal regulation of nitric oxide synthase. Cell Biochem Biophys. 2005;43:439–49. doi: 10.1385/CBB:43:3:439. [DOI] [PubMed] [Google Scholar]
- 59.Szabo C, Ischiropoulos H, Radi R. Peroxynitrite: biochemistry, pathophysiology and development of therapeutics. Nat Rev Drug Discov. 2007;6:662–80. doi: 10.1038/nrd2222. [DOI] [PubMed] [Google Scholar]
- 60.Forrester MT, Stamler JS. A classification scheme for redox-based modifications of proteins. Am J Respir Cell Mol Biol. 2007;36:135–7. doi: 10.1165/rcmb.2006-001ED. [DOI] [PubMed] [Google Scholar]
- 61.Sugita H, Kaneki M, Tokunaga E, Sugita M, Koike C, Yasuhara S, Tompkins RG, Martyn JAJ. Inducible nitric oxide synthase plays a role in LPS-induced hyperglycemia and insulin resistance. Am J Physiol Endocrinol Metab. 2002;282:E386–94. doi: 10.1152/ajpendo.00087.2001. [DOI] [PubMed] [Google Scholar]
- 62.Yasukawa T, Tokunago E, Ota H, Sugita H, Maryn JAJ, Keneki M. S-nitrosylation-dependent inactivation of Akt/protein kinase B in insulin resistance. J Biol Chem. 2005;2890:7411–8. doi: 10.1074/jbc.M411871200. [DOI] [PubMed] [Google Scholar]
- 63.Fujimoto M, Shimizu N, Kunii K, Martyn JA, Ueki K, Kaneki M. A role for iNOS in fasting hyperglycemia and impaired insulin signaling in the liver of obese diabetic mice. Diabetes. 2005;54:1340–8. doi: 10.2337/diabetes.54.5.1340. [DOI] [PubMed] [Google Scholar]
- 64.Sugita H, Fujimoto M, Yasukawa T, Shimizu N, Sugita M, Yasuhara S, Martyn JA, Kaneki M. Inducible nitric-oxide synthase and NO donor induce insulin receptor substrate-1 degradation in skeletal muscle cells. J Biol Chem. 2005;280:14203–11. doi: 10.1074/jbc.M411226200. [DOI] [PubMed] [Google Scholar]
- 65.Tannous M, Rabini RA, Vignini A, Moretti N, Fumelli P, Zielinski B, Mazzanti L, Mutus B. Evidence for iNOS-dependent peroxynitrite production in diabetic platelets. Diabetologia. 1999;42:539–44. doi: 10.1007/s001250051192. [DOI] [PubMed] [Google Scholar]
- 66.Torres SH, De Sanctis JB, de LBM, Hernandez N, Finol HJ. Inflammation and nitric oxide production in skeletal muscle of type 2 diabetic patients. J Endocrinol. 2004;181:419–27. doi: 10.1677/joe.0.1810419. [DOI] [PubMed] [Google Scholar]
- 67.Elizalde M, Ryden M, van Harmelen V, Eneroth P, Gyllenhammar H, Holm C, Ramel S, Olund A, Arner P, Andersson K. Expression of nitric oxide synthases in subcutaneous adipose tissue of nonobese and obese humans. J Lipid Res. 2000;41:1244–51. [PubMed] [Google Scholar]
- 68.Perreault M, Marette A. Targeted disruption of inducible nitric oxide synthase protects against obesity-linked insulin resistance in muscle. Nat Med. 2001;7:1138–43. doi: 10.1038/nm1001-1138. [DOI] [PubMed] [Google Scholar]
- 69.Laaksonen DE, Niskanen L, Nyyssonen K, Punnonen K, Tuomainen TP, Valkonen VP, Salonen R, Salonen JT. C-reactive protein and the development of the metabolic syndrome and diabetes in middle-aged men. Diabetologia. 2004;47:1403–10. doi: 10.1007/s00125-004-1472-x. [DOI] [PubMed] [Google Scholar]
- 70.Festa A, D'Agostino R, Jr., Tracy RP, Haffner SM. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131–7. doi: 10.2337/diabetes.51.4.1131. [DOI] [PubMed] [Google Scholar]
- 71.Nakatani Y, Kaneto H, Kawamori D, Yoshiuchi K, Hatazaki M, Matsuoka TA, Ozawa K, Ogawa S, Hori M, Yamasaki Y, Matsuhisa M. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. J Biol Chem. 2005;280:847–51. doi: 10.1074/jbc.M411860200. [DOI] [PubMed] [Google Scholar]
- 72.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–76. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 73.Tamatani M, Matsuyama T, Yamaguchi A, Mitsuda N, Tsukamoto Y, Taniguchi M, Che YH, Ozawa K, Hori O, Nishimura H, Yamashita A, Okabe M, Yanagi H, Stern DM, Ogawa S, Tohyama M. ORP150 protects against hypoxia/ischemia-induced neuronal death. Nat Med. 2001;7:317–23. doi: 10.1038/85463. [DOI] [PubMed] [Google Scholar]
- 74.Kuwabara K, Matsumoto M, Ikeda J, Hori O, Ogawa S, Maeda Y, Kitagawa K, Imuta N, Kinoshita T, Stern DM, Yanagi H, Kamada T. Purification and characterization of a novel stress protein, the 150-kDa oxygen-regulated protein (ORP150), from cultured rat astrocytes and its expression in ischemic mouse brain. J Biol Chem. 1996;271:5025–32. doi: 10.1074/jbc.271.9.5025. [DOI] [PubMed] [Google Scholar]
- 75.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–40. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang C, Kawauchi J, Adachi MT, Hashimoto Y, Oshiro S, Aso T, Kitajima S. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem Biophys Res Commun. 2001;289:718–24. doi: 10.1006/bbrc.2001.6044. [DOI] [PubMed] [Google Scholar]
- 77.Werstuck GH, Lentz SR, Dayal S, Hossain GS, Sood SK, Shi YY, Zhou J, Maeda N, Krisans SK, Malinow MR, Austin RC. Homocysteine-induced endoplasmic reticulum stress causes dysregulation of the cholesterol and triglyceride biosynthetic pathways. J Clin Invest. 2001;107:1263–73. doi: 10.1172/JCI11596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, Mori M. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest. 2002;109:525–32. doi: 10.1172/JCI14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim H, Shim J, Han PL, Choi EJ. Nitric oxide modulates the c-Jun N-terminal kinase/stress-activated protein kinase activity through activating c-Jun N-terminal kinase kinase. Biochemistry. 1997;36:13677–81. doi: 10.1021/bi970837f. [DOI] [PubMed] [Google Scholar]
- 80.Umansky V, Hehner SP, Dumont A, Hofmann TG, Schirrmacher V, Droge W, Schmitz ML. Co-stimulatory effect of nitric oxide on endothelial NF-kappaB implies a physiological self-amplifying mechanism. Eur J Immunol. 1998;28:2276–82. doi: 10.1002/(SICI)1521-4141(199808)28:08<2276::AID-IMMU2276>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 81.Uehara T, Nakamura T, Yao D, Shi ZQ, Gu Z, Ma Y, Masliah E, Nomura Y, Lipton SA. Snitrosylated protein-disulphide isomerase links protein misfolding to neurodenegeration. Nature. 2006;441:513–7. doi: 10.1038/nature04782. [DOI] [PubMed] [Google Scholar]
- 82.Gribble FM. Metabolism: A higher power for insulin. Nature. 2005;434:965–6. doi: 10.1038/434965a. [DOI] [PubMed] [Google Scholar]
- 83.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 84.Elmquist JK, Marcus JN. Rethinking the central causes of diabetes. Nat Med. 2003;9:645–7. doi: 10.1038/nm0603-645. [DOI] [PubMed] [Google Scholar]
- 85.Yamada T, Katagiri H, Ishigaki Y, Ogihara T, Imai J, Uno K, Hasegawa Y, Gao J, Ishihara H, Niijima A, Mano H, Aburantani H, Asano T, Oka Y. Signals from intra-abdominal fat modulate insulin and leptin sensitivity through different mechanism: neuronal involvement in food-intake regulation. Cell Metab. 2006;3:223–9. doi: 10.1016/j.cmet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 86.Murphy KG, Bloom SR. Gut hormones and the regulation of energy homeostasis. Nature. 2006;444:854–9. doi: 10.1038/nature05484. [DOI] [PubMed] [Google Scholar]
- 87.Cota S, Proulx K, Seeley R. The role of CNS fuel sensing in energy and glucose regulation. Gastroenterology. 2007;132:2158–68. doi: 10.1053/j.gastro.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 88.Ikezu T, Yasuhara S, Granneman JG, Kraemer FB, Okamoto T, Tompkins RG, Martyn JAJ. A unique mechanism of desensitization to lipolysis mediated by beta(3)- adrenoceptor in rats with thermal injury. Am J Physiol. 1999;277:E316–24. doi: 10.1152/ajpendo.1999.277.2.E316. [DOI] [PubMed] [Google Scholar]
- 89.Boden G, Hoeldtke RD. Nerves, fat, and insulin resistance. N Engl J Med. 2003;349:1966–7. doi: 10.1056/NEJMcibr035229. [DOI] [PubMed] [Google Scholar]
- 90.Kreier F, Kap YS, Mettenleiter TC, van Heijningen C, van der Vliet J, Kalsbeek A, Sauerwein HP, Fliers E, Romijn JA, Buijs RM. Tracing from fat tissue, liver, and pancreas: a neuroanatomical framework for the role of the brain in type 2 diabetes. Endocrinology. 2006;147:1140–1147. doi: 10.1210/en.2005-0667. [DOI] [PubMed] [Google Scholar]
- 91.Buijs RM, Kreier F. The metabolic syndrome: a brain disease? J Neuroendocrinol. 2006;8:715–6. doi: 10.1111/j.1365-2826.2006.01456.x. [DOI] [PubMed] [Google Scholar]
- 92.Giordano A, Song CK, Bowers RR, Ehlen JC, Frontini A, Cinti S, Bartness TJ. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1243–55. doi: 10.1152/ajpregu.00679.2005. [DOI] [PubMed] [Google Scholar]
- 93.Berthoud HR, Fox EA, Neuhuber WL. Vagaries of adipose tissue innervation. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1240–42. doi: 10.1152/ajpregu.00428.2006. [DOI] [PubMed] [Google Scholar]
- 94.Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449:721–5. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 95.Sternberg EM. Neural regulation of innate immunity: a coordinated nonspecific host response to pathogens. Nat Rev Immunol. 2006;6:318–28. doi: 10.1038/nri1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–31. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 97.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–32. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 98.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 99.Gaede P, Lund-Andersen H, Parving HH, Pederson O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–91. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 100.Wu RR, Zhao JP, Jin H, Shao P, Fang MS, Guo XF, He YQ, Liu YJ, Chen JD, Li LH. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain. JAMA. 2008;299:185–93. doi: 10.1001/jama.2007.56-b. [DOI] [PubMed] [Google Scholar]
- 101.Shepherd PR, Kahn BB. Glucose transporters and insulin action--implications for insulin resistance and diabetes mellitus. N Engl J Med. 1999;341:185–57. doi: 10.1056/NEJM199907223410406. [DOI] [PubMed] [Google Scholar]
- 102.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004;350:2549–57. doi: 10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 103.DeMaria EJ. Bariatric surgery for morbid obesity. N Engl J Med. 2007;356:2176–83. doi: 10.1056/NEJMct067019. [DOI] [PubMed] [Google Scholar]
- 104.Sjostrom L, Narbro K, Sjostrom CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, Bengtsson C, Dahlgren S, Gummesson A, Jacobson P, Karlsson J, Lindroos AK, Lonroth H, Naslund I, Olbers T, Stenlof K, Torgerson J, Agren G, Carlsson LM. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–52. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 105.Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, Skinner S, Proietto J, Bailey M, Anderson M. Adjustable gastric banding and conventional therapy for type 2 diabetes. JAMA. 2008;299:316–23. doi: 10.1001/jama.299.3.316. [DOI] [PubMed] [Google Scholar]
- 106.Detaille D, Guigas B, Chauvin C, Batandier C, Fontaine E, Wiernsperger N, Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–87. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 107.Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–18. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- 108.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bouhlel MA, Derudas B, Rigamonti E, Dievart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, Staels B, Chinetti-Gbaguidi G. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6:137–43. doi: 10.1016/j.cmet.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 110.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, Eagle AR, Vats D, Brombacher F, Ferrante AW, Chawla A. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447:1116–20. doi: 10.1038/nature05894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–71. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 112.Moller DE. New drug targets for type 2 diabetes and the metabolic syndrome. Nature. 2001;414:821–7. doi: 10.1038/414821a. [DOI] [PubMed] [Google Scholar]
- 113.Oliveros H, Villamor E. Obesity and mortality in critically ill adults: A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:515–21. doi: 10.1038/oby.2007.102. [DOI] [PubMed] [Google Scholar]
- 114.Lang CH, Dobrescu C. Sepsis-induced increases in glucose uptake by macrophage-rich tissues persist during hypoglycemia. Metabolism. 1991;40:585–93. doi: 10.1016/0026-0495(91)90048-2. [DOI] [PubMed] [Google Scholar]
- 115.Mendoza A, Kim YN, Chernoff A. Hypoglycemia in hospitalized adult patients without diabetes. Endocr Pract. 2005;11:91–6. doi: 10.4158/EP.11.2.91. [DOI] [PubMed] [Google Scholar]
- 116.Kiryluk K, Isom R. Thiazolidinediones and fluid retention. Kidney Int. 2007;72:762–8. doi: 10.1038/sj.ki.5002442. [DOI] [PubMed] [Google Scholar]
- 117.Bolen S, Feldman L, Vassy J, Wilson L, Yeh HC, Marinopoulos S, Wiley C, Selvin E, Wilson R, Bass EB, Brancati FL. Systematic review: Comparative effectiveness and safety of oral medications for type 2 diabetes mellitus. Ann Intern Med. 2007;147:386–99. doi: 10.7326/0003-4819-147-6-200709180-00178. [DOI] [PubMed] [Google Scholar]
- 118.Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, Moerer O, Gruendling M, Oppert M, Grond S, Olthoff D, Jaschinski U, John S, Rossaint R, Welte T, Schaefer M, Kern P, Kuhnt E, Kiehntopf M, Hartog C, Natanson C, Loeffler M, Reinhart K. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 119.Vanhorebeek I, Langouche L, Van den Berghe G. Tight blood glucose control: What is the evidence? Crit Care Med. 2007;35:S496–502. doi: 10.1097/01.CCM.0000278051.48643.91. [DOI] [PubMed] [Google Scholar]
- 120.Preiser JC, Devos P. Clinical experience with tight glucose control by intensive insulin therapy. Crit Care Med. 2007;35:S503–7. doi: 10.1097/01.CCM.0000278046.24345.C7. [DOI] [PubMed] [Google Scholar]
- 121.Zauner A, Nimmerrichter P, Anderwald C, Bischof M, Shiefermeier M, Ratheiser K, Schneeweiss B, Zauner C. Severity of insulin resistance in critically ill medical patients. Metabolism. 2007;56:1–5. doi: 10.1016/j.metabol.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 122.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290:1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 123.McGlinch BP, Que FG, Nelson JL, Wrobleski DM, Grant JE, Collazo-Clavell ML. Perioperative care of patients undergoing bariatric surgery. Mayo Clin Proc. 2006;81:S25–33. doi: 10.1016/s0025-6196(11)61178-6. [DOI] [PubMed] [Google Scholar]
- 124.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, Van Wijngaerden E, Bobbaers H, Bouillon R. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 125.Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in the surgical intensive care unit. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]