Abstract
Previous studies demonstrated that naïve plasma has inherent capabilities to enhance bacterial opsonization and phagocyte killing but not all plasma is equally effective. This raised the question of whether plasma constituents other than opsonins may play a role. Adenosine receptor antagonists have been shown to modulate cytokine response and survival in mice after a bacterial challenge. We investigated whether selective adenosine receptor blockade would influence the ability of naïve plasma to effectively control bacterial growth. Colonic bacteria, and thioglycollate elicited peritoneal macrophages and neutrophils were obtained from naïve mice. Stock murine plasma from naïve was purchased and categorized as having high plasma enhanced bacterial killing capacity using our previously described methods. Bacteria and plasma were incubated in order to allow for opsonization and then added to macrophages previously exposed to selected adenosine receptor antagonists; ZM 241385: A2A, MRS1754: A2B, DPCPX: A1, and MRS1220: A3. The final mixture was plated on blood agar plates in aerobic and anaerobic conditions and bacterial colony forming units quantified after 24 hours. This study demonstrated that exogenous adenosine was able to significantly decrease phagocyte killing of cecal bacteria. Blocking adenosine receptors with selective antagonists altered the bacterial killing capacity of plasma. Selectively blocking the A1,A2A, or A2B receptors proved most beneficial at reversing the effect of adenosine. Consistent with previous work, only macrophage killing of bacteria could be modulated by adenosine receptor blockade since neutrophils were unaffected. These data demonstrate that adenosine decreases macrophage killing of enteric bacteria and that this effect is mediated through the adenosine receptors.
Keywords: adenosine, adenosine receptor antagonist, plasma enhanced killing, sepsis, phagocytosis
Introduction
Sepsis continues to be a large personal and economic burden; it has an incidence of approximately 750,000 patients/year with mortality close to 210,000 deaths/year (1, 2). One interesting fact about sepsis is that the mortality rate is about 40%, indicating that some people are able to recover from a septic challenge and while others succumb. A major problem facing the medical world today is the emergence of multidrug resistant bacteria. The increasing number drug resistant bacteria is far outpacing the development of effective antibiotics, leaving clinicians with a limited arsenal of antibiotics at their disposal (3, 4). This creates a need to evaluate other ways to augment the immune system defense against invading pathogens. A murine study was able to identify that a pre-existing plasma factor, probably IgG, could be used to predict mortality in the murine cecal ligation and puncture (CLP) model. Plasma collected days prior to the onset of sepsis would enhance neutrophil killing of enteric plasma in those mice who would survive sepsis (5). This could be helpful in possibly determining which patients would need more aggressive management of their septic episode. However, the previous study did not fully identify the nature of the factor within the plasma that enhances bacterial killing.
One specific factor that can modulate the immune response is adenosine. Adenosine is an endogenous purine nucleoside rapidly released from cells upon ATP degradation under conditions of stress, hypoxia, or inflammation (6). Adenosine receptors are broken into 2 categories: Stimulatory G protein coupled and Inhibitory G protein coupled. A2A and A2B are G stimulatory protein coupled and are expressed mostly by macrophages and monocytes. Adenosine binding to these receptors causes increased cAMP resulting in inhibition of these cells. It also stops the release of pro-inflammatory cytokines; IFN-γ, TNF-α, IL-12 and causes the release of IL-10 and VEGF. A1 and A3 are inhibitory G protein coupled and inhibit adenylyl cyclase activity, decrease TNF-α, IL-12 and KC production, stop mast cell degranulation and inhibit tissue factor expression (7). In aggregate, adenosine functions as a strong stop signal to the immune system as it develops an inflammatory response to an infectious stimulus. Adenosine's role has been studied in a variety of experimental models ranging from infectious (cecal ligation and puncture, CLP) to ischemic. In the CLP model pharmacologic antagonists or genetic deletion of the receptors A2A and A2B has been shown to improved survival (8, 9). Interestingly A1 and A3 knockout mice have been found to do worse during an infectious insult such as bacterial peritonitis (10, 11). In the ischemia realm A1 receptor agonists have been shown to be protect the kidney from injury (12).
Adenosine, which has the power to enhance or subdue the immune response, represents an ideal candidate to examine as an option for the current sepsis problem. Further evidence suggesting a role for adenosine is provided by data indicating that some bacteria such as Staphylococcus aureus and Bacillus anthracis make adenosine as a way of evading their killing (13). Based on this prior work, we hypothesized that adenosine, even in the presence of plasma that promoted increased bacterial killing, may still play a role in the cellular response necessary to kill cecal bacteria. This action if present should then be able to be prevented or reversed by using an adenosine receptor antagonist.
Materials and Methods
Animals
Adult female ICR mice (from Harlan-Sprague Dawley, Inc., Indianapolis, IN) were used. Mice were acclimatized to our housing room for at least 5 days before surgery. This was a temperature controlled room with 12 hours light- 12 hours dark diurnal cycle. They were provided food and water ad libitum for the entire duration of the experiment. The experiments were approved by Boston University Animal Care and Use Committee.
Bacteria, Neutrophil and Macrophage collection
Mice were injected with 3mL of 3% thioglycollate intraperitoneally and 4 or 96 hours later were sacrificed after being given a mixture of ketamine/xylazine intraperitoneally (2.175mg and 0.325mg respectively), timing varied in order to obtain neutrophils or macrophages The abdominal area was sterilized using chlorhexidine prep solution, a midline incision was made and the peritoneal cavity lavaged with 5mL of Hank's Balanced Salt Solution (HBSS). The cecum was then identified and resected at the level of the ileocecal valve and placed into 5mL of HBSS. The peritoneal cells collected were then centrifuged, red blood cells were lysed with 3 ml of ACK lysis buffer (Invitrogen) and cells were re-suspended in HBSS and counted using Beckman-Coulter particle counter model ZF (Coulter Electronics, Hialeah, FL). The cecum was homogenized (Brinkmann, Polytron PT 3000). The bacteria were re-suspended in HBSS and quantified using the McFarland standard with measurement at 565 nm. Each experimental replicate was performed with a new cecal bacteria preparation. The colony forming units (CFU) were verified by plating aliquots in aerobic and anaerobic conditions. Each experimental replicate performed included a control in which the bacteria were plated by themselves in order to confirm appropriate bacterial growth. The results of bacterial growth are a sum of the anaerobic and aerobic plates.
Plasma
Naïve outbred ICR plasma was bought from Lampire Biological (Pipersville, PA). A plasma enhanced killing assay (PEK) was performed in order (described below) to ascertain the PEK value of the plasma. The stock of plasma was then aliquoted and stored at −20°C until it was used. Based on prior studies the PEK value of the plasma would be categorize as high killing capacity.
Plasma Enhanced Killing Assay (PEK)
Equal volumes of plasma (diluted to 50% with HBSS) and cecal bacteria (7.5×106 CFU total) were incubated together for 30 minutes at 37°C while shaking in order to allow for opsonization. The mixture was the placed on ice for 15 minutes to stop the process. The thioglycollate elicited macrophages were then added to the opsonized bacteria in a ratio of 10 bacteria per macrophage or neutrophil and placed back in the 37°C shaker for 1 hour. The final mixture was the plated on blood agar plates (Fisher Scientific) in two dilutions and placed at 37°C in both aerobic and anaerobic conditions for 24 hours. Bacterial colonies were then recorded.
Adenosine and Adenosine receptor antagonists
Adenosine was purchased from Acros Organics (New Jersey, US). A1 (DPCPX), A2A (ZM241385, A2B (MRS1754), and A3 (MRS1220) receptor antagonists were purchased from Tocris Bioscience (Minneapolis, MN). The final concentration of adenosine used in the PEK assay was 300nM. The adenosine receptor antagonists were diluted to concentrations that would allow for specific receptor antagonism with minimal crossover to other receptors based on previous publication (14). The following concentrations were used: A1 (10nM), A2A (30nM), A2B (10nM), A3 (10nM). These agents were used in a slightly modified PEK assay. During the ice incubation of the opsonized bacteria the thioglycollate elicited macrophages or neutrophils were incubated with adenosine for 15 minutes in a 37°C shaker and then the PEK assay continued as described above. For the experiments utilizing adenosine and adenosine receptor antagonists the cells were first exposed to the antagonists for 15 minutes in the 37°C shaker and then the adenosine was added and the cells were kept in the shaker for an additional 15 minutes.
Phagocytosis assay
pHrodo™ Red E. coli BioParticles, Invitrogen (Grand Island, NY) were opsonized with the supplied opsonizing reagent. The opsonized E. coli was then mixed with thioglycollate elicited macrophages which were collected in the same manner and exposed to adenosine or adenosine plus a receptor antagonist as described above. The final mixture was then analyzed by flow cytometry using a FACS Calibur (BD). The data are expressed as percent total mean fluorescence intensity.
Statistical Analysis
Statistics were performed using the computer program Prism 5 (GraphPad Software, San Diego, CA). A P value <0.05 was considered statistically significant. One-way ANOVA followed by Dunnett's Multiple Comparison Test was used to compare multiple groups. Unpaired students T-test were used when two groups were compared. Paired T-test was used to compare flow cytometry data after it was normalized to the control group.
Results
To conduct the in vitro phagocytosis assays we needed large populations of cells. Thioglycollate has been historically used to cause sterile peritonitis and phagocytic cell recruitment. It is also useful in its ability to select for specific cell populations depending on the time from injection to cell collection (15, 16). Peritoneal lavages performed 96 hours (4 days) after thioglycollate injection resulted in an exudate consisting of 84.5% macrophages compared to 54% neutrophils present 4 hours after injection (Figure 1). The effect of thioglycollate on macrophages has been debated, but most studies show some alterations in receptor expression and morphology (17, 18) indicating possible activation but without increased phagocytic capacity. This data allows us to confidently interpret the result of a plasma enhanced killing assay being due to the experimental treatments of the cells.
Figure 1.
A. Peritoneal lavage cell percentage at different times after intraperitoneal thioglycollate injection. Mice were injected with 3% thioglycollate and peritoneal lavages were performed at 4 hours or and 96 hours (4 days) after injection. Four hours after injection the cell population consisted of 54% neutrophils and after 96 hours (4 days) the cells were 84% macrophages. Cells were counted under a microscope and classified by morphology. Each time point consisted of 5 peritoneal lavages and the data are mean ± SEM. B. Representative cytospin slides of peritoneal lavages at 4 and 96 hours after thioglycollate injection.
In order to document that plasma will enhance phagocyte killing of enteric bacteria we incubated bacteria with macrophages without plasma and macrophages with plasma separately. Figure 2A demonstrates that macrophages without plasma carry out minimal bacterial killing compared to bacteria only. In the presence of plasma, macrophages were able to reduce the bacterial load to half (7×105 vs 3.2×105 CFU). These data confirm that factors within the plasma will enhance bacterial killing.
Figure 2.
A. Plasma enhanced killing assay. The presence of plasma enhances macrophage killing of bacteria. Optimal killing occurs when both plasma and macrophages are used in the assay. N=10 for each group, data are mean ± SEM. *p<0.05 when comparing macrophages alone versus macrophages with plasma. B. Adenosine decreases bacterial killing. Adenosine at a concentration of 300 nM decreases bacterial killing by peritoneal macrophages in the presence of plasma. N=5 for each column. *p<0.05 Comparing plasma alone vs plasma plus 300 nM adenosine. Bacterial colony forming units reported were the sum of aerobic and anaerobic growth.
The immune response has checks and balances to prevent bystander damage during a response to injurious agents such as Damage Associated Molecular Patterns (DAMPS) and Pathogen Associated Molecular Patterns (PAMPS) (19). Adenosine is one of these checks since it is released by the host system when cells are destroyed in order to alert the immune cells that excessive damage has occurred. Physiological levels of adenosine in murine and human plasma are reported to be less than 1μM and adenosine has the capacity of activating receptors A1, A2A, A3 within these levels (20, 21). To verify that adenosine at the appropriate concentrations will alter bacterial killing, we exposed macrophages to various concentrations of adenosine (300nM – 3μM) and then incubated the macrophages with the opsonized bacteria. Macrophages that had been exposed to 300 nM adenosine prior to bacteria showed decreased capacity to kill bacteria (Figure 2B). Increasing the adenosine concentration to 3000 nM did not further decrease the ability of macrophages to kill bacteria (data not shown). Adenosine's effect in this experiment was not dose dependent as it has been reported in other publications (22).
Cells have four different adenosine receptors in their membrane and adenosine receptor antagonists are able to block the inhibitory adenosine signal. All four receptors are expressed on immune cells but the concentration of adenosine required to activate them varies significantly with A2BR requiring much higher concentrations for activation (23). For the purpose of depicting their role in bacterial killing, thioglycollate elicited macrophages or neutrophils were exposed to the selected adenosine receptor antagonists at concentration that would only affect the desired receptor. Blocking the A2BR had the greatest capacity to augment bacterial killing in the presence of adenosine. Blocking the A1 or A2A receptors independently also overcame the inhibitory effect of adenosine (Figure 3). These results correlate with previous work which showed that A2B receptor knockout mice exhibited greater phagocytic activity than wild type ones (24).
Figure 3.
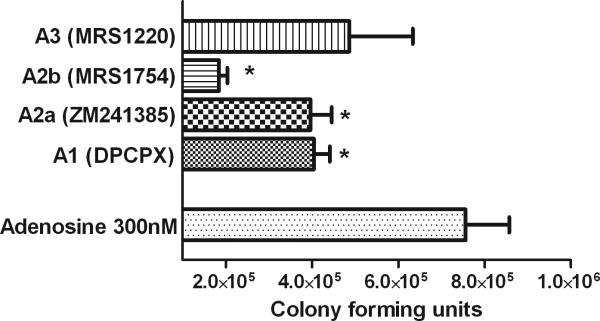
Adenosine receptor antagonists reverse adenosine augmented bacterial killing. Selected adenosine receptor antagonists reversed the effect of adenosine and improve bacterial killing. Macrophages exposed to A1, A2A, and A2B receptor antagonists prior to incubation with bacteria were able to significantly reduce the CFU count when compared to plasma that contained adenosine alone (300nM). All experimental groups contained plasma, macrophages, bacteria, and adenosine 300nM. *p<0.05 compared to adenosine 300 nM. N=5 for each group, data are mean ± SEM.
The effect of adenosine was also tested on neutrophils. The inhibitory effect of adenosine was cell specific as thioglycollate elicited neutrophils exposed to adenosine did not suffer the same decrease in killing capacity as the macrophages. Addition of the A2BR antagonist did not alter bacterial killing by plasma, Figure 4.
Figure 4.

Neutrophil plasma enhanced killing. Adenosine did not decrease thyoglycollate elicited neutrophil bacterial killing. Blocking the adenosine receptors has no effect on bacterial killing. N=5 for each group, data are mean ± SEM.
Immune cells have many pathways in which they can carry out the killing of bacteria. One pathway is the phagocytosis and destruction of bacteria within lysosomes. An assay has been developed which allows the determination of when the phagocytozed bacteria have been incorporated into the phagolysosome. These bacteria emit a detectable light when in the presence of a low pH atmosphere such as in the phagolysosome. Macrophages were treated in the same manner as in the previous experiments by exposing them to adenosine plus one of the receptor antagonists. Representative flow histograms show that adenosine shifts the mean fluorescence intensity to the left, indicating that macrophages exposed to adenosine showed decreased phagolysosome fusion (Figure 5A). Since both the MRS1754 (A2B) and ZM 241385 (A2A) receptor antagonists significantly blocked the effects of adenosine they were selected for further study. Antagonizing the adenosine A2A and A2B receptors prevented the ability of adenosine to decrease the incorporation of bacteria into macrophage phagolysosomes (Figure 5B). This assay cannot determine whether this occurred through decreased phagocytosis or decreased phagolysosome fusion.
Figure 5.

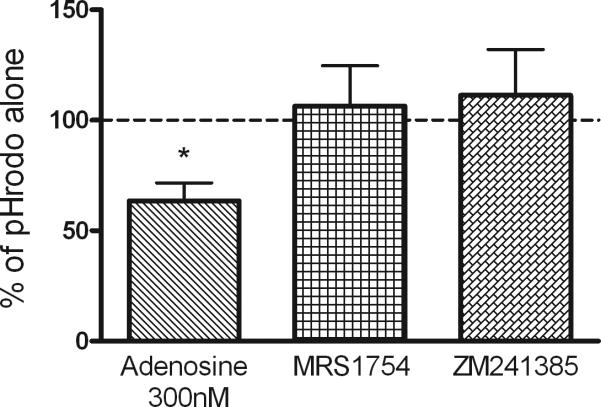
A. Representative histogram of the effect of adenosine and the receptor antagonists on macrophage phagocytosis of pHrodo™ Red E. coli BioParticles. B. Adenosine reduced phagolysosome creation by 36% when compared to the control group (pHrodo alone). Adenosine was able to diminish significantly the phagocytosis capacity of macrophages and utilizing adenosine receptors A2B inhibition with MRS 1754 or A2A inhibition with ZM241385 returns the measurement of phagolysosome fusion back to normal levels, indicated by the horizontal dashed line. *p<0.05 compared to no adenosine dashed line.
Discussion
Adenosine has been implicated in affecting various cellular functions. The surrounding microenvironment of tumor cells is hypoxic which causes an elevated level of adenosine with resulting suppression of antitumor cells by the increased extracellular adenosine. One group demonstrated greater tumor rejection in mice that had the A2AR genetically deleted or pharmacologically blocked (25). Many sepsis models, ranging from LPS, bacterial inoculations, to CLP have been tested in order to examine the role of adenosine receptors with conflicting results (8, 26). In the cardiac system adenosine is used in for the treatment of supraventricular tachycardia and is a potent vasodilator which promotes its use in cardiac stress testing (27). In the realm of autoimmune diseases adenosine and its receptors have been implicated in conditions such as rheumatoid arthritis (RA). One study found an increased number of A1 and A3 receptors in patients with RA when compared to healthy volunteers and suggesting a compensatory mechanism to augment the anti-inflammatory properties of adenosine (28). These contradictions are probably dependent on the model that was used.
Our results demonstrated that adenosine at 300nM was able to reduce the plasma enhanced killing (PEK) of bacteria by macrophages. In order to elucidate the mechanism of reduced bacterial killing caused by adenosine, flow cytometry using labeled E. coli was performed. This demonstrated that adenosine decreases the phagolysosome creation in macrophages as shown by decreased fluorescence in cells. Antagonizing adenosine receptors (A1, A2A, A2B) prevented this decrease. These results agree with previous studies but differ in the fact that A2BR antagonism resulted in better bacterial killing than A2A (29, 30). We realize that the PEK assay is an end assay in which bacterial growth in a agar plate from an experimental group is compared to a control plate and the results represented as killing capacity. This limits the assay in determining how the bacteria are killed: decreased phagocytosis of bacteria vs. decreased phagolysome fusion.
Although adenosine receptors are present on neutrophils, 300nM adenosine did not impact its killing capacity nor did blocking the receptors improve it. Neutrophils carry out their immune functions by phagocytosis, release of extracellular enzymes and antimicrobial granule contents, and neutrophil extracellular trap (NET) formation. They are able to produce and be affected by adenosine. All four adenosine receptors are present on neutrophils and each one carries a varying function (31). Adenosine at different concentrations can alter chemotaxis by inhibiting release of the neutrophil chemoattractant CXCL8 from endothelial cells and reduces expression of adhesion molecules endothelial selectin (E-selectin) and VCAM-1 on the endothelial cell surface (32). Neutrophils have an increased concentration of A3 receptors at the leading edge while A2a receptors are more globally located, these two receptors have different affinities and this combination allows adenosine to help direct the cells to the point of inflammation (33). Adenosine also serves as a signal for neutrophil survival versus apoptosis depending on which receptor becomes activated (34, 35). We did not look at adenosine's function on chemotaxis since the neutrophils used were collected after thioglycollate injection and we were attempting to decipher their effect on bacterial killing. It could very well be that the concentration of adenosine used was not high enough to promote the anti-inflammatory signal seen in the previous studies.
Adenosine plays an important role in the modulation of an inflammatory response. It has been shown to influence the activity of the immune response by promoting an anti-inflammatory state in phagocytic cells through the production of anti-inflammatory cytokines such as IL10 and the decrease of pro-inflammatory cytokines as well as phagocytosis. Studies have shown that antagonizing adenosine receptors improved survival in polymicrobial sepsis with improved bacterial clearance (8, 24). Ischemia/reperfusion models improved organ protection from injury using adenosine analogs to maintain an anti-inflammatory environment. In the experiments above we showed that even in the presence of plasma that promotes bacterial killing, adenosine can block the phagocytic function of macrophages and that antagonizing specific receptors returns their function. These data support the role of adenosine in the immune response as protecting the host system by attenuating inflammation in order to prevent bystander damage.
Acknowledgments
This work was supported by NIH grant RO1 GM 082962 and T32 GM 86308.
Footnotes
This work was performed in the Department of Pathology and Laboratory Medicine, Boston University School of Medicine, 670 Albany Street, Room 441, Boston, MA 02118, USA.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–10. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–54. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Aminov RI. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol. 2010;1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares J, Bernardini A, Garcia-Leon G, Corona F, M BS, Martinez JL. The intrinsic resistome of bacterial pathogens. Front Microbiol. 2013;4:103. doi: 10.3389/fmicb.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moitra R, Beal DR, Belikoff BG, Remick DG. Presence of preexisting antibodies mediates survival in sepsis. Shock. 2012;37(1):56–62. doi: 10.1097/SHK.0b013e3182356f3e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spicuzza L, Di Maria G, Polosa R. Adenosine in the airways: implications and applications. Eur J Pharmacol. 2006;533(1-3):77–88. doi: 10.1016/j.ejphar.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5(3):247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belikoff B, Hatfield S, Sitkovsky M, Remick DG. Adenosine negative feedback on A2A adenosine receptors mediates hyporesponsiveness in chronically septic mice. Shock. 2011;35(4):382–7. doi: 10.1097/SHK.0b013e3182085f12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemeth ZH, Csoka B, Wilmanski J, Xu D, Lu Q, Ledent C, Deitch EA, Pacher P, Spolarics Z, Hasko G. Adenosine A2A receptor inactivation increases survival in polymicrobial sepsis. J Immunol. 2006;176(9):5616–26. doi: 10.4049/jimmunol.176.9.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gallos G, Ruyle TD, Emala CW, Lee HT. A1 adenosine receptor knockout mice exhibit increased mortality, renal dysfunction, and hepatic injury in murine septic peritonitis. Am J Physiol Renal Physiol. 2005;289(2):F369–76. doi: 10.1152/ajprenal.00470.2004. [DOI] [PubMed] [Google Scholar]
- 11.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R959–69. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]
- 12.Park SW, Kim M, Kim JY, Brown KM, Haase VH, D'Agati VD, Lee HT. Proximal tubule sphingosine kinase-1 has a critical role in A1 adenosine receptor-mediated renal protection from ischemia. Kidney Int. 2012;82(8):878–91. doi: 10.1038/ki.2012.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thammavongsa V, Kern JW, Missiakas DM, Schneewind O. Staphylococcus aureus synthesizes adenosine to escape host immune responses. J Exp Med. 2009;206(11):2417–27. doi: 10.1084/jem.20090097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beukers MW, Meurs I, Ijzerman AP. Structure-affinity relationships of adenosine A2B receptor ligands. Med Res Rev. 2006;26(5):667–98. doi: 10.1002/med.20069. [DOI] [PubMed] [Google Scholar]
- 15.Baron EJ, Proctor RA. Elicitation of peritoneal polymorphonuclear neutrophils from mice. J Immunol Methods. 1982;49(3):305–13. doi: 10.1016/0022-1759(82)90130-2. [DOI] [PubMed] [Google Scholar]
- 16.Cook AD, Braine EL, Hamilton JA. The phenotype of inflammatory macrophages is stimulus dependent: implications for the nature of the inflammatory response. J Immunol. 2003;171(9):4816–23. doi: 10.4049/jimmunol.171.9.4816. [DOI] [PubMed] [Google Scholar]
- 17.Longbrake EE, Lai W, Ankeny DP, Popovich PG. Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem. 2007;102(4):1083–94. doi: 10.1111/j.1471-4159.2007.04617.x. [DOI] [PubMed] [Google Scholar]
- 18.Leijh PC, van Zwet TL, ter Kuile MN, van Furth R. Effect of thioglycolate on phagocytic and microbicidal activities of peritoneal macrophages. Infect Immun. 1984;46(2):448–52. doi: 10.1128/iai.46.2.448-452.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Said-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J. 2012;35(6):437–49. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008;7(9):759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredholm BB. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007;14(7):1315–23. doi: 10.1038/sj.cdd.4402132. [DOI] [PubMed] [Google Scholar]
- 22.Barnholt KE, Kota RS, Aung HH, Rutledge JC. Adenosine blocks IFN- gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation. J Immunol. 2009;183(10):6767–77. doi: 10.4049/jimmunol.0900331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007;113(2):264–75. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belikoff BG, Hatfield S, Georgiev P, Ohta A, Lukashev D, Buras JA, Remick DG, Sitkovsky M. A2B adenosine receptor blockade enhances macrophage-mediated bacterial phagocytosis and improves polymicrobial sepsis survival in mice. J Immunol. 2011;186(4):2444–53. doi: 10.4049/jimmunol.1001567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, Chen JF, Jackson EK, Apasov S, Abrams S, Sitkovsky M. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A. 2006;103(35):13132–7. doi: 10.1073/pnas.0605251103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan GW, Fang G, Linden J, Scheld WM. A2A adenosine receptor activation improves survival in mouse models of endotoxemia and sepsis. J Infect Dis. 2004;189(10):1897–904. doi: 10.1086/386311. [DOI] [PubMed] [Google Scholar]
- 27.Arumugham P, Figueredo VM, Patel PB, Morris DL. Comparison of intravenous adenosine and intravenous regadenoson for the measurement of pressure-derived coronary fractional flow reserve. EuroIntervention. 2013;8(10):1166–71. doi: 10.4244/EIJV8I10A180. [DOI] [PubMed] [Google Scholar]
- 28.Varani K, Padovan M, Vincenzi F, Targa M, Trotta F, Govoni M, Borea PA. A2A and A3 adenosine receptor expression in rheumatoid arthritis: upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res Ther. 2011;13(6):R197. doi: 10.1186/ar3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157(10):4634–40. [PubMed] [Google Scholar]
- 30.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006;317(1):172–80. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 31.Barletta KE, Ley K, Mehrad B. Regulation of neutrophil function by adenosine. Arterioscler Thromb Vasc Biol. 2012;32(4):856–64. doi: 10.1161/ATVBAHA.111.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouma MG, van den Wildenberg FA, Buurman WA. Adenosine inhibits cytokine release and expression of adhesion molecules by activated human endothelial cells. Am J Physiol. 270(2 Pt 1):C522–9. 1996. doi: 10.1152/ajpcell.1996.270.2.C522. [DOI] [PubMed] [Google Scholar]
- 33.Junger WG. Purinergic regulation of neutrophil chemotaxis. Cell Mol Life Sci. 2008;65(16):2528–40. doi: 10.1007/s00018-008-8095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohno Y, Sei Y, Koshiba M, Kim HO, Jacobson KA. Induction of apoptosis in HL-60 human promyelocytic leukemia cells by adenosine A(3) receptor agonists. Biochem Biophys Res Commun. 1996;219(3):904–10. doi: 10.1006/bbrc.1996.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yasui K, Agematsu K, Shinozaki K, Hokibara S, Nagumo H, Nakazawa T, Komiyama A. Theophylline induces neutrophil apoptosis through adenosine A2A receptor antagonism. J Leukoc Biol. 2000;67(4):529–35. doi: 10.1002/jlb.67.4.529. [DOI] [PubMed] [Google Scholar]





