Abstract
In a placebo-controlled trial, we examined the efficacy of a 6-month (“extended”) transdermal nicotine therapy vs. the 8-week (“standard”) therapy in 471 Caucasian smokers with either normal or reduced rates of nicotine metabolism as determined at pretreatment. Extended therapy was superior to standard therapy in genotypic or phenotypic reduced metabolizers (RMs) of nicotine but not in normal metabolizers (NMs). RMs of nicotine are candidates for extended transdermal nicotine therapy, whereas an alternative therapeutic approach may be needed for those with normal rates of nicotine metabolism.
Tobacco dependence is a chronic disorder that may require extended therapy. Indeed, smokers who receive extended (6-month) therapy with transdermal nicotine are approximately twice as likely to be abstinent at the end of treatment than those receiving the standard (8-week) therapy that is usually prescribed.1 However, extended therapy is more costly, and there is substantial interindividual variability in treatment outcome.1 There is therefore a strong rationale for identifying pretreatment biomarkers that can be used to inform decisions about treatment duration. Genotypic or phenotypic measures of the rate of nicotine clearance have promise in this regard.2
Nicotine is metabolized to cotinine, and cotinine is metabolized to 3′-hydroxycotinine, primarily by CYP2A6.3 Approximately 60% of the variability in nicotine metabolism is heritable,4 and reduced-activity and inactive variants of the CYP2A6 gene associated with slower nicotine clearance have been well characterized.5 Among smokers receiving a standard dose of transdermal nicotine, those with such genetic variants achieve higher therapeutic doses of nicotine than those without such variants, at comparable levels of treatment compliance.6 Correspondingly, a phenotypic measure of the ratio of the nicotine metabolites derived from smoking (plasma 3′-hydroxycotinine/cotinine) predicts the efficacy of transdermal nicotine therapy across independent clinical trials.7,8 Lower values of this nicotine metabolite ratio (NMR), indicating reduced nicotine metabolism, are associated with more successful smoking cessation. On the basis of the greater responsiveness to transdermal nicotine shown by slow metabolizers of nicotine, we tested the hypothesis that this subgroup, defined by CYP2A6 genotype or NMR, would be more likely to benefit from a 6-month therapy with transdermal nicotine than from an 8-week therapy, as compared with normal metabolizers (NMs) of nicotine. We refer to these treatment arms as “extended” and “standard,” respectively; however, we recognize that there is variability in duration of administration across settings and countries.
RESULTS
Descriptive data
In this study, 243 participants were assigned to standard therapy and 228 were assigned to extended therapy. We assigned the participants to one of two NMR phenotype groups, defined by the first quartile of the NMR vs. the second to fourth quartiles: (i) reduced metabolizer phenotype (RM-P) (n = 118, 25%) and (ii) NM phenotype (NM-P) (n = 353, 75%). Our decision to dichotomize the NMR in this fashion was based on the results of our prior validation study,8 which showed that the main difference in quit rates was between the first quartile (slowest metabolizers) and the second through fourth quartiles. We also created two genotype-defined groups: (i) RM CYP2A6 genotype (RM-G), consisting of all individuals with variant (i.e., not CYP2A6*1/*1) genotypes (n = 109, 23.1%), and (ii) NM genotype (NM-G) (n = 362, 76.9%).9 These RM-G and NM-G groupings allowed us to compare the utility of the genotype and phenotype groupings, as these provided comparable group sizes (23.1 vs. 25%, respectively) with similar CYP2A6 activity (i.e., NMR and plasma nicotine levels; see below).
Of the total cohort, 58% were men, and the mean age was 44.6 years (SD = 10.4). The mean number of cigarettes smoked per day was 22.1 (SD = 9.0), and the mean Fagerström Test for Nicotine Dependence score was 5.28 (SD = 2.16). On average, NM-P participants were older (P = 0.009) and more likely to be women (P = 0.017), as compared with RM-Ps. There were no significant differences in demographic or smoking variables by treatment arm or genotype group.
Inactive and reduced-activity variants of genotypes commonly found among Caucasians were found at expected frequencies (Table 1);6,10 each variant genotype was found to be in Hardy–Weinberg equilibrium. The expected gene–dose effect on the NMR was observed: those with CYP2A6*1/*1 had a higher mean NMR than those with CYP2A6*1/*2, which in turn was higher than in those homozygous for CYP2A6*2 (P = 0.001); the *1/*1 group also has a higher mean NMR than the CYP2A6*9 group (P < 0.0001, Table 1).
Table 1.
Frequency of CYP2A6 genotypes and their associated baseline mean NMR ± SD (n = 471)
| Allele | Genotype | Observed frequency (n) | Baseline mean 3HC/COTa | SD | % Mean | P valueb |
|---|---|---|---|---|---|---|
| Normal metabolizer genotypec | *1/*1 | 362 | 0.42 | 0.19 | 100 | Reference |
| CYP2A6*2 | *1/*2 | 21 | 0.24 | 0.10 | 57 | <0.0001 |
| *2/*2 | 3 | 0.11 | 0.08 | 29 | ||
| CYP2A6*4 | *1/*4 | 9 | 0.31 | 0.17 | 74 | 0.01 |
| CYP2A6*9 | *1/*9 | 58 | 0.29 | 0.11 | 69 | <0.0001 |
| *9/*9 | 3 | 0.15 | 0.08 | 36 | ||
| CYP2A6*12 | *1/*12 | 11 | 0.21 | 0.09 | 50 | <0.0001 |
| Two or more different variant alleles | *2/*9 | 1 | 0.65 | — | 155 | <0.0001 |
| *4/*9 | 1 | 0.04 | — | 10 | ||
| *4/*12 | 1 | 0.02 | — | 5 | ||
| *2/*2/*9 | 1 | 0.32 | — | 76 | ||
| Reduced metabolizer genotype | All variants | 109 | 0.26 | 0.13 | 62 | <0.001 |
The baseline mean NMR for each genotype is expressed in absolute terms and as a percentage of the CYP2A6*1/*1 group mean.
3HC, 3′-hydroxycotinine; ANOVA, analysis of variance; COT, cotinine; NM, normal metabolizer; NMR, nicotine metabolite ratio; RM, reduced metabolizer.
Baseline mean 3HC/COT ratios (NMR) reported in the table were pre-log-transformed.
For statistical analysis, the ratios were log-transformed to obtain normal distribution. ANOVA or Student’s t-test was used to compare the log-transformed mean 3HC/COT ratio from each variant genotype to the reference group (composed of CYP2A6*1/*1 individuals only). Individuals with two or more different variant alleles were grouped for analysis.
Of the 471 participants, 364 were in the same group by both genotype and phenotype, another 58 participants were NM by genotype but RM by phenotype, and the remaining 49 were RM by genotype but NM by phenotype.
Treatment-related plasma nicotine levels
In smokers with verified abstinence at 1 week after quitting smoking (CO ≤ 10 p.p.m.), we measured plasma nicotine levels arising from the treatment with transdermal nicotine. The abstinent participants in the RM-G group (n = 81) had significantly higher plasma nicotine levels from treatment (17.8 ng/ml (SD = 8.0)) as compared with the abstinent participants in the NM-G group (n = 242) (14.9 ng/ml (SD = 7.2), P = 0.003). Likewise, plasma nicotine levels were higher among abstinent RM-P participants (n = 88) than among the NM-P ones (n = 235) (18.1 ng/ml (SD = 9.3) vs. 14.8 ng/ml (SD = 6.5), P = 0.003). Compliance was similar in the groups, with the rate of daily patch use being only slightly higher in RM-Gs than in NM-Gs (3.6%, P = 0.20), and slightly higher in RM-Ps than in NM-Ps (2.5%, P = 0.34); it is clear, therefore, that the higher levels of plasma nicotine in RM-Gs and RM-Ps (relative to the respective normal groups) cannot be attributed to differences in compliance.
Efficacy of extended therapy vs. standard therapy
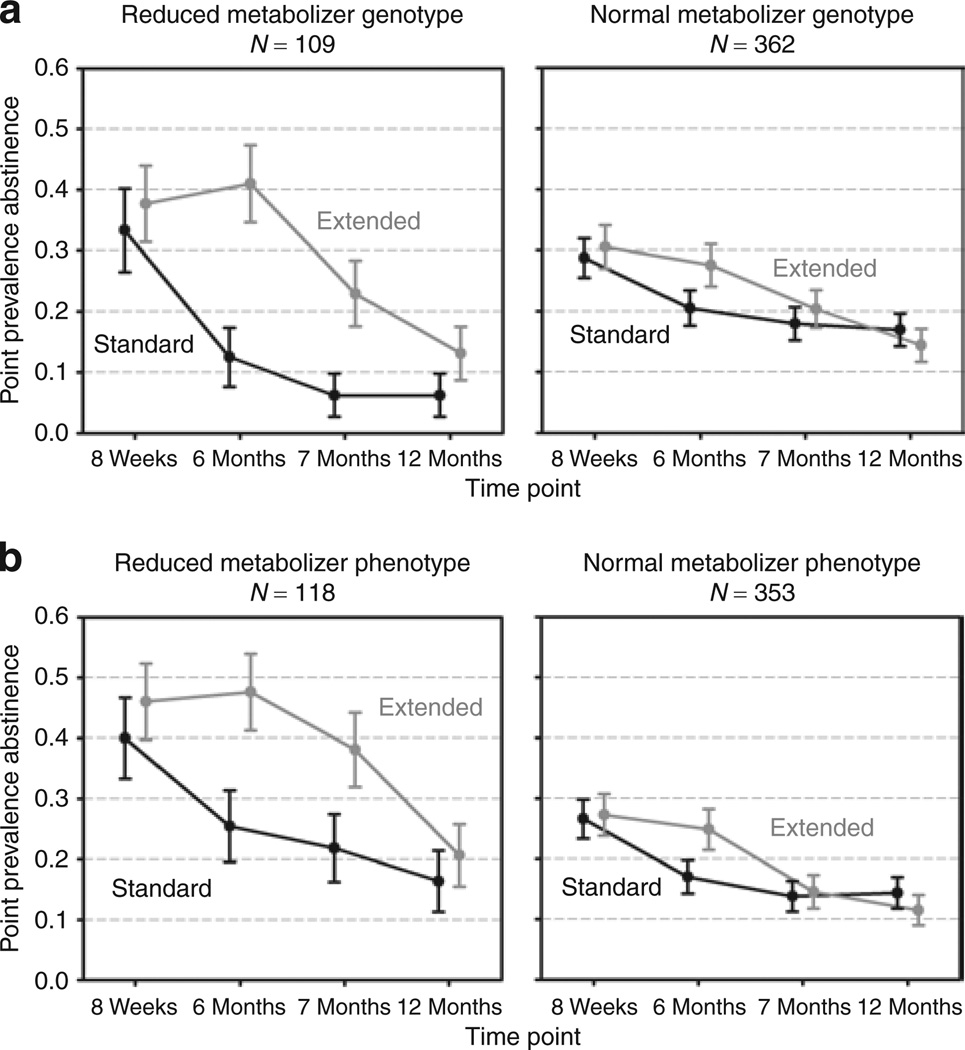
As reported in Table 2 and illustrated in Figure 1a, at 24 weeks (end of the extended treatment phase), there was a significant interaction between CYP2A6 genotype and treatment duration (P = 0.04), indicating the greater relative efficacy of extended therapy vs. standard therapy for RM-Gs (P = 0.002) but not for NM-Gs (P = 0.13). This interaction effect was no longer statistically significant after the medication period ended (i.e., 52 weeks). For NMR phenotype, as shown in Table 2 and Figure 1b, there was a significant treatment effect in RM-Ps (P = 0.02) but not in NM-Ps (P = 0.078) at 24 weeks; however, the effect of the interaction of NMR phenotype with treatment duration was not statistically significant (P = 0.346).
Table 2.
Longitudinal logistic regression models of effects of extended vs. standard duration therapy on quit rates
| Reduced metabolizer genotype (n = 109) | Normal metabolizer genotype (n = 362) | Genotype × treatment interactionb | ||||
| Time point | ORa (95% CI) | P value | ORa (95% CI) | P value | ORa (95% CI) | P value |
| CYP2A6 genotype model | ||||||
| 8 Weeksc | 1.17 (0.53–2.59) | 0.70 | 1.08 (0.68–1.70) | 0.74 | 1.09 (0.43–2.72) | 0.86 |
| 24 Weeksd | 4.78 (1.74–13.13) | 0.002 | 1.47 (0.90–2.41) | 0.13 | 3.25 (1.05–10.05) | 0.04 |
| 28 Weeks | 4.36 (1.13–16.83) | 0.032 | 1.16 (0.63–1.97) | 0.59 | 3.77 (0.88–16.04) | 0.07 |
| 52 Weeks | 2.27 (0.56–9.26) | 0.25 | 0.82 (0.46–1.46) | 0.49 | 2.78 (0.61–12.69) | 0.19 |
| Reduced metabolizer phenotype (n = 118) | Normal metabolizer phenotype (n = 353) | Phenotype × treatment interaction | ||||
| Time point | ORa (95% CI) | P value | ORa (95% CI) | P value | ORa (95% CI) | P value |
| NMR phenotype model | ||||||
| 8 Weeksc | 1.21 (0.58–2.50) | 0.62 | 1.02 (0.63–1.65) | 0.93 | 1.18 (0.49–2.84) | 0.71 |
| 24 Weeksd | 2.54 (1.15–5.60) | 0.021 | 1.60 (0.95–2.71) | 0.08 | 1.58 (0.61–4.11) | 0.35 |
| 28 Weeks | 2.09 (0.90–4.82) | 0.09 | 1.04 (0.57–1.91) | 0.89 | 2.00 (0.71–5.64) | 0.19 |
| 52 Weeks | 1.27 (0.48–3.31) | 0.63 | 0.76 (0.41–1.44) | 0.41 | 1.66 (0.52–5.26) | 0.39 |
CI, confidence interval; FTND, Fagerstrom Test for Nicotine Dependence; NMR, nicotine metabolite ratio; OR, odds ratio.
Odds ratio and 95% confidence interval for comparison of extended vs. standard therapy; models are controlled for time point, age, sex, and FTND score.
Significance based on Wald χ2.
End of standard treatment period.
End of extended treatment period.
Figure 1.
The impact of reduced nicotine metabolism on the efficacy of extended vs. standard duration transdermal nicotine therapy. (a) Differences between CYP2A6 genotype groups as regards response to extended vs. standard therapy (RM-G = reduced metabolism genotype; NM-G = normal metabolism genotype). (b) Differences between baseline nicotine metabolite ratio (3HC/cotinine) phenotype groups with respect to response to extended vs. standard therapy (RM-P = reduced metabolism phenotype; NM-P = normal metabolism phenotype). 3HC, 3′-hydroxycotinine.
In addition to longitudinal modeling of extended therapy vs. standard therapy within genotype or NMR group, we also examined whether there were genotype or NMR phenotype effects on abstinence rates at the end of 8-weeks of standard therapy (independent of treatment group, because all the participants were on active patch for the first 8 weeks). There was a significant difference in abstinence rates between the RM-Ps and NM-Ps (χ2 = 10.99, P = 0.001), as reported previously.8 For genotype, by contrast, the difference was not significant (χ2 = 1.5, P = 0.13). Consistent with the aforementioned analysis, at 24 weeks, the effect of CYP2A6 genotype on abstinence was significant only in the extended therapy group (χ2 = 3.76, P = 0.039) and not in the standard therapy group (χ2 = 1.61, P = 0.14). Likewise, the effect of NMR phenotype was significant only in the extended therapy group (χ2 = 11.02, P = 0.001) and not in the standard therapy group (χ2 = 1.97, P = 0.12).
DISCUSSION
The results show that smokers with reduced nicotine metabolism benefit more than NMs from extended (6-month) transdermal nicotine therapy as compared to standard (8-week) therapy. At the end of extended therapy, the treatment effect was significant among RMs by genotype and phenotype but not among NMs; however, the group-by-treatment interaction was significant only for the genotype measure. The substantial benefits of extended therapy for RMs were maintained during the treatment period, and quit rates exceeded the 6-month quit rates achieved with 12 weeks of bupropion or varenicline therapy.11 However, these benefits for RMs dissipate once treatment ends, suggesting that they may benefit more from even longer treatment.
The higher treatment-related plasma nicotine levels among RMs as compared with NMs may contribute to the greater quitting success in RMs during transdermal nicotine therapy. In addition, in RMs, the pharmacokinetics of transdermal nicotine (i.e., stable nicotine levels) may be more similar to that of nicotine derived from smoking, whereas NMs may be more accustomed to intercigarette variation (peaks and troughs) in nicotine levels from smoking. It appears that the benefit of transdermal nicotine is maintained among RMs but only as long as therapy is continued (Figure 1). The differences in outcome between RMs and NMs cannot be attributed to differences in dependence or smoking rate because these variables were controlled for in the models and are not strongly associated with nicotine metabolism rate, as shown in earlier studies.12,13
As we reported previously,1 significant benefits of extended vs. standard therapy are observed in the full cohort when nicotine metabolism rate is not considered. However, a prior study reported no benefits of an extended transdermal therapy regimen.14 In addition to differences in study design and outcome assessment, it is possible that the lack of concordance of findings is partly attributable to variability in nicotine clearance rates across the two study populations. Assessment of nicotine metabolism rate in clinical trials of smoking cessation treatment may provide valuable information for interpretation of effects within and between studies.
A limitation of this study is that the study sample was restricted to Caucasians to minimize population stratification in the genotype assessment. In other populations, this benefit of extended treatment may be even more important because RMs of nicotine represent a substantially larger portion of the African-American and Asian populations.5,15,16 Similar studies in these populations are warranted to test the generalizability of these findings. Another limitation is the lack of data collected immediately following the change from active to placebo patch in the standard therapy group, leaving open questions as to the mechanisms responsible for differential efficacy.
We have shown that the benefit of extending transdermal nicotine therapy to 6 months is greatest among smokers with reduced nicotine metabolism. NMs who benefit less from standard or extended transdermal nicotine therapy may be good candidates for higher-dose nicotine replacement or for non-nicotine therapies for smoking cessation. A placebo-controlled trial of bupropion showed a substantial benefit of therapy with this drug for NMs, particularly those with the highest metabolism rates.17 If further studies confirm these results, determination of nicotine metabolism rate can be used to tailor the type, dose, and length of smoking cessation treatment in clinical practice.
METHODS
Participants
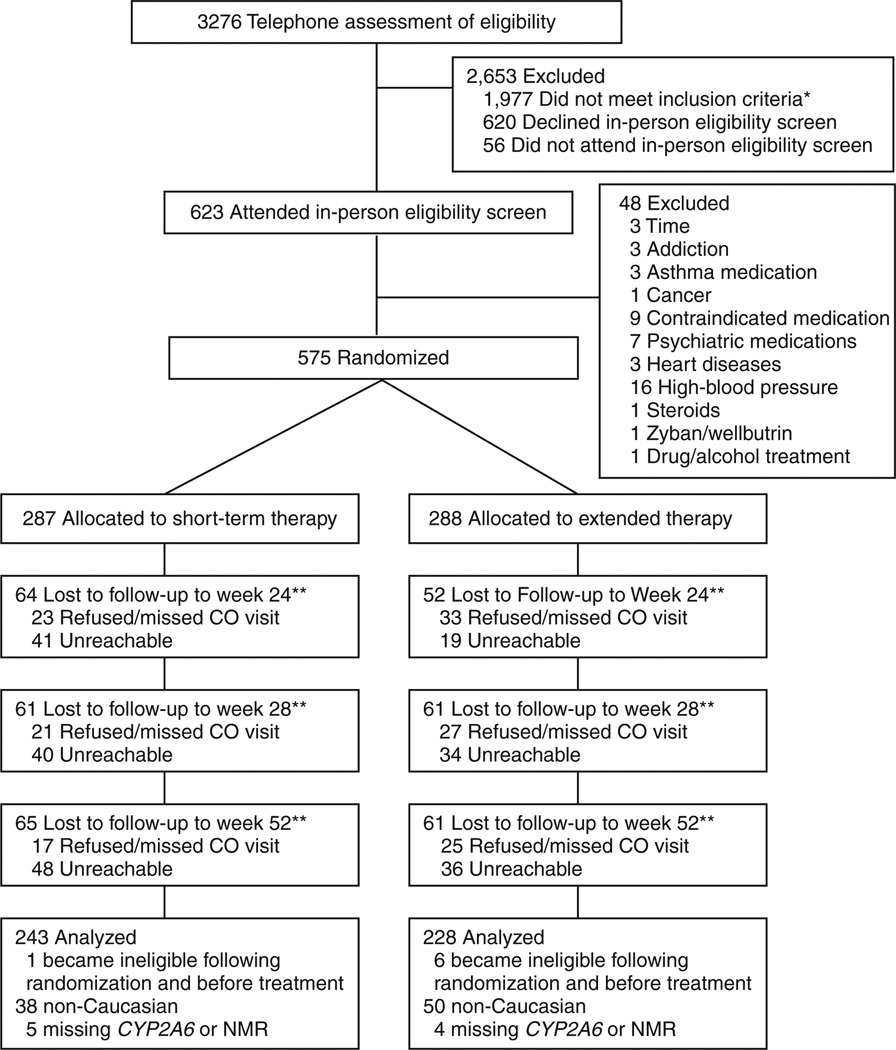
Individuals who responded to advertisements for a free smoking cessation program and provided written informed consent were screened for eligibility. Eligible participants were 18–65 years of age, reported smoking more than 10 cigarettes per day, had no prior history of a DSM-IV (Diagnostic and Statistical Manual of Mental Disorders, 4th ed.) axis I psychiatric or substance use disorder, and were not using psychotropic medications. The full exclusion criteria can be found elsewhere.1 The study was restricted to 471 Caucasian smokers (Figure 2).
Figure 2.
Flowchart of study participation. CO, carbon monoxide; NMR, nicotine metabolite ratio.
Procedures
Participants’ smoking rates and nicotine dependence were reported on the basis of the Fagerström Test for Nicotine Dependence.18. They were randomized to one of two treatment arms using a computer-generated randomization scheme accessible only to the senior programmer. At baseline, participants provided samples for the NMR plasma assay and DNA for genotyping.
As previously described,1 treatment was initiated on the target quit date. All participants received 8 weeks of open-label 21-mg transdermal nicotine (Nicoderm CQ; GlaxoSmithKline, Research Triangle Park, NC). Thereafter, those who were randomized to standard treatment received placebo patches for 16 weeks, and those randomized to extended treatment received 21-mg nicotine patches for 16 weeks. The study condition was concealed from participants and staff.
The NMR was measured at baseline using liquid chromatography-mass spectrometry.19 In participants who were abstinent from smoking at 1 week, plasma nicotine levels were assessed by gas chromatography.6
DNA samples were genotyped for the following functionally impaired variant CYP2A6 alleles prominent in Caucasians: CYP2A6 *2, *9, and *12,5,6,15 as well as CYP2A6*4, using the assay16 that detects the *4A/C, and D–F variants, suitably modified to use the reverse primer R0 in the first amplification16 and R6 (previously described as “2A6reverse”)20 in the second amplification. CYP2A6*9 contains a change in the TATA box that reduces transcription, *12 and *4 are hybrid CYP2A7/CYP2A6 alleles, and *2 contains a single amino acid change.
Follow-up telephone surveys were conducted at 8 weeks and at 6, 7, and 12 months after the target quit date. Of the total study group, 411 (87.3%) participants completed the 6-month survey, 397 (84.3%) completed the 7-month survey, and 387 (82.2%) completed the 12-month survey. As compared to 6-month noncompleters, survey completers tended to be older (P = 0.013), smoked fewer cigarettes per day at baseline (P = 0.018), had higher Fagerström test scores (P = 0.001), and were more likely to be in the standard-therapy group (P = 0.005).
The primary outcome was 7-day point prevalence of abstinence.21 Self-reported smoking status was assessed during the follow-up surveys using a validated timeline follow-back assessment.22 Participants who reported on the survey that they had not smoked (even a puff) were asked to come to the clinic to provide a CO breath sample for biochemical verification. Consistent with guidelines,23 participants who failed to respond to the survey, or who failed to provide a CO sample (n = 57, 48, and 42 at 6, 7, and 12 months, respectively), or who provided a CO sample >10 p.p.m. (n = 15, 9, and 11 at 6, 7, and 12 months, respectively) were considered to be smokers at that time point.
Differences in demographic characteristics by treatment arm, CYP2A6 genotype, and NMR group were assessed using χ2-tests, one-way analysis of variance, or t-tests. We fitted longitudinal logistic regression models of abstinence, using generalized estimating equations with an unstructured correlation, incorporating time points (8 weeks, 6 months, 7 months, and 12 months), treatment condition, group (e.g., RM-P vs. NM-P), and the group-by-treatment interaction at each time point. The models were controlled for sex, age, and Fagerström test score. Design codes were used to estimate adjusted odds ratios for treatment that were specific to time point and metabolism group, and treatment effects were tested using z-scores. Interactions were tested by comparing treatment odds ratios post hoc between metabolism groups, using the Wald χ2-test with one degree of freedom (z-test).
ACKNOWLEDGMENTS
This research was supported by grants from the National Cancer Institute and the National Institutes on Drug Abuse (P50 CA/DA 84718 and P50CA143187 to C.L.; DA 020830 to N.B., R.F.T., and C.L.; and DA12393 to N.B.); the Canadian Institutes of Health Research (MOP 86471); the Centre for Addiction and Mental Health; the Canadian Research Chair (to R.F.T.); and the Scholar Program for Interdisciplinary Capacity Enhancement Related Research (to M.M.).
C.L. has served as a consultant for and/or received research support from Pfizer, AstraZeneca, Novartis, and GlaxoSmithKline. R.F.T. owns shares in and participates in Nicogen Research Inc., a company focused on novel smoking cessation treatment approaches. No Nicogen funds were used in this work and no other Nicogen participants reviewed the manuscript. R.F.T. has also consulted for Novartis. N.B. is a paid consultant for several pharmaceutical companies that market smoking cessation medications, and has been a paid expert witness against tobacco companies in litigation related to nicotine addiction. This research was not supported by industry funds.
Footnotes
CONFLICT OF INTEREST
The other authors declared no conflict of interest.
References
- 1.Schnoll RA, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann. Intern. Med. 2010;152:144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray R, Tyndale RF, Lerman C. Nicotine dependence pharmacogenetics: role of genetic variation in nicotine-metabolizing enzymes. J. Neurogenet. 2009;23:252–261. doi: 10.1080/01677060802572887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benowitz NL. Clinical pharmacology of nicotine: implications for understanding, preventing, and treating tobacco addiction. Clin. Pharmacol. Ther. 2008;83:531–541. doi: 10.1038/clpt.2008.3. [DOI] [PubMed] [Google Scholar]
- 4.Swan GE, Lessov-Schlaggar CN, Bergen AW, He Y, Tyndale RF, Benowitz NL. Genetic and environmental influences on the ratio of 3′hydroxycotinine to cotinine in plasma and urine. Pharmacogenet. Genomics. 2009;19:388–398. doi: 10.1097/FPC.0b013e32832a404f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mwenifumbo JC, Tyndale RF. Molecular genetics of nicotine metabolism. Handb. Exp. Pharmacol. 2009:235–259. doi: 10.1007/978-3-540-69248-5_9. [DOI] [PubMed] [Google Scholar]
- 6.Malaiyandi V, Lerman C, Benowitz NL, Jepson C, Patterson F, Tyndale RF. Impact of CYP2A6 genotype on pretreatment smoking behaviour and nicotine levels from and usage of nicotine replacement therapy. Mol. Psychiatry. 2006;11:400–409. doi: 10.1038/sj.mp.4001794. [DOI] [PubMed] [Google Scholar]
- 7.Lerman C, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin. Pharmacol. Ther. 2006;79:600–608. doi: 10.1016/j.clpt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol. Biochem. Behav. 2009;92:6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee AM, Jepson C, Shields PG, Benowitz N, Lerman C, Tyndale RF. CYP2B6 genotype does not alter nicotine metabolism, plasma levels, or abstinence with nicotine replacement therapy. Cancer Epidemiol. Biomarkers Prev. 2007;16:1312–1314. doi: 10.1158/1055-9965.EPI-07-0188. [DOI] [PubMed] [Google Scholar]
- 10.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales D, et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 12.Mooney ME, Li ZZ, Murphy SE, Pentel PR, Le C, Hatsukami DK. Stability of the nicotine metabolite ratio in ad libitum and reducing smokers. Cancer Epidemiol. Biomarkers Prev. 2008;17:1396–1400. doi: 10.1158/1055-9965.EPI-08-0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnstone E, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin. Pharmacol. Ther. 2006;80:319–330. doi: 10.1016/j.clpt.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Tønnesen P, et al. Higher dosage nicotine patches increase one-year smoking cessation rates: results from the European CEASE trial. Collaborative European Anti-Smoking Evaluation. European Respiratory Society. Eur. Respir. J. 1999;13:238–246. doi: 10.1034/j.1399-3003.1999.13b04.x. [DOI] [PubMed] [Google Scholar]
- 15.Ho MK, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin. Pharmacol. Ther. 2009;85:635–643. doi: 10.1038/clpt.2009.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mwenifumbo JC, et al. Novel and established CYP2A6 alleles impair in vivo nicotine metabolism in a population of Black African descent. Hum. Mutat. 2008;29:679–688. doi: 10.1002/humu.20698. [DOI] [PubMed] [Google Scholar]
- 17.Patterson F, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin. Pharmacol. Ther. 2008;84:320–325. doi: 10.1038/clpt.2008.57. [DOI] [PubMed] [Google Scholar]
- 18.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br. J. Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey D, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin. Pharmacol. Ther. 2004;76:64–72. doi: 10.1016/j.clpt.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Fukami T, Nakajima M, Sakai H, McLeod HL, Yokoi T. CYP2A7 polymorphic alleles confound the genotyping of CYP2A6*4A allele. Pharmacogenomics J. 2006;6:401–412. doi: 10.1038/sj.tpj.6500390. [DOI] [PubMed] [Google Scholar]
- 21.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob. Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 22.Brown R, Burgess E, Sales S, Whiteley J. Reliability and validity of a smoking timeline follow-back interview. Psychol. Addict. Behav. 1988;12:101–112. [Google Scholar]
- 23.SRNT Subcommittee on Biochemical Verification. Biochemical verification of tobacco use and cessation. Nicotine Tob. Res. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]