Abstract
Reward enhancement by nicotine has been suggested as an important phenomenon contributing toward tobacco abuse and dependence. Reinforcement value is a multifaceted construct not fully represented by any single measure of response strength. The present study evaluated the changes in the reinforcement value of a visual stimulus in 16 male Sprague–Dawley rats using the reinforcer demand technique proposed by Hursh and Silberberg. The different parameters of the model have been shown to represent differing facets of reinforcement value, including intensity, perseverance, and sensitivity to changes in response cost. Rats lever-pressed for 1-min presentations of a compound visual stimulus over blocks of 10 sessions across a range of response requirements (fixed ratio 1, 2, 4, 8, 14, 22, 32). Nicotine (0.4 mg/kg, base) or saline was administered 5 min before each session. Estimates from the demand model were calculated between nicotine and saline administration conditions within subjects and changes in reinforcement value were assessed as differences in Q0, Pmax, Omax, and essential value. Nicotine administration increased operant responding across the entire range of reinforcement schedules tested, and uniformly affected model parameter estimates in a manner suggesting increased reinforcement value of the visual stimulus.
Keywords: nicotine, rat, reinforcer demand, reward
Introduction
Tobacco use is the leading cause of preventable death and disease worldwide, making it the largest single contributor to the global burden of disease (World Health Organization, 2004). The rewarding effects of nicotine are commonly accepted as the principal driving mechanism responsible for tobacco addiction (United States Department of Health and Human Services, 1988). The role of nicotine and reward in establishing and maintaining tobacco dependence is commonly conceptualized in three ways: (a) nicotine is a primary reinforcer, possessing inherent rewarding properties of its own that influence behavior (Corrigall and Coen, 1989; Donny et al., 1998); (b) nicotine-associated stimuli acquire conditioned rewarding properties by virtue of their association with the rewarding effects of nicotine, and these also influence behavior (Rose et al., 2000; Caggiula et al., 2001; Wilkinson and Bevins, 2008); and (c) nicotine functions as a reward-enhancer, increasing the reinforcement value of other rewarding stimuli in its presence (Donny et al., 2003; Bevins and Palmatier, 2004; Chaudhri et al., 2006a, 2006b; Caggiula et al., 2009).
All three of these facets are important in understanding the nature of nicotine reward and tobacco dependence. However, the reward-enhancing effects of nicotine have received considerable attention recently as it appears to fill a gap in our current understanding of tobacco dependence. Namely, a growing body of evidence suggests that the primary reinforcing effects of nicotine and associated conditioned reward it imbues to environmental stimuli cannot account for the entirety of tobacco dependence (Donny et al., 2003; Chaudhri et al., 2006a, 2006b; Palmatier et al., 2006). Recent studies on the role of nicotine in tobacco addiction have shown that nicotine also has robust reinforcement-enhancing properties. Briefly, in a study by Donny et al. (2003) using the nicotine self-administration procedure, rats were trained to press a lever to receive infusions of 0.03 mg/kg nicotine or of 0.9% saline. Some of these rats received infusions coupled with the presentation of a 1-min visual stimulus (VS), whereas the remaining rats received infusions without any other consequence. Additional groups of rats were allowed to press the active lever for the VS only, and received either nicotine or saline infusions independent of their lever pressing. Active lever response rates were significantly elevated in rats receiving nicotine infusions and the VS, irrespective of whether or not nicotine delivery was contingent upon their behavior. This finding has now been replicated in several studies, using a variety of different procedures and routes of nicotine administration (e.g. Chaudhri et al., 2006a, 2006b; Palmatier et al., 2006; Raiff and Dallery, 2006, 2009; Caggiula et al., 2009).
The finding that nicotine leads to an increase in response rates for a VS in rats has been interpreted as an inherent ability of nicotine to increase the reinforcing effectiveness of other stimuli (Chaudhri et al., 2006a, 2006b; Caggiula et al., 2009). There is good reason to accept this conclusion, as response rate is a well documented and widely used measure of reinforcement value and response strength (Skinner, 1938; Killeen and Hall, 2001; Hursh and Silberberg, 2008). However, response rate is only one of several putative measures of reinforcement value. A large body of literature has repeatedly shown that various accepted measures of reinforcement value often lead to conflicting conclusions about the strength of a given response (Herrnstein, 1961, 1970; Nevin, 1974, 1995; Killeen and Hall, 2001; Hursh and Silberberg, 2008). Unfortunately, little attention has been paid to the application of different measures of response strength to the reinforcement-enhancing effects of nicotine (but see Cassidy and Dallery, 2012).
An increasingly popular method of analyzing the reinforcement value of a wide variety of rewards relies on the application of economic demand models to the operant behavior of animals (Hursh, 1980, 1993; Hursh and Silberberg, 2008). This approach generally involves training subjects to respond for a putative reinforcer over several blocks of sessions, between which the response requirement per reinforcer delivery (unit cost) is varied. Once subjects have experienced all test conditions of interest, the data are analyzed and demand curves are generated by applying the following mathematical model:
| (1) |
where Q represents units of consumption, Q0 represents units of consumption at the lowest price of the demand curve, C represents the cost requirement [fixed-ratio (FR) value], k is a constant that specifies the number of log units spanned by the demand curve, e represents the base of the natural logarithm, and a is a free parameter that is adjusted to minimize the differences between the predictions of the equation and the demand curve to which it is being fit. The a value is of particular interest as it represents the rate of change in elasticity of demand and thus provides a simple index of essential value (a component of reinforcement value independent of magnitude; see Hursh and Winger, 1995; Hursh and Silberberg, 2008). In addition, the measures of Pmax and Omax, which represent the unit cost of unitary elasticity (where the curve shifts from inelastic to elastic), and the level of maximal behavioral output, respectively, are also derived from the predictions of the model.
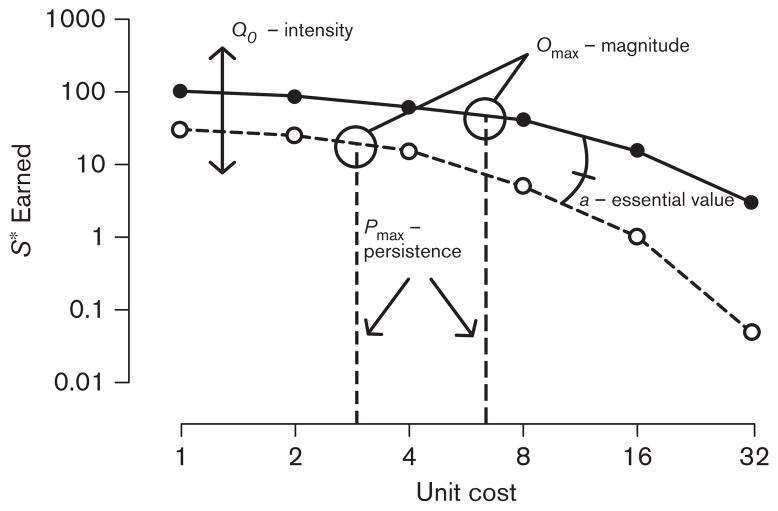
The benefit of such an approach is the ability to assess the value of a reinforcer across a variety of response requirements, instead of a single unit cost, yielding a more comprehensive assessment of the strength of a reinforcer. Furthermore, many of the parameters and derivations of the model serve as reliable indices that may represent different facets of reinforcer value as a construct. Specifically, the values of Q0, Pmax, Omax, and a may be believed to, respectively, represent the intensity of demand for the reinforcer, perseverance of responding for the reinforcer in the face of fluctuations in price, maximal behavioral performance sustainable by the reinforcer at this magnitude, and essential value of the reinforcer. Figure 1 shows each of these indices and how they relate to the model as a whole. Note that these indices are not entirely independent of one another. For instance, Pmax and Omax necessarily correspond to the same unit cost on the demand curve, as the point of unitary elasticity is always where maximal behavioral output is predicted, although different levels of responding between conditions that yield a similar Pmax can be observed. In the same vein, both Pmax and a represent elements of elasticity of demand, that is, the point of unitary elasticity and the rate of change in elasticity of demand, respectively. Therefore, we urge caution in interpreting these indices as representing independent elements of the construct of reinforcement value. Rather, these indices represent elements that differentially represent different facets of value that may or may not co-vary with each other in differing degrees. Most importantly, the application of an economic demand model provides a method to quantifiably compare the value of alternative rewards, or of the same reward under differing conditions, along a variety of indices.
Fig. 1.
Hypothetical data representing two typical demand curves plotted as the number of reinforcers earned as a function of fixed-ratio schedule (unit cost). The effects of experimental condition on the models estimates of Q0, Pmax, Omax, and a are shown.
The aim of the present experiment was to extend previous research on the reinforcement-enhancing effects of nicotine by applying an economic demand approach to quantifiably compare the performance of rats responding for VS between conditions of noncontingent administration of saline or nicotine. The present experiment aimed to provide an insight into the following questions: (a) Is the conclusion that nicotine changes the reinforcement value of other stimuli supported using different measures of response strength? (b) Does nicotine differentially affect different aspects of the behavioral performance (i.e. intensity, persistence and flexibility) to produce its response-enhancing effects? (c) Does the application of an economic demand approach provide a more informative analysis for assessing changes in reinforcement value in the case of the reward-enhancing effects of drugs?
Methods
Subjects
Sixteen male Sprague–Dawley rats (Harlan, Indianapolis, Indiana, USA), weighing 296 g on average (±5.6 SEM), were individually housed in clear polycarbonate tubs lined with wood shavings in a temperature-controlled and humidity-controlled colony. Water was continuously available in the home cage. Rats were provided 20–25 g of laboratory chow daily; previous studies from this lab have shown that rats maintain healthy rates of growth while still encouraging exploratory behavior under these feeding conditions. Sessions were conducted during the light phase of an automated 12 : 12 h light/dark cycle. Experimental protocols were approved by the University of Nebraska-Lincoln IACUC and follow the ‘Guide for the Care and Use of Laboratory Animals’ (National Research Council, 1996).
Apparatus
Sessions were conducted in eight behavioral conditioning chambers (ENV-008CT; Med Associates Inc., St. Albans, Vermont, USA); measuring 30.5×24.1×21.0 cm (l×w×h) enclosed in light-attenuating and sound-attenuating cubicles fitted with a fan to mask noise and provide airflow. Sidewalls were aluminum; the ceiling and front and back walls were clear polycarbonate. One sidewall featured a dipper receptacle, occupying a 5.2×5.2×3.8 cm (h×w×d) recessed space, into which a dipper arm, when raised, provided access to 0.1 ml of a 26% sucrose solution (w/v) in the receptacle. Retractable operant response levers were located on either side of the dipper receptacle ~5 cm from the chamber floor. Each lever required 147nN of force for the microswitch close and the computer to record a response. White 28 V, 100-mA DC lamps were located 7 cm above each lever, hereafter referred to as the right and left lever lights. Two external 28 V, 100-mA DC lamps were also located 10 cm above the conditioning chamber but within the light-attenuating and sound-attenuating cubicle, hereafter referred to as the houselight. An infrared emitter/detector unit positioned 4 cm above the rod floor bisected the chamber 14.5 cm from the sidewall featuring the dipper receptacle and functioned to monitor locomotor activity during experimental sessions. Data collection and presentation of experimental events were controlled through a personal computer with Med Associates interface and software (MedPC for Windows, IV) located in the same room as the conditioning chambers.
Drugs
(−)Nicotine hydrogen tartrate (Sigma, St Louis, Missouri, USA) was dissolved in 0.9% saline and adjusted to a pH of 7.0±0.2 with a dilute NaOH solution. Nicotine was injected subcutaneously as 0.4mg base/kg at a volume of 1 ml/kg. Saline was also injected subcutaneously at a volume of 1 ml/kg.
Habituation and lever training
All rats were habituated to the conditioning chambers and trained to lever press for sucrose deliveries over four daily 60-min sessions. These sessions delivered a non-contingent 4-s access to a 26% sucrose solution (w/v) on a variable time (VT) schedule. In addition, sucrose deliveries were available contingent upon lever pressing on a FR1 schedule on either lever. To ensure equal exposure to reinforcement on each lever, both levers were presented at session initiation and the first press on either lever retracted that lever until a response was made on the opposite lever, resulting in the reintroduction of the former lever and retraction of the latter. The VTschedule controlling noncontingent sucrose deliveries was faded out across the four training sessions in the following sequence: VT 45 s; VT 90 s; VT 180 s; and no VT (all sucrose deliveries were response contingent). These sessions were conducted with constant houselight illumination. Following the fourth session of lever training, no sucrose was available at any time for the rest of the experiment.
On the day following the fourth session of lever training/ habituation, rats received training to press the active lever for a 1-min VS. The VS consisted of a 5-s presentation of the right and left lever lights concurrent with a 60-s termination of otherwise constant houselight illumination. This stimulus arrangement resembles that used in previous experiments examining the reinforcement-enhancing effects of nicotine and has been shown to be weakly reinforcing (Donny et al., 2003; Palmatier et al., 2006; Chaudhri et al., 2007). Assignment of levers as active or inactive was randomized and counter-balanced across rats. Responding on the inactive lever was recorded, but had no programmed consequences. Presentations of the VS were programmed on a FR1 schedule across the 10 sessions. To habituate the rats to the injection protocol, and to reduce the initial response-suppressing effects of nicotine, all rats received an injection of 0.4 mg/kg nicotine 15 min after the completion of each experimental session during this training period.
Demand curve determination
Following the VS-training period, VS presentations were provided on a FR schedule for responses on the active lever over blocks of 10 sessions, each session lasting 60 min. During these sessions, rats received 0.4 mg/kg nicotine or saline 5 min before placement in the chambers. Sessions were arranged such that each rat experienced five nicotine and five saline sessions per 10-session block, in a pseudorandomized order, with no more than two sessions of each type in succession. Session order was constrained such that on any given day, half the rats received saline whereas the remainder received nicotine before the session. Each rat also received the opposite injection 15min following each session, ensuring that each rat received each injection type daily. The response requirement per VS presentation on the active lever was escalated across blocks of 10 sessions using the following sequence: 1, 2, 4, 8, 14, 22, and 32.
Drugs
(−)Nicotine hydrogen tartrate (Sigma) was dissolved in 0.9% saline and adjusted to a pH of 7.0±0.2 with a dilute NaOH solution. Nicotine was injected subcutaneously as 0.4 mg base/kg at a volume of 1 ml/kg. Saline was also injected subcutaneously at a volume of 1 ml/kg.
Data analysis
Data were organized and analyzed using the Microsoft Excel 2007 (Microsoft, Redmond, Washington, USA), GraphPad Prism 5 (GraphPad Software Inc., La Holla, California, USA), and SPSS 19 software packages (IBM, Armonk, New York, USA). Only data from the last three nicotine and the last three saline sessions of each block were analyzed to more accurately represent stable performance under each reinforcement schedule. Although the main objective of the present study was to evaluate the effects of nicotine in the behavioral economic demand model, the data were also analyzed using more traditional measures of response strength and reinforcement value to provide adequate comparison. To this end, the total responses made on each lever, the number of VS presentations earned, and locomotor activity were analyzed using repeated-measures analysis of variance (ANOVA) across sessions and across FR schedules. All post-hoc analyses were carried out using Fisher’s least-significant difference (reported as minimum mean differences; LSDmmd).
Data from each FR schedule were averaged across the three nicotine or three saline sessions for each rat and demand for the VS was assessed under conditions of nicotine and saline administration by fitting the demand model developed by Hursh and Silberberg (2008) to the data of each rat [Eq. (1)]. Paired t-tests were used to evaluate the significance in differences between nicotine and saline conditions for Q0, a, Pmax, and Omax.
Analysis of the demand curves was also carried out following the normalization method proposed by Hursh and Silberberg (2008). This method normalizes consumption as a proportion of maximal consumption (Q/Q0) and standardizes price as the total cost required to maintain Q0 at each response requirement (Q0×C). The utility of this technique is that it allows for simple comparisons between conditions where consumption and elasticity are dependent on the scalar properties of the reinforcer (i.e. magnitude, amount, dose); when this is the case, the demand curves will converge on top of each other and model estimates will be similar (Hursh and Silberberg, 2008).
Results
All 16 rats acquired lever pressing by the end of the fourth session of habituation and lever-training session, earning on average 59.8 (±6.84 SEM) sucrose deliveries a session. Over the following 10-session VS-training period, lever pressing decreased initially, but eventually stabilized, with a successful discrimination between active and inactive levers emerging after the third session (data not shown). The two-way repeated-measures ANOVA (Lever ×Sessions) showed significant main effects of Lever [F(1, 135)=23.13, P<0.001] and Sessions [F(9, 135)= 19.26, P<0.001], but no significant interaction [F(9, 135)= 1.49, NS].
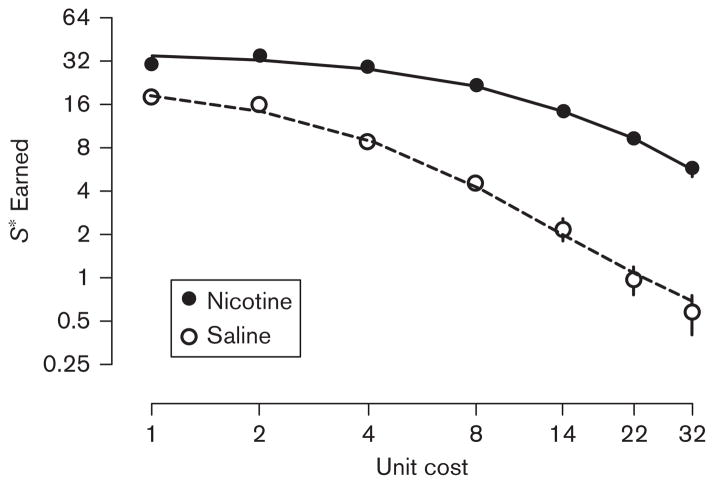
Figure 2 shows the number of VS presentations earned as a function of unit cost (response requirement) for all rats across the 70-session demand-assessment period, between nicotine and saline pretreatment conditions. Rats earned more VS presentations when pretreated with 0.4 mg/kg nicotine than when administered saline across all response requirements. The three-way repeated-measures ANOVA (Drug×Cost×Sessions) showed significant main effects of Drug [F(1, 180)=229.8, P<0.001] and of Cost [F(6, 180)=91.64, P<0.001], but not of Sessions [F(2, 180)=2.93, P=0.069]. A significant Drug×Cost interaction was also found [F(6, 180)=26.23, P<0.001]. Post-hoc analyses showed a greater effect of response requirement under saline conditions than under nicotine conditions, and significant differences in VS presentations earned at every unit cost assessed (LSDmmd=2.21). The Drug×Sessions interaction was also significant [F(2, 180)=4.70, P<0.02]. Post-hoc analysis showed that VS presentations earned during the first session were significantly lower than during the two following sessions only under the nicotine condition (LSDmmd=0.833). Neither the Cost×Sessions interaction nor the Drug×Cost×Sessions interaction were significant (P’s ≥ 0.065).
Fig. 2.
Number of visual stimulus presentations earned between nicotine and saline administration conditions as a function of fixed-ratio schedule (unit cost). The filled and open circles represent data from the nicotine and saline administration sessions, respectively, averaged over the last three sessions of each type. The solid and dashed lines represent the predicted values of the reinforcer demand model when fit to the mean data from the nicotine and saline conditions, respectively. Note that both axes are plotted in logarithmic space.
Active lever-pressing data (not shown) followed a trend similar to that of VS presentations earned: higher levels of lever pressing were observed following pretreatment with 0.4mg/kg nicotine than saline across all response requirements tested. A three-way repeated-measures ANOVA (Drug×Cost×Sessions) of active lever pressing showed significant main effects of Drug [F(1, 180)=130.6, P<0.001] and of Cost [F(6, 180)=13.23, P<0.001], but not of Sessions (F<1). A significant Drug×Cost interaction was also found [F(6, 180)=20.54, P<0.001]. Post-hoc analyses showed a greater effect of cost under the saline condition than under the nicotine condition, and significant differences in active lever pressing across all unit costs tested (LSDmmd=28.51). Neither the Drug×Sessions, Sessions×Cost, nor Drug×Cost×Sessions interactions were significant (P’s ≥ 0.497).
Inactive lever pressing (not shown) followed a considerably different trend compared with VS presentations earned and active lever pressing. Low levels of inactive lever pressing were observed across response requirements and drug-administration conditions (15.1±1.37 SEM), with a tendency toward higher levels of inactive lever pressing following nicotine administration than saline. Closer analysis of the inactive lever pressing data (Drug×Cost ×Sessions) indicated a significant main effect of Drug [F(1, 180)=47.02, P<0.001] but not of Cost [F(6, 180)=1.27, NS] or of Sessions [F<1]. A significant Drug×Cost interaction was also found [F(6, 180)=4.65, P<0.001]. Post-hoc analyses showed that inactive lever pressing increased marginally with response requirement and this increase was greater under the nicotine condition than under the saline condition. Levels of inactive lever pressing were significantly different between nicotine and saline conditions at every response requirement tested except FR1 (LSDmmd=5.64).
Locomotor activity (not shown) showed no trends across response requirement or across sessions within blocks of response requirements, but was consistently higher following nicotine administration than after saline administration. A repeated-measure three-way ANOVA (Drug ×Cost×Sessions) of the locomotor activity data indicated a significant main effect of Drug [F(1, 180)= 145.5, P<0.001], with nicotine pretreatment inducing greater levels of locomotor activity than saline. No other significant main effects or interactions were found for locomotor activity (P’s ≥ 0.146).
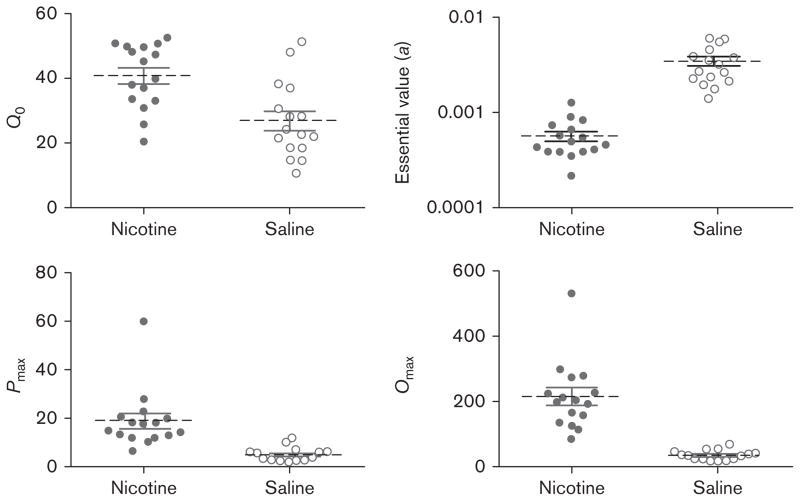
As detailed earlier, the reinforcer demand model was fit to the data of individual rats and estimates of the values of Q0, a, Pmax, and Omax were calculated from the model for each rat between nicotine and saline conditions (Fig. 3). Because the model cannot accommodate zero values at a given unit cost, the lowest possible nonzero value under the present test conditions (0.3333; i.e. 1 VS presentation over 3 days) was substituted in these instances. The model fits the data well for both the nicotine (R2=0.986) and the saline conditions (R2=0.996). Table 1 presents the estimates of the aforementioned model parameters for each rat, between drug conditions. Significant differences were found between drug conditions for each parameter: Q0, Pmax, and Omax [t’s(15) ≥ 7.54, P<0.001] were higher under nicotine conditions than under saline conditions, and a was lower for nicotine than for saline conditions [t(15)=7.62, P<0.001].
Fig. 3.
Values for the model estimates of Q0, a, Pmax, and Omax between nicotine and saline conditions from fits of the model to the data from individual rats. The solid circles (left) and open circles (right) represent the data from nicotine and saline administration sessions, respectively. Q0 is represented in units of visual stimulus presentations earned. Pmax and Omax are represented in the number of lever presses. a is represented in arbitrary units and is inversely correlated with reinforcer value.
Table 1.
Model parameter estimates and variance accounted for by applying the reinforcer demand model to the nicotine and saline data from all 16 rats
| Rat # | Saline
|
Nicotine
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q0 | a | Pmax | Omax | R2 | Q0 | a | Pmax | Omax | R2 | |
| 7256 | 22.6 | 0.00276 | 5.9 | 39.6 | 0.958 | 52.4 | 0.00036 | 17.7 | 276.6 | 0.956 |
| 7257 | 28.1 | 0.00663 | 2.0 | 16.5 | 0.899 | 33.0 | 0.00056 | 19.9 | 195.7 | 0.950 |
| 7258 | 18.5 | 0.00326 | 6.0 | 33.3 | 0.938 | 25.9 | 0.00068 | 20.7 | 160.5 | 0.929 |
| 7259 | 14.6 | 0.00229 | 12.1 | 51.5 | 0.876 | 37.0 | 0.00039 | 27.9 | 301.2 | 0.895 |
| 7260 | 10.9 | 0.00497 | 7.2 | 23.2 | 0.942 | 20.6 | 0.00084 | 22.6 | 136.6 | 0.629 |
| 7261 | 30.6 | 0.00360 | 2.8 | 26.6 | 0.913 | 39.9 | 0.00075 | 10.3 | 127.4 | 0.719 |
| 7262 | 36.9 | 0.00129 | 5.9 | 68.9 | 0.901 | 50.7 | 0.00040 | 14.0 | 224.6 | 0.939 |
| 7263 | 14.8 | 0.00673 | 3.7 | 16.3 | 0.983 | 48.1 | 0.00129 | 6.0 | 85.1 | 0.883 |
| 7264 | 48.0 | 0.00177 | 3.8 | 56.4 | 0.926 | 50.6 | 0.00036 | 18.2 | 281.1 | 0.973 |
| 7265 | 24.2 | 0.00391 | 3.4 | 25.2 | 0.955 | 45.3 | 0.00059 | 12.0 | 167.6 | 0.982 |
| 7266 | 18.5 | 0.00216 | 10.3 | 55.2 | 0.955 | 30.8 | 0.00022 | 59.8 | 534.8 | 0.484 |
| 7267 | 22.1 | 0.00265 | 6.0 | 39.8 | 0.772 | 37.9 | 0.00050 | 18.3 | 209.1 | 0.963 |
| 7268 | 28.2 | 0.00401 | 2.9 | 25.0 | 0.957 | 49.6 | 0.00044 | 15.0 | 227.1 | 0.943 |
| 7269 | 38.3 | 0.00240 | 3.3 | 38.9 | 0.953 | 47.3 | 0.00047 | 13.5 | 198.9 | 0.935 |
| 7270 | 51.3 | 0.00196 | 2.7 | 44.3 | 0.981 | 49.6 | 0.00042 | 13.1 | 206.1 | 0.981 |
| 7271 | 21.4 | 0.00620 | 2.6 | 16.8 | 0.950 | 33.6 | 0.00091 | 11.3 | 114.8 | 0.990 |
Discussion
The present study found that nicotine administration increased operant lever pressing for a VS, similar to previously reported results (Donny et al., 2003; Chaudhri et al., 2006a; Raiff and Dallery, 2006, 2009; Caggiula et al., 2009). Importantly, the present results extend previous findings by systematically evaluating the reward-enhancing effects across a range of reinforcement schedules and by using a reinforcer demand model to quantify the differences between the behavioral performance under differing drug conditions. The finding that nicotine enhanced responding for the VS across the entire range of reinforcement schedules supports the notion that nicotine increases the reinforcement value of the VS (cf. Chaudhri et al., 2006a, 2006b). However, nicotine did not uniformly increase lever pressing for the VS across each response requirement tested. Rather, the magnitude of this effect varied with the changes in unit cost. Interestingly, nicotine increased responding for the VS at unit costs at which responding under saline was almost abolished (FR22 and FR32).
The advantage of the behavioral economic approach adopted for the present study (cf. Hursh and Silberberg, 2008) is that it allows us to quantifiably compare different aspects of the operant performance across response requirements. These include the intensity of demand for the reinforcer when it costs nothing (Q0), persistence of responding for the reinforcer (Pmax), the maximal behavior output maintained by the reinforcer (Omax), and the essential value of the reinforcer after accounting for sensitivity to changes in unit cost (a). Previous studies have shown that these parameters may vary independently and represent different facets of reinforcement value [for a review, see Hursh and Silberberg (2008)]. For example, Hursh and Winger (1995) showed how scalar differences in consumption resulting from variations in reinforcer magnitude can differentially affect different parameters of the reinforcedemand equation. Briefly, adult rhesus monkeys were trained to self-administer intravenous infusions of alfentanil at doses of 0.0003, 0.001, 0.003, and 0.01 mg/kg, and demand curves were generated from performance across a range of FR schedules (Hursh and Winger, 1995; Winger et al., 1996). Analysis of the demand curves showed large differences in Q0, Pmax, and Omax between doses of alfentanil, but similar values of a, indicating that the essential value of the reinforcer remains constant despite large shifts in the levels of consumption of the reinforcer caused by differences in reinforcer magnitude. In support of this conclusion, when the demand curves for alfentanil were evaluated after normalizing consumption as a proportion of Q0 and, normalizing price as the product of response requirement and Q0, the four demand curves representing each dose of alfentanil converged on top of each other and values of Pmax and Omax became similar (Hursh and Winger, 1995).
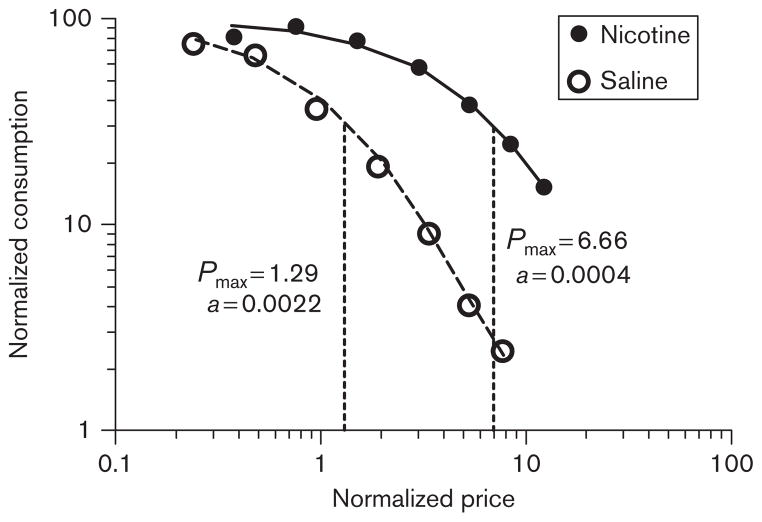
In contrast to Hursh and Winger (1995), in the present study, we observed differences in all four model estimates. This outcome indicates that the differences observed in the present study are not because of changes in the perceived reinforcing magnitude of the VS generated by nicotine administration. Figure 4 graphically demonstrates this point by showing the resultant demand curves after applying the same normalizing operations on consumption and price proposed in previous studies (Hursh and Winger, 1995; Hursh and Silberberg, 2008). The curves generated under nicotine and saline conditions do not converge, nor do the estimates of Pmax and Omax become similar, suggesting that the observed enhancement of responding by nicotine in the present study does not reflect a scalar enhancement of the perceived magnitude of the reinforcing VS that is analogous to changes in drug dose.
Fig. 4.
Demand curves from the mean normalized data expressed as normalized consumption as a function of normalized price. Normalized consumption was calculated as the number of visual stimulus presentations earned divided by Q0. Normalized price was calculated as the product of response requirement and Q0. Filled and open circles represent the data from the nicotine and the saline sessions, respectively.
The present findings support previous claims that nicotine enhances the strength of other reinforcing stimuli through nonassociative means. Note that this entire study was carried out within subjects, and that the data comprising the nicotine and saline demand curves come from the same rats receiving nicotine or saline pretreatment in a pseudo-alternating manner across sessions. Such a procedure is unlikely to engender effective conditioning between the VS and the nicotine stimulus because both VS and nicotine were delivered in the absence of one another during half the sessions (i.e. the saline pretreatment sessions). The finding that nicotine reliably enhanced lever pressing above levels observed following saline administration supports this assumption. The present findings, combined with previously published reports from other laboratories (see the Introduction section), provide strong evidence against the suggestion that a purely associative learning process is responsible for the reward-enhancing effects of nicotine. Furthermore, the present results are equally difficult to explain from the perspective of lasting neuronal changes resulting from repeated nicotine exposure that exert their effects in the absence of nicotine. That is, nicotine exposure alone is inadequate to account for the present findings because we do not observe reliable increases in levels of operant responding for the VS merely as a function of sessions and nicotine experience during saline sessions.
An alternative interpretation of the present results relies on the well-documented and reliable effect of nicotine exposure to increase baseline rates of locomotor activity (Bevins and Besheer, 2001; Bevins et al., 2001; Frenk and Dar, 2004). Indeed, such an account makes several studies on the reward-enhancing effects of nicotine harder to interpret, especially when one considers the possible interaction of locomotor-stimulating effects and learned place-preference within the experimental apparatus centered around the operandum that produces reinforcement. That is, rats may possibly develop a preference for the spatial location they previously occupied at the time of reinforcement delivery, and any locomotor-activating effects of nicotine may be disproportionately expressed within that same spatial location. Because this location is shared by the active lever, locomotor-activating effects by nicotine may reveal themselves as more responding on that lever. Although the present study does not systematically examine this issue, and thus cannot directly refute its plausibility, there are several observations that make a locomotor-activation account alone inadequate. First, initial lever training with liquid sucrose was arranged to force equal experience with both levers producing reward, reducing the likelihood of rats developing a preference for one lever over the other before VS-lever training. Further, the observed differences in locomotor activity between nicotine and saline sessions did not vary systematically with increases in the response requirement for the VS. Although increases in inactive lever pressing occurred with increases in response cost, these increases were observed under both nicotine and saline conditions, and were only marginally higher on nicotine sessions. Moreover, although an effect of nicotine on general chamber locomotor activation (i.e. center beam breaks) was observed in the present experiment, this effect did not vary across sessions or as a function of response cost. These findings, in combination with previous findings from other laboratories (cf. Donny et al., 2003; Chaudhri et al., 2006a, 2006b; Palmatier et al., 2006; Raiff and Dallery, 2006, 2009; Barrett and Odum, 2011; Cassidy and Dallery, 2012), indicate that the locomotor-activating effects of nicotine are a minor contributor, at best, toward the enhancement in operant responding observed in the present experiment. For example, Raiff and Dallery (2008) found that although nicotine administration did lead to an increase in the response rates for conditioned reinforcers in an observing response procedure, nicotine exerted no effects of food-maintained responding. Furthermore, nicotine did not lead to an increase in the rates of response associated with either conditioned or unconditioned reinforcement during extinction, suggesting that the enhancement in operant responding by nicotine is not adequately explained as a tendency to increase responding that has been reinforced in the past (Frenk and Dar, 2004). Rather, the present results are more consistent with a reward-enhancement account of the effects of nicotine on operant responding, but do not rule out possible contributions from the locomotor-activating effects of nicotine.
The present study provides a method for evaluating the reward-enhancing effects of compounds using a behavioral economic reinforcer demand approach. Although the individual estimates of Q0, Pmax, Omax, and a did not yield results that differed in interpretation in the present study, which does not preclude their utility in evaluating the specific effects of compounds on different facets of reinforcement value in future studies. For instance, the mechanisms by which nicotine exerts its reward-enhancing effects remain unclear, as also how these mechanisms differ from those underlying the primary reinforcing effects of nicotine. Nicotine acts on several receptor subtypes in the cholinergic system, in addition to exerting modulating effects on other receptor systems (Brunzell and Picciotto, 2009). Some of these receptor subtypes or modulatory roles may have differential effects on the various facets of reinforcer value, and these may be reflected in differences between specific model estimates. Although such a conjecture at this point is certainly speculative, using a methodology that has the ability to detect such differences is favorable to using one that lacks this capability. Furthermore, the present results and methodology provide a far richer picture of the reward-enhancing effects of nicotine by virtue of the repeated assessment across a wide range of reinforcement schedules under multiple conditions, and provides a technique for quantifiably comparing differences in performance along multiple indices that represent different facets of reinforcement value.
Several questions remain on the nature of the reward-enhancing effects of nicotine for future studies to investigate. The present study will hopefully provide a powerful procedure for examining these questions. For instance, previous work has shown that the reward-enhancing effects of nicotine depend, in part, upon the degree of initial reinforcement value of the stimulus to be enhanced (Palmatier et al., 2007). The reinforcer demand procedure used in the present study provides a novel approach toward quantifiably investigating this effect. The reinforcer demand approach is unique by allowing the investigator to compare rates of reinforcement across a range of response requirements between conditions where the reinforcer differs in initial value. The estimates of the model allow for the possibility of detecting differences in intensity of demand for the reinforcer, persistence of responding for the reinforcer, maximal behavioral output generated by the reinforcer, and essential value of the reinforcer, which may vary independently between conditions. Our hope is that the application of such an approach may enrich the knowledge that we currently possess on what appears to be an important piece of the puzzle of nicotine reward, leading to a better understanding of tobacco addiction and informing better treatment procedures.
Acknowledgments
The authors thank the National Institute on Drug Abuse, whose funding made this research possible (DA018114 and DA023951). The authors also thank Steven Hursh for developing and providing us with the tool by which we fit the reinforcer demand model. The authors also thank Greg Madden for his insightful comments on the manuscript, and Stephanie Savala for help with conducting experimental sessions.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- Barrett ST, Odum AL. The effects of repeated exposure on the reward-enhancing effects of nicotine. Behav Pharmacol. 2011;22:283–290. doi: 10.1097/FBP.0b013e3283473c25. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J. Individual differences in rat locomotor activity are diminished by nicotine through stimulation of central nicotine acetylcholine receptors. Physiol Behav. 2001;72:237–244. doi: 10.1016/s0031-9384(00)00413-3. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cogn Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Besheer J, Pickett KS. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol Biochem Behav. 2001;68:135–145. doi: 10.1016/s0091-3057(00)00451-2. [DOI] [PubMed] [Google Scholar]
- Brunzell DH, Picciotto MR. Molecular mechanisms underlying the motivational effects of nicotine. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use. New York: Springer Science and Business Media; 2009. pp. 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, et al. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual reinforcement model. In: Bevins RA, Caggiula AR, editors. The motivational impact of nicotine and its role in tobacco use. New York: Springer Science and Business Media; 2009. pp. 91–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J. Effects of economy type and nicotine on the essential value of food in rats. J Exp Anal Behav. 2012;97:183–202. doi: 10.1901/jeab.2012.97-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by primary reinforcing and reinforcementenhancing effects of nicotine. Psychopharmacology. 2006a;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology. 2006b;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, et al. Self-adminstered nicotine and noncontingent nicotine enhance reinforced operant responsing in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology. 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib M, et al. Operant responding for a visual stimulus in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Frenk H, Dar R. Reward potentiation or behavioral activation? A comment on Donny et al. Psychopharmacology (Berl) 2004;171:472–473. doi: 10.1007/s00213-003-1622-8. [DOI] [PubMed] [Google Scholar]
- Herrnstein RJ. Relative and absolute strength of response as a function of frequency of reinforcement. J Exp Anal Behav. 1961;4:264–272. doi: 10.1901/jeab.1961.4-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrnstein RJ. On the law of effect. J Exp Anal Behav. 1970;13:243–266. doi: 10.1901/jeab.1970.13-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Economic concepts for the analysis of behavior. J Exp Anal Behav. 1980;34:219–238. doi: 10.1901/jeab.1980.34-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR. Behavioral economics of drug self-administration: an introduction. Drug Alcohol Depend. 1993;33:165–172. doi: 10.1016/0376-8716(93)90058-x. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–198. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Winger G. Normalized demand for drugs and other reinforcers. J Exp Anal Behav. 1995;64:373–384. doi: 10.1901/jeab.1995.64-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen PR, Hall SS. The principal components of response strength. J Exp Anal Behav. 2001;75:111–134. doi: 10.1901/jeab.2001.75-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council. Guide for the care and use of laboratory animals. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Nevin JA. Response strength in multiple schedules. J Exp Anal Behav. 1974;21:389–408. doi: 10.1901/jeab.1974.21-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevin JA. Behavioral economics and behavioral momentum. J Exp Anal Behav. 1995;64:385–395. doi: 10.1901/jeab.1995.64-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, et al. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology. 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, et al. The reinforcement enhancing-effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated injections. Psychopharmacology. 2007;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Exp Clin Psychopharmacol. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. The generality of nicotine as a reinforcer enhancer in rats: effects on responding maintained by primary and conditioned reinforcers and resistance to extinction. Psychopharmacology. 2008;201:305–314. doi: 10.1007/s00213-008-1282-9. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Responding maintained by primary reinforcing visual stimuli is increased by nicotine administration in rats. Behav Process. 2009;82:95–99. doi: 10.1016/j.beproc.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Skinner BF. The behavior of organisms. New York: Appleton Century Crofts; 1938. [Google Scholar]
- United States Department of Health and Human Services. Nicotine Addiction: a report of the surgeon general. Rockville, MD: US Department of Health and Human Services, Office of the Assistant Secretary for Health, Office on Smoking and Health; 1988. [Google Scholar]
- Wilkinson JL, Bevins RA. Intravenous nicotine conditions a place preference in rats using an unbiased design. Pharmacol Biochem Behav. 2008;88:256–264. doi: 10.1016/j.pbb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winger G, Woods JH, Hursh SR. Behavior maintained by alfentanil or nalbuphine in rhesus monkeys: fixed-ratio and time-out changes to establish demand curves and relative reinforcing effectiveness. Exp Clin Psychopharmacol. 1996;4:131–140. [Google Scholar]
- World Health Organization. Neuroscience of psychoactive substance use and dependence. Geneva, Switzerland: World Health Organization; 2004. [Google Scholar]