Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is curative treatment, albeit in a minority of patients with accelerated (AP) or blast phase (BP) chronic myeloid leukemia (CML). Imatinib (IM) has transient but significant activity in advanced phases of CML, which may permit early allografting for responding patients. To identify prognostic factors in allograft recipients previously treated with IM, we analyzed 449 allogeneic HSCT performed between 1999–2004 in advanced phase CML using data reported to the Center for International Blood and Marrow Transplant Research. CML patients in second chronic phase (CP2, n=184), AP (n=185), and BP (n=80) received HLA-identical sibling (27%), related (3%), or matched or mismatched unrelated donor (70%), peripheral blood (47%) or bone marrow (53%) HSCT after myeloablative (78%) or non-myeloablative (22%) conditioning. 52% in CP2, 49% in AP, and 46% in BP received IM pre-HSCT. Disease-free survival was 35–40% for CP2, 26–27% for AP and 8–11% for BP. Cumulative incidence of acute and chronic GVHD and TRM were not affected by stages of CML or pre-HSCT IM exposure. Multivariate analyses showed that conventional prognostic indicators remain the strongest determinants of transplant outcomes. In conclusion, there are no new prognostic indicators of outcomes of allogeneic HSCT for advanced phase CML in the IM era.
Keywords: Imatinib, allogeneic transplantation, chronic myeloid leukemia, accelerated phase, blast phase, outcomes
INTRODUCTION
Imatinib mesylate (IM) is a potent and selective inhibitor of the tyrosine kinase activity of BCR-ABL with substantial, albeit transient, activity in advanced phase chronic myeloid leukemia (CML). Treatment of accelerated (AP) and blast phase (BP) CML with single agent IM is associated with hematological responses in 50–70%;(1–4) better results than those achieved with chemotherapy alone.(5) These rapid and high response rates have often allowed patients with a suitable donor to proceed early with allografting. Results of allogeneic hematopoietic stem cell transplantation (HSCT) in this particular patient population have been reported, (6–14) and have consistently shown a lack deleterious or beneficial effect of IM on transplant outcomes. However, analyses of prognostic factors were not feasible in these reports given the relatively small numbers of patients with advanced phase CML. We therefore sought to analyze outcomes of allogeneic hematopoietic stem cell transplantation (HSCT) in advanced phase CML in the IM era specifically focusing on prognostic indicators.
MATERIAL AND METHODS
Data Source
A formal affiliation of the research division of the National Marrow Donor Program (NMDP), the International Bone Marrow Transplant Registry and the Autologous Blood and Marrow Transplant Registry led to establishment of the CIBMTR in 2004. The CIBMTR is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic HSCTs to the Statistical Center at the Medical College of Wisconsin in Milwaukee or the NMDP Coordinating Center in Minneapolis. Approximately two-thirds of all active transplantation centers worldwide report data to the registry. The registry database includes information on 40–45% of all patients who have received an allotransplant since 1970, with annual updates. Compliance is assessed by periodic audits and accuracy of data is ensured by computerized record checks, physician review of submitted data and on-site audits. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with HIPAA regulations as determined by the Institutional Review Board and Privacy Officer of Medical College of Wisconsin.
Patient Selection and Definitions
The patient population consisted of sibling or unrelated allogeneic HCT recipients with advanced phase CML transplanted between 1999 and 2004 reported to the CIBMTR. A total of 449 cases with CML beyond first chronic phase and complete research data available within the CIBMTR database were identified. Those who received bone marrow (BM) or peripheral blood stem cells (PBSC) from a sibling or other relative or from an unrelated donor (URD) were selected for analysis. Donor and recipient HLA matching were defined using best available HLA-matching data.(15)
Patients were conditioned with myeloablative or non-myeloablative regimens, and received methotrexate + calcineurin inhibitor +/− other drugs for graft-versus-host disease (GVHD) prophylaxis. The population was restricted to patients with no prior transplantation.
Advanced phase CML was defined as second chronic phase (CP2), accelerated phase (AP) and blast phase (BP, myeloid or lymphoid or undifferentiated) at the time of HSCT. Accelerated phase was defined on the CIBMTR case report forms by any of the following: anemia (hemoglobin < 8 g/dL), leukocytosis (WBC > 100 × 109/L), thrombocytopenia (platelets < 100 × 109/L), thrombocytosis (platelets > 1,000 × 109/L) or splenomegaly unresponsive to busulfan or hydroxyurea, extramedullary disease, clonal marrow cytogenetic abnormality(ies) in addition to the original Ph-chromosome, blood or marrow blasts >10%, blood or marrow blasts plus promyelocytes > 20%, and/or blood basophils+ eosinophils > 20%. Patients with more advanced findings were classified as blast phase. CP2 was defined as return to a second chronic phase or remission after successful treatment of advanced phase. Disease status was captured at the time of diagnosis and immediately before the conditioning regimen started.
Study-specific supplemental forms were sent to all CIBMTR reporting centers to collect additional information that included: time of initiation/stopping of IM, starting dose, maximal dose as well as IM dose reductions, hematological, cytogenetic and molecular responses, toxicity associated with IM, addition of other agents to IM prior to conditioning, and reason for transplantation. Data on post-transplant use of IM was also collected. Center responses to those supplemental forms were 68%. Transplant centers providing data on 80% or more of eligible patients receiving IM during the study period were included.
Study Endpoints
Primary outcomes were overall survival (OS) and leukemia-free survival (LFS, survival in continuous complete remission). Secondary outcomes included survival at 30 and 100 days, treatment-related mortality (TRM), and grades II to IV acute GVHD and chronic GVHD. Chronic GVHD was assessed in patients surviving more than 90 days with evidence of engraftment. TRM is defined as death in continued remission; patients were censored at relapse, or for those in continuous remission, at last follow-up. For LFS, patients were considered treatment failures at the time of hematological or cytogenetic relapse or death from any cause; patients alive were censored at the last follow-up evaluation.
Statistical Analysis
Multivariate analyses were done using the Cox proportional hazards regression model with a stepwise selection procedure to identify clinical variables that were associated with particular outcomes. Potential covariates included patient age, sex and race, Karnofsky performance status, time from diagnosis to HCT, donor type, donor-recipient sex match and cytomegalovirus (CMV) serological status, human leukocyte antigen (HLA) matching grade, type of conditioning regimen, graft source, year of transplantation, and GVHD prophylaxis. Potential interactions between significant covariates were assessed and no significant interactions were present. Because of multiple testing, a p-value < 0.01 was considered statistically significant. In a separate subgroup analysis, factors associated with survival and TRM were assessed among patients who received pre-HSCT IM. Potential predictors included reason for proceeding to HCT, best response to IM prior to HCT, duration of IM treatment, and interval between IM discontinuation and HCT, in addition to the other potential clinical predictors listed above. Adequate details about cytogenetic and molecular burden of disease just prior to transplantation were not available. The product limit estimator proposed by Kaplan-Meier was used to estimate the median and range of the follow-up time. The probabilities of overall survival and leukemia-free survival for all patients were calculated using the Kaplan-Meier estimator, with the variance estimated by Greenwood’s formula. Patients were censored at date of last known follow-up. Cumulative incidence estimates were calculated for other endpoints to account for competing risks.
RESULTS
Patients
Between 1/1999 and 12/2004, 449 patients with CML in AP (n=185), CP2 (n=184) and BP (n=80) at the time conditioning regimen begun met the study eligibility criteria. Pre-transplant IM administration (IM+) was reported in 91 AP, 96 CP2 and 37 BP patients. Patient and transplant characteristics are summarized in Tables 1 and 2. Fifty-three patients had a documented history of blast transformation prior to transplantation, but were in CP2 at the time of conditioning. Forty one AP, 41 CP2, and 13 BP patients were prepared for transplantation with a reduced intensity conditioning regimen. For patients who received IM pre-transplant, the median daily doses of pre-transplant IM were 600 mg administered for a median duration of 11 (range 1–54), 7 (range 1–60), and 8 (range 1–36) months in patients with AP, CP2 and BP, respectively. For patients with AP, CP2 and BP, 29%, 52%, and 54% were planned HSCT procedures, while 62%, 46%, and 41% were transplanted for IM failure, respectively. IM was continued up to 4 weeks pre-transplant in the majority of patients. Information on post-transplant administration of IM was only available in patients who received pre-transplant IM. Of those 214 patients, 56 (25%) received post-transplant IM for relapse prophylaxis (20%), persistent disease (27%), relapsed disease (48%) or for unspecified reasons (5%).
Table 1.
Characteristics of CML patients who underwent an allogeneic HSCT for AP, CP2, and BP, between 1999 to 2004, and reported to the CIBMTR.
| Advanced phase CML (N=449) | |||
|---|---|---|---|
| Disease status at conditioning | AP (N=185) | CP2 (N=184) | BP (N=80) |
| Age, median (range), years | 43 (12–70) | 40 (7–69) | 41 (7–61) |
| Male sex | 116 (63) | 116 (63) | 48 (60) |
| Race white | 143 (77) | 145 (79) | 57 (71) |
| Karnofsky score > 80% at transplant | 127 (69) | 123 (67) | 38 (48) |
| CML phase at diagnosis | |||
| CP | 54 (29) | 53 (29) | 27 (34) |
| AP | 18 (10) | 5 (3) | 3 (4) |
| BP | 4 (2) | 12 (6) | 6 (7) |
| missing | 109 (59) | 114 (62) | 44 (55) |
| CP2 at conditioning with prior BP | NA | 53 (29) | NA |
| CP2 at conditioning no prior BP | NA | 20 (11) | NA |
| CP2 at conditioning prior BP unknown | NA | 111 (60) | NA |
| Phenotype of blast transformation | |||
| Lymphoid | NA | 21 (40) | 21 (26) |
| Myeloid only | NA | 19 (36) | 42 (53) |
| Unspecified | NA | 13 (24) | 17 (21) |
| Pre-HSCT IM administration | 91 (49) | 96 (52) | 37 (46) |
| Duration of IM therapy (months) | 11 (1–54) | 7 (1–60) | 8 (1–36) |
| Reasons to proceed to HSCT | |||
| Planned transplant | 26 (29) | 49 (52) | 20 (54) |
| Intolerance to imatinib | 9 (9) | 2 (2) | 2 (5) |
| Imatinib therapy failure | 56 (62) | 43 (46) | 15 (41) |
| Interval last dose IM - transplant (months) | |||
| 0–1 | 60 (65) | 56 (58) | 18 (49) |
| 1–3 | 15 (16) | 13 (13) | 9 (24) |
| > 3 | 13 (13) | 20 (20) | 7 (20) |
| Unknown | 3 (3) | 7 (7) | 3 (8) |
Table 2.
Transplant characteristics of AP, CP2, and BP CML
| Advanced phase CML (N=449) | |||
|---|---|---|---|
| Disease status at conditioning | AP (N=185) | CP2 (N=184) | BP (N=80) |
| Time from diagnosis to HSCT, median (range), months | 18 (2–120) | 14 (2–180) | 18 (1–150) |
| Time from diagnosis to transplant ≤12 months | 67 (36) | 83 (45) | 26 (33) |
| Donor/recipient gender match | |||
| F-M | 34 (18) | 49 (27) | 18 (23) |
| Other | 151 (82) | 135 (73) | 62 (77) |
| Donor | |||
| HLA-identical sibling | 52 (28) | 46 (25) | 26 (33) |
| Related mismatched | 6 (3) | 4 (2) | 3 (4) |
| Unrelated | 127 (69) | 134 (73) | 51 (63) |
| Donor-recipient CMV status | |||
| +/+ | 60 (34) | 56 (34) | 28 (38) |
| +/− | 28 (16) | 15 (9) | 8 (11) |
| −/+ | 41 (23) | 45 (26) | 22 (30) |
| −/− | 46 (27) | 51 (31) | 15 (21) |
| Myeloablative Conditioning | 144 (78) | 143 (78) | 67 (84) |
| Non-myeloablative Conditioning | 41 (22) | 41 (22) | 13 (16) |
| TBI for conditioning regimen | 81 (44) | 102 (55) | 40 (50) |
| BM Graft | 106 (57) | 91 (49) | 37 (46) |
| PB Graft | 79 (43) | 93 (51) | 43 (54) |
| GVHD prophylaxis | |||
| MTX+Calcineurin Inhibitor ± others | 139 (76) | 142 (76) | 57 (73) |
| Other * | 46 (24) | 42 (76) | 23 (27) |
| Year of transplant 1999–2000 | 66 (36) | 54 (29) | 33 (41) |
| Year of transplant 2001–2002 | 45 (24) | 62 (34) | 28 (35) |
| Year of transplant 2003–2004 | 74 (40) | 68 (37) | 19 (24) |
| Median follow-up of survivors, months | 38 (4–95) | 45 (3–87) | 46 (23–88) |
Other includes: CSA +/− other, FK 506 +/− other, steroids + MTX, cellcept+ MTX, rapamycin + MTX, extracorporeal phototherapy + MTX, T cell depletion (n=19), and none
Outcomes
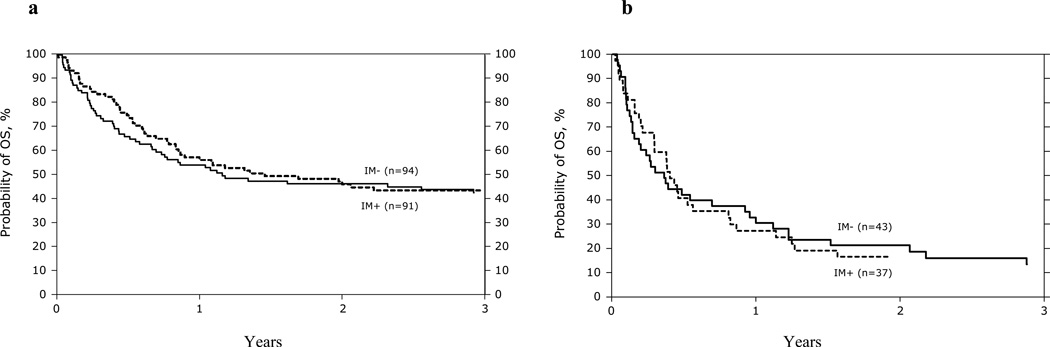
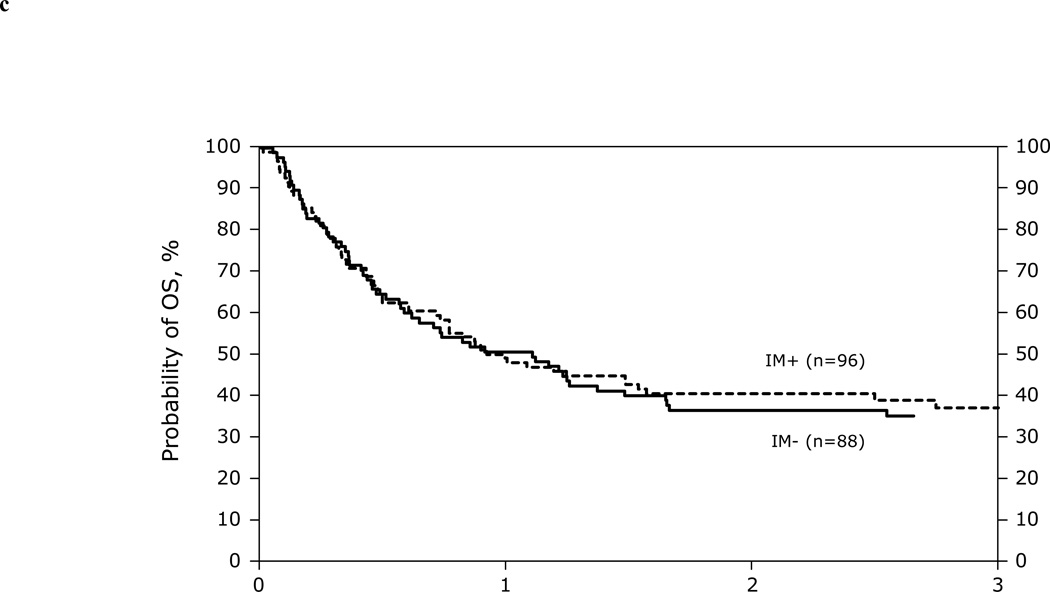
Univariate Probabilities of day 100 acute GVHD, and 1 and 3 years chronic GVHD, TRM, LFS, and OS are listed in Table 3. Kaplan-Meier plots for overall survival for patients in AP, BP and CP2 are shown in Figure 1. Table 4 summarizes causes of death. Recurrence of the primary disease and GVHD/infection were the most common causes of death in all phases of CML.
Table 3.
Univariate probabilities of transplant outcomes among allogeneic transplant recipients with AP, CP2, and BP
| AP (N=185) | CP2 (N=184) | BP (N=80) | |
|---|---|---|---|
| Outcome | Prob (95% CI) | Prob (95% CI) | Prob (95% CI) |
| Acute GVHD @ 100 days, grades (2–4) | 49 (42–56) | 51 (44–58) | 40 (30–51) |
| Chronic GVHD | |||
| @ 1 year | 51 (44–58) | 52 (45–59) | 22 (14–32) |
| @ 3 years | 54 (46–61) | 54 (46–61) | 22 (14–32) |
| TRM | |||
| @ 1 year | 34 (28–41) | 33 (26–40) | 46 (35–58) |
| @ 3 years | 37 (30–44) | 39 (32–46) | 54 (42–65) |
| Relapse | |||
| @ 1 year | 24 (18–30) | 29 (22–35) | 34 (24–45) |
| @ 3 years | 26 (20–33) | 34 (27–41) | 36 (26–48) |
| LFS | |||
| @ 1 year | 42 (35–49) | 38 (31–45) | 20 (11–29) |
| @ 3 years | 37 (30–44) | 27 (20–34) | 10 (4–17) |
| Overall survival | |||
| @ 1 year | 55 (48–60) | 50 (42–57) | 29 (19–39) |
| @ 3 years | 43 (35–50) | 36 (29–43) | 14 (8–23) |
Abbreviations: TRM = treatment-related mortality; LFS = leukemia-free survival; Prob = probability; CI = confidence interval.
Figure 1.
Kaplan-Meier curves depicting survival of AP (1A), CP2 (1B), and BP (1C) patients
Table 4.
Causes of Death
| AP (N=185) | CP2 (N=184) | BP (N=80) | |
|---|---|---|---|
| Primary disease | 28 (26) | 39 (33) | 29 (43) |
| GVHD | 19 (18) | 32 (27) | 7 (10) |
| Infection | 24 (22) | 15 (13) | 9 (13) |
| Organ Failure | 15 (14) | 9 (8) | 8 (12) |
| ARDS | 2 (2) | 4 (4) | 2 (3) |
| IpN | 6 (6) | 4 (3) | 3 (4) |
| Graft rejection | 4 (4) | 4 (3) | 3 (4) |
| Secondary malignancy | 1 (1) | 1 (1) | 0 |
| Hemorrhage | 2 (2) | 2 (2) | 3 (4) |
| Vascular | 2 (2) | 0 | 0 |
| Toxicity | 1 (1) | 3 (3) | 2 (3) |
| Other** | 4 (4) | 4 (3) | 2 (3) |
Abbreviations: GVHD = Graft-vs-Host Disease; IpN = interstitial pneumonia; ARDS = adult respiratory distress syndrome.
Others include (N=10): Cardio pulmonary arrest (n=3); Toxic epidermal (n=1); Suicide (n=2); Autoimmune hemolytic (n=1); Cause unknown (n=3)
Prognostic Factors
Cox proportional hazards regression models were constructed to assess factors that affected overall survival (OS), leukemia-free survival (LFS), TRM, relapse, acute and chronic GVHD using the variables summarized in the Supplemental Table. Time from diagnosis to transplant , KPS, and degree of HLA matching independently predicted OS, relapse and LFS; while GVHD prophylaxis, CMV serostatus, sex mismatch, conditioning regimen, and degree of HLA matching affected GVHD and TRM (Table 5). Pre-transplant IM had neither a positive nor a negative association with transplant outcomes, including acute and chronic GVHD. A subset analysis in patients who received pre-transplant IM was performed. Duration of pre-transplant IM (dichotomized at 3 months and 6 months) did not affect acute or chronic GVHD, TRM, DFS or OS, while reason to proceed with transplantation was significantly associated with TRM. Indeed the relative risk for TRM was 1.8 (95%CI: 1.0–3.4) for patients receiving allograft for IM failure as compared to those with a planned transplant (p=0.03). In the CP2 group with reported prior BP, patients who received pre-transplant IM (n=23) had a better 1 (61 vs. 44%, p=0.22) and 3 years (41 vs. 26%, p=0.26) survivals when compared to those who had no pre-transplant IM (n=30), however, these differences were not statistically significant. Finally, outcomes of the 95 patients conditioned with reduced-intensity were, within each phase of the disease at the time of conditioning comparable, to the 354 who received conventional myeloablative conditioning.
Table 5.
Multivariate analysis of transplant outcomes among allogeneic transplant recipients with AP, CP2, and BP
| OS | LFS | TRM | Relapse | ||
|---|---|---|---|---|---|
| Variable | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| AP | Interval diagnosis to transplant | ||||
| ≤12 months | 1.0 | 1.0 | - | 1.0 | |
| > 12 months | 1.55 (1.01–2.40) p=0.048 | 2.10 (1.34–3.31) p=0.001 | 3.24 (1.53–6.86) p=0.002 | ||
| KPS | - | - | |||
| > 80% | 1.0 (overall p=0.024) | 1.0 (overall p=0.0013) | |||
| ≤80% | 1.83 (1.18–2.82) p=0.007 | 1.74 (1.15–2.64) p=0.009 | |||
| Unknown | 1.13 (0.52–2.44) p=0.76 | 1.80 (0.87–3.74) p=0.11 | |||
| Recipient Age | - | - | - | ||
| ≤30 | 1.0 (overall p=0.035) | ||||
| 31–40 | 0.45 (0.23–0.86) p=0.016 | ||||
| 41–50 | 0.76 (0.43–1.32) p=0.33 | ||||
| >50 | 0.95 (0.54–1.67) p=0.87 | ||||
| GVHD prophylaxis | - | - | - | ||
| CsA+MTX +/− others | 1.0 (overall p=0.03) | ||||
| FK+MTX +/− others | 0.67 (0.33–1.34) p=0.25 | ||||
| CsA +/− others | 1.36 (0.57–3.28) p=0.4 | ||||
| Others | 2.05 (1.13–3.73) p=0.02 | ||||
| Pre-transplant IM (No vs. Yes) | 1.35 (0.88–2.06) p=0.17 | 1.43 (0.95–2.14) p=0.087 | 1.05 (0.64–1.72) p=0.85 | 1.02 (0.57–1.85) p=0.94 | |
| CP2 | HLA matching | - | - | ||
| HLA identical sibling | 1.0 (overall P=0.04) | 1.0 (overall p=0.008) | |||
| Well matched unrelated | 1.22 (0.72–2.05) p=0.46 | 1.55 (0.75–3.22) p=0.23 | |||
| Partially matched unrelated | 1.29 (0.76–2.17) p=0.34 | 1.17 (0.55–2.5) p=0.69 | |||
| Mismatched unrelated | 2.43 (1.36–4.35) p=0.003 | 3.40 (1.63–7.11) p=0.001 | |||
| Unknown unrelated | 1.07 (0.46–2.47) p=0.88 | 1.47 (0.52–4.17) p=0.47 | |||
| Donor/Recipient Sex Mismatch | - | - | - | ||
| M/M | 1.0 (overall p=0.039) | ||||
| M/F | 0.75 (0.36–1.59) p=0.45 | ||||
| F/M | 0.41 (0.2–0.86) p=0.02 | ||||
| F/F | 1.29 (0.68–2.45) p=0.43 | ||||
| Pre-transplant IM (No vs. Yes) | 0.98 (0.67–1.42) p=0.9 | 0.92 (0.65–1.30) p=0.62 | 0.89 (0.54–1.46) p=0.63 | 0.85 (0.51–1.42) p=0.55 | |
| BP | Donor/Recipient CMV Match | - | - | - | |
| D+/R+ | 1.0 (overall p=0.01) | ||||
| D+/R− | 5.07 (2.04–12.59) p=0.0005 | ||||
| D−/R+ | 1.94 (1.00–3.76) p=0.049 | ||||
| D−/D− | 1.39 (0.67–2.89) p=0.38 | ||||
| unknown | 2.22 (0.81–6.07) p=0.12 | ||||
| Donor/Recipient Sex Mismatch | - | - | - | ||
| M/M | 1.0 (overall p=0.03) | ||||
| M/F | 0.82 (0.43–1.58) p=0.56 | ||||
| F/M | 2.22 (1.18–4.19) p=0.01 | ||||
| F/F | 1.44 (0.63–3.29) p=0.39 | ||||
| Pre-transplant IM (No vs. Yes) | 1.05 (0.65–1.69) p=0.85 | 0.86 (0.52–1.43) p=0.56 | 1.42 (0.75–2.69) p=0.28 | 0.52 (0.24–1.11) p=0.09 | |
Abbreviations: OS= overall survival, LFS = leukemia-free survival, TRM = transplant-related mortality, aGVHD = acute graft-cersus-host disease, cGVHD = chronic graft-versus-host disease, RR= relative risk, CI= confidence interval, KPS= karnofsky performance status, CI = calcineurin inhibitors, MA = myeloablative, NMA = non-myeloablative, IM= imatinib, M = male donor, F = female, D = donor, R = recipient, MTX= methotrexate
Note: Pre-transplant exposure to imatinib was forced into each model.
DISCUSSION
For patients with CP CML, treatment with IM is associated with improved survival through high rates of sustained cytogenetic and molecular remissions, (16) while the impact of single agent IM on outcomes of advanced phase CML, despite high initial responses, is less impressive. HSCT therefore remains an essential part of the therapeutic armamentarium for patients with advanced phases of CML. Several papers have addressed the effects of pre-transplant IM on outcomes of transplantation, (6–9, 11–13, 17) and the safety profile of IM is now well established for all phases of the disease. Addressing prognostic factors in the IM era for advanced phase CML has been limited by the relatively small number of patients in single center reports. Additionally, outcomes of those patients who respond well to IM and then proceed in remission with allogeneic transplantation are not well-defined. The CIBMTR database offers the advantage of a large number of patients with extensive data which permits multivariate analyses.
Our analysis included 449 CML patients with advanced phase CML and confirmed that pre-transplant IM was not associated with deleterious or beneficial effects on post-transplant outcomes. Among the studies that have reported an impact of pre-transplant IM on post-transplant outcomes, three have included historical controls.(6–8) In a study that included 145 IM+ to 231IM− allograft recipients with CML, Oehler et al (6) reported comparable 3 year OS for the 73 advanced phase IM+ (60 CP2/AP and 13BP) and the 48 IM− (38 CP2/AP and 10 BP). Deininger et al,(8) analyzed 70 CML and 21 Ph+ ALL and compared outcomes to historical controls identified in the EBMT database. Pre-transplant IM did not influence overall survival, progression-free survival or non-relapse mortality, while a trend towards higher relapse mortality and significantly less chronic GVHD was observed in the IM+ group (OR=0.44, p=0.027). Finally, in 30 Philadelphia chromosome positive leukemias including 16 advanced phase CML, outcomes were similar in 48 controls that did not receive prior IM.(7)
Previously reported and well-recognized prognostic indicators such as disease phase, age, donor type, donor/recipient sex mismatch, and time from diagnosis to transplant (18, 19) were found in our analysis to affect post-transplant outcomes in this patient population.. Disappointingly, the outcomes of allografting advanced phase CML remain poor and have not improved over time and with the availability of IM. Indeed, 35–43% of CP2, 26–37% of AP, and 8–16% of BP patients are alive and in remission 3 years post-transplant. However, CP2 patients defined as remission or chronic phase after prior AP or BP, had comparable outcomes to AP and more favorable outcomes than BP patients. Therefore it is possible that newer therapies, and/or more potent tyrosine kinase inhibitors that increase the response rates and the achievement to CP2 may improve outcomes of patients with BP CML. Interestingly, comparable survivals were observed after transplantation using a reduced intensity or a myeloablative conditioning. Our results are comparable to outcomes reported by the EBMT on allogeneic transplantation using reduced intensity conditioning in advanced phase CML.(20)
This study, similar to all registry analyses, has its inherent limitations: it is a retrospective study, with a relatively short follow-up (median 3 years). Additionally, requested data from transplant centers was often incompletely reported: 53% and 68% had no information on prior BP in the IM+ and IM− cohorts. Information on patients identified at conditioning as AP or BP with prior transient remission after treatment with IM was not available. Patients were classified as AP according to the criteria previously defined in the CIBMTR case report forms and was not done according to the WHO classification. Although a supplemental data questionnaire was sent to transplant centers to determine the reasons patients proceeded with transplantation (planned, or IM resistance), reasons that often led to delays in transplantation in the IM group were unclear. To circumvent this particular limitation, we analyzed the impact of duration of IM therapy prior to transplantation on outcomes and no effects were found. Of interest, information on post-transplant use of IM in patients who did not receive pre-transplant IM was not available, and could potentially have affected our results. Finally, data on the presence of BCR/ABL mutations was unavailable.
This analysis, in our opinion, is still relevant despite the widespread use of imatinib and other tyrosine kinase inhibitors. Indeed, and in contrast to chronic phase CML patients who are more likely to proceed to allogeneic HSCT after IM-resistance and exposure to second generation kinase inhibitors (21, 22), patients presenting at diagnosis with advanced phases of CML usually do so after exposure to front-line IM. This is the approach recommended by the European Leukemia Net (23), and the National Cancer Center Network guidelines (24). This study provides updated outcomes analysis of allografting for these patients. Additionally, one can speculate that with higher remission rates associated with IM as compared to chemotherapy, (5) it is possible, albeit impossible to demonstrate, that a higher fraction of patients reach CP2 after treatment with IM and therefore are able to undergo transplantation and achieve better outcomes than if they had remained in BP.
In conclusion, in this largest cohort of patients with advanced phase CML, CP2 and AP patients had similar outcomes following allogeneic HSCT; whereas outcomes of BP patients, were dismal unless CP2 can be achieved. Conventional prognostic indicators remain the major determinants of transplant outcomes in the IM era. Time from diagnosis to allogeneic HSCT less than 12 months is a modifiable variable that is associated with better outcomes and early planning for transplantation in appropriate patients may be beneficial.
Supplementary Material
ACKNOWLEDGEMENTS
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
AUTHOR DISCLOSURES
Drs. J. Cortes and D.A. Rizzieri have received research funding from Novartis. Dr Rizzieri also received an honorarium from Novartis. Dr Jeffrey Szer received a consultant or advisory role compensation from Novartis.
The authors are grateful for Drs. Manuel M. Abecasis, Joseph Antin, Brian J Bolwell, Matthew Carabasi, Francisco Cervantes, Richard A. Champlin, Edward Copelan, Gregory A. Hale, Jane Liesveld, Mark R. Litzow; Eduardo Olivaria, and Harry C. Schouten, for their thoughtful comments and suggestions.
Footnotes
Disclaimer: Presented as a poster at the Annual Meeting of the CIBMTR in February 2009
REFERENCES
- 1.Talpaz M, Silver RT, Druker BJ, Goldman JM, Gambacorti-Passerini C, Guilhot F, et al. Imatinib induces durable hematologic and cytogenetic responses in patients with accelerated phase chronic myeloid leukemia: results of a phase 2 study. Blood. 2002;99(6):1928–1937. doi: 10.1182/blood.v99.6.1928. [DOI] [PubMed] [Google Scholar]
- 2.Kantarjian HM, O'Brien S, Cortes JE, Smith TL, Rios MB, Shan J, et al. Treatment of philadelphia chromosome-positive, accelerated-phase chronic myelogenous leukemia with imatinib mesylate. Clin Cancer Res. 2002;8(7):2167–2176. [PubMed] [Google Scholar]
- 3.Sureda A, Carrasco M, de Miguel M, Martinez JA, Conde E, Sanz MA, et al. Imatinib mesylate as treatment for blastic transformation of Philadelphia chromosome positive chronic myelogenous leukemia. Haematologica. 2003;88(11):1213–1220. [PubMed] [Google Scholar]
- 4.Sawyers CL, Hochhaus A, Feldman E, Goldman JM, Miller CB, Ottmann OG, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99(10):3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, Cortes J, O'Brien S, Giles FJ, Albitar M, Rios MB, et al. Imatinib mesylate (STI571) therapy for Philadelphia chromosome-positive chronic myelogenous leukemia in blast phase. Blood. 2002;99(10):3547–3553. doi: 10.1182/blood.v99.10.3547. [DOI] [PubMed] [Google Scholar]
- 6.Oehler VG, Gooley T, Snyder DS, Johnston L, Lin A, Cummings CC, et al. The effects of imatinib mesylate treatment before allogeneic transplantation for chronic myeloid leukemia. Blood. 2007;109(4):1782–1789. doi: 10.1182/blood-2006-06-031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perz JB, Khorashad JS, Marin D, Apperley JF, Olavarria E. Imatinib preceding allogeneic stem cell transplantation in chronic myeloid leukemia. Haematologica. 2006;91(8):1145–1146. [PubMed] [Google Scholar]
- 8.Deininger M, Schleuning M, Greinix H, Sayer HG, Fischer T, Martinez J, et al. The effect of prior exposure to imatinib on transplant-related mortality. Haematologica. 2006;91(4):452–459. [PubMed] [Google Scholar]
- 9.Bornhauser M, Kroger N, Schwerdtfeger R, Schafer-Eckart K, Sayer HG, Scheid C, et al. Allogeneic haematopoietic cell transplantation for chronic myelogenous leukaemia in the era of imatinib: a retrospective multicentre study. Eur J Haematol. 2006;76(1):9–17. doi: 10.1111/j.0902-4441.2005.t01-1-EJH2321.x. [DOI] [PubMed] [Google Scholar]
- 10.Zaucha JM, Prejzner W, Giebel S, Gooley TA, Szatkowski D, Kalwak K, et al. Imatinib therapy prior to myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36(5):417–424. doi: 10.1038/sj.bmt.1705087. [DOI] [PubMed] [Google Scholar]
- 11.Shimoni A, Kroger N, Zander AR, Rowe JM, Hardan I, Avigdor A, et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia. 2003;17(2):290–297. doi: 10.1038/sj.leu.2402808. [DOI] [PubMed] [Google Scholar]
- 12.Tiribelli M, Marin L, Calistri E, Geromin A, Damiani D, Fanin R. Imatinib mesylate (Glivec) pre-treatment does not have a negative effect on outcome of allogenic hematopoietic stem cell transplantation in Philadelphia-positive leukemias. Bone Marrow Transplant. 2004;34(9):827–828. doi: 10.1038/sj.bmt.1704687. [DOI] [PubMed] [Google Scholar]
- 13.Wassmann B, Pfeifer H, Scheuring U, Klein SA, Gokbuget N, Binckebanck A, et al. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (SCT) in relapsed or refractory Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL) Leukemia. 2002;16(12):2358–2365. doi: 10.1038/sj.leu.2402770. [DOI] [PubMed] [Google Scholar]
- 14.Saussele S, Lauseker M, Gratwohl A, Beelen DW, Bunjes D, Schwerdtfeger R, et al. Allogeneic hematopoietic stem cell transplantation (allo SCT) for chronic myeloid leukemia in the imatinib era: evaluation of its impact within a subgroup of the randomized German CML Study IV. Blood. 2010;115(10):1880–1885. doi: 10.1182/blood-2009-08-237115. [DOI] [PubMed] [Google Scholar]
- 15.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Druker BJ, Guilhot F, O'Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Kukreja M, Wang T, Giralt SA, Szer J, Arora M, et al. Impact of prior imatinib mesylate on the outcome of hematopoietic cell transplantation for chronic myeloid leukemia. Blood. 2008;112(8):3500–3507. doi: 10.1182/blood-2008-02-141689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gratwohl A, Hermans J, Goldman JM, Arcese W, Carreras E, Devergie A, et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet. 1998;352(9134):1087–1092. doi: 10.1016/s0140-6736(98)03030-x. [DOI] [PubMed] [Google Scholar]
- 19.Passweg JR, Walker I, Sobocinski KA, Klein JP, Horowitz MM, Giralt SA. Validation and extension of the EBMT Risk Score for patients with chronic myeloid leukaemia (CML) receiving allogeneic haematopoietic stem cell transplants. Br J Haematol. 2004;125(5):613–620. doi: 10.1111/j.1365-2141.2004.04955.x. [DOI] [PubMed] [Google Scholar]
- 20.Crawley C, Szydlo R, Lalancette M, Bacigalupo A, Lange A, Brune M, et al. Outcomes of reduced-intensity transplantation for chronic myeloid leukemia: an analysis of prognostic factors from the Chronic Leukemia Working Party of the EBMT. Blood. 2005;106(9):2969–2976. doi: 10.1182/blood-2004-09-3544. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Giles F, Wunderle L, Bhalla K, O'Brien S, Wassmann B, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med. 2006;354(24):2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 22.Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 23.Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27(35):6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. http://www.nccn.org. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.