Abstract
The zebrafish has been proposed as a model organism to study genetic effects influencing behaviour and also as a tool with which the mechanisms of the action of alcohol (ethanol or EtOH) in the vertebrate brain may be investigated. In the current study we exposed zebrafish from two genetically distinct strains (WIK and TU) to a computer animated image of a natural predator of this species, the Indian leaf fish. We measured the subjects’ behavioural responses in the presence of different acute doses of alcohol (0.00, 0.25, 0.50, and 1.00% vol/vol) using an observation based event-recording method. We found fish of both strains to exhibit an atypical predator inspection response during the presentation of the animated predator image coupled with a classical fear response, increased jumping frequency. We found numerous alcohol induced behavioural changes and more importantly also revealed alcohol induced strain dependent changes as well, including different dose-response trajectories for WIK versus TU in predator inspection response, general swimming activity, location of swimming (top vs. bottom half of the tank) and freezing. The results suggest that zebrafish of the TU strain may be more tolerant at least to lower doses of alcohol as compared to WIK. The characterization of strain differences in zebrafish will aid the identification of possible molecular mechanisms involved in alcohol’s actions in the vertebrate brain.
Keywords: Alcohol, ethanol, ethyl alcohol, fear. anxiety, zebrafish, strain comparison
1. Introduction
The widespread acceptance of alcohol (ethanol, ethyl alcohol or EtOH) use may obscure the risks associated with the consumption of this drug of abuse. Alcohol related injuries account for over 35% of motor vehicle accidents and approximately 50% of all acts of violence [1–2]. The deleterious consequences of the use and abuse of alcohol partially stems from the effect of this drug on risk-taking behaviour. For example, acute alcohol exposure has been shown to attenuate fear-responses (defined as the instantaneous reaction towards an imminent threat) in a dose-dependent manner, an effect that has been replicated in model organisms including the zebrafish [3]. Importantly, genetic differences in individual susceptibility towards alcohol’s effects have been demonstrated in humans [4–5] and such differences may offer a way to unravel the biological mechanisms underlying alcohol’s actions in the brain.
Animal species with high genetic homology to humans such as the mouse, rat and nonhuman primates have been successfully applied in the investigation of the biochemical and genetic correlates of alcohol’s effects [6–7]. However, various factors other than evolutionary proximity to humans should also be considered when selecting an animal model [8]. Genetic differences in alcohol induced behavioural responses have been identified in zebrafish and numerous authors have argued that this species may be successfully employed in alcohol research [9, 10].
The zebrafish (Danio rerio) has been argued to be an optimal candidate model organism as it offers a compromise between practical simplicity and system complexity [11]. Zebrafish are highly prolific, resilient and one of the lower order vertebrate species with which complex brain function and behaviour may be studied in the laboratory [12]. Their small size (4cm long) and natural propensity to aggregate in groups make zebrafish easy to keep in the laboratory. Administration of water soluble drugs such as alcohol allows non-invasive drug delivery making this species ideal for behavioural analysis of drug effects [13]. Alcohol is efficiently absorbed by the blood vessels of the gills and skin [14–15], and blood and brain alcohol levels reach equilibrium with the external alcohol concentration within 30–40 min after immersion [9, 16]. Furthermore, complex behaviours such as those indicative of fear can be easily induced and assessed in zebrafish while being exposed to alcohol [3, 13, 15].
Past studies assessing fear responses in zebrafish have employed the use of an automated predator-paradigm in which zebrafish were presented with an animated image of their natural predator and the spontaneous behavioural responses of the subjects to the image were quantified (3, 17–20]. Typical fear responses induced by predator-images include changes in postural and motor patterns such as increased jumping frequency, reduced overall swimming activity, and increased immobility [21]. Although innate, these behaviours can be modified by alcohol in a dose-dependent manner [3]. Zebrafish exposed to higher alcohol concentrations (0.75% or 1.00% v/v ethanol) exhibit reduction of these responses towards aversive stimuli [3, 13, 15]. Previous studies have already revealed significant strain differences in response to acute alcohol exposure [9–10]. However, the TU and WIK strains, two of the most frequently used genetically well defined and highly homozygous strains [22], have not been compared. Furthermore, information on the anxiolytic action of acute alcohol administration in zebrafish is now being compiled [23]. Most studies do not provide a detailed time course analysis of the behavioural changes that occur in the course of acute alcohol administration; despite the fact that alcohol administered acutely is known to have a biphasic temporal effect (initially stimulatory and subsequently depressive) on brain function and behaviour; a trajectory already demonstrated in zebrafish [24]. In the current study we are attempting to address these questions and investigate the effect of acute alcohol administration in a predator-presentation (fear inducing) paradigm on the temporal changes in behaviour of zebrafish from two strains, TU and WIK.
2. Methods
2.1 Animals, Housing and Maintenance
A total of 144 adult zebrafish (Danio rerio) of the Tübingen (TU) and WIK (n = 72 WIK; n= 72 TU) strains were used for this study. Originating from progenitors obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon), experimental fish were bred, raised and housed under controlled conditions in our facility (University of Toronto Mississauga Vivarium, Mississauga ON, Canada). The general maintenance procedures and housing conditions for fry, juveniles and adults were as previously described [25]. Briefly, fish of all stages of development were kept in system water (deionized water supplemented with 60 mg/l Instant Ocean Sea Salt [Big Al’s Pet Store, Mississauga, Ontario, Canada]) whose temperature was maintained by thermostat controlled heaters at 28 C°. The pH of this water was 7.0 and conductivity was 300 μS/cm. Fish were raised from embryo stage to adulthood (4 months old) in 37 litre tanks (25 fish per tank). The fish of the two strains were raised and kept in the same holding room under identical conditions side by side. The water of the holding tanks was oxygenated using an air compressor pumping air via rubber tubing through an air stone, and it was filtered by overhang filters (Emperor 280 Power filter, Marineland, Blacksburg, Virginia, USA), which contained a mechanical filter sponge, a bio-wheel for biological filtration (large surface area with oxygenation to provide optimal growth for beneficial bacteria), and an activated carbon container (chemical filtration). Each tank was coated on the bottom and back surface with white sheets of corrugated plastic, and an artificial plant was placed in the housing tank to match experimental conditions and to reduce novelty during testing. The tanks were illuminated by fluorescent light tubes from the ceiling with lights turned on at 0900 hours and off at 2300 hours. Fish were fed three times a day alternating between a mixture of ground freeze-dried krill and flake food supplemented with live nauplii of brine shrimp (Artemia salina). Behavioural experiments were conducted once the fish reached sexual maturity (approximately 4 months post-fertilization). All methods employed were pre-approved by the Local Animal Care Committee of the University of Toronto Mississauga following guidelines from the Canadian Council on Animal Care.
2.2 Experimental Design
To examine the time-course of zebrafish fear responses and the effect of alcohol on these responses, a predator paradigm was utilized. We employed a 2 × 4 between subject experimental design with 2 strains (TU and WIK) and 4 acute alcohol does (0.00%, 0.25%, 0.50%, and 1.00% v/v). Individual fish from both strains were randomly assigned to one of four acute alcohol exposure treatment groups (n= 18, TU; n= 18, WIK in each group). The testing environment was a 56 L tank measuring 76 cm × 23 cm × 32 cm (length × width × height). This elongated tank was chosen for testing because past studies have shown zebrafish to maintain a distance of approximately 50 cm away from the stimulus screen that showed the aversive predator stimulus [20]. The experimental tank was illuminated by a 15W fluorescent light tube placed above the tank. Both the back and bottom of the test tank were coated with white sheets of corrugated plastic in order to enhance contrast and reduce glare for video tracking analysis. Flat LCD computer monitors (17 in. Samsung SyncMaster 132N) were placed adjacent and parallel to the short side walls of the test tank on both left and right side. Each monitor was connected to a Dell Vostro 1000 laptop running a custom made software application allowing presentation of an animated predator (stimulus). The stimulus presented was a 15 cm long colour photograph (side view) of an Indian leaf fish (a sympatric predator) moving with a velocity of 0.3 cm/sec horizontally across the screen within a range of predetermined heights (between 2cm–15cm measured from the bottom) (see Figure 1). That is, in addition to the horizontal movement, small vertical movements were also added to the image in order to mimic the natural wave actions in the predator’s position induced in the water column [25]. Stimulus side presentation (left or right monitor) was randomized and counterbalanced between treatment groups. The monitor side that did not present the predator image remained a blank (black) screen for the entire trial.
Figure 1.

Image of the predator (Indian leaf fish, Nandus nandus) that was shown during animated predator image presentation.
2.3 Testing Procedure
All tests were conducted between 1000 hours and 2000 hours by an experimenter blind to the treatment group designations. Testing area was divided from the housing area by a black divider (curtain) to minimize the effect of extraneous cues that could influence the behaviour of the experimental fish. A Canon Vixia high definition camera placed in front of the observation tank recorded the behaviour of the experimental zebrafish. Alcohol, in doses corresponding to each treatment group, was added to the experimental tank prior to testing. Behavioural recording began as soon as the fish was introduced into the observation tank. Each recording session lasted 60 minutes, an alcohol immersion period previously determined to be sufficiently long to achieve maximal blood and brain alcohol levels. The stimulus was presented for one minute every five minutes during the recording session, i.e., from minute four to five, minute nine to ten ect. Two identical experimental set ups ran in parallel to increase throughput.
2.4 Quantification of Behaviour
The digital video-recordings were converted into AVI format using iSkysoft video converter and stored on an external hard drive. Files were replayed and analyzed using a manual event recording software application (Observer XT; Noldus, Wageningen, The Netherlands). Motor and posture patterns previously described [18, 20] were quantified. These were as follows Swimming (continuous locomotion activity with the use of the pectoral and caudal fins that does not include contact with the glass or bottom surface of the tank); freezing (a motionless state during which only the gills and eyes may move, occurring only on the bottom of the tank); and jumping (a single forceful leap using the caudal fin). The total duration in seconds of all behavioural measures was analyzed except in the case of jumping, which was measured as number of occurrences (frequency).
In addition to motor and posture patterns, we also measured the location of the experimental fish in the tank. We divided the test tank into two equal virtual horizontal segments, top and bottom as well as into three equal vertical segments, stimulus side (side close to where the stimulus was presented), middle, and the plant side (the side where plant was located, opposite to where the stimulus was shown). Fish were accepted as being in the quadrant once the head of the fish (up to the gill opening) had crossed the imaginary line. To assess location preferences, we calculated the difference between the time spent performing a behaviour near the stimulus versus the plant area of the tank, as well the top versus the bottom area of the tank and calculated these values for one minute intervals of the recording session.
2.5 Statistical Analysis
Data were analyzed using SPSS version 21 for Windows. A repeated measures 3-way ANOVA was conducted with time (60 levels: 60 × 1 minute intervals), strain (2 levels: WIK and TU), and alcohol concentration (4 levels: 0.00%, 0.25%, 0.50%, and 1.00%). This analysis provides an overall assessment of the effects and the interactions among them. However, it does not allow us to compare groups at specific time points since post-hoc multiple comparisons (e.g. the Tukey HSD test), are not appropriate for repeated measures designs. To circumvent this problem, we calculated the average for the one-minute intervals immediately preceding the stimulus presentation (pre-stimulus intervals), the average of one-minute intervals immediately following the stimulus presentation interval (post-stimulus intervals) and the average of the one-minute stimulus presentation intervals. Subsequently, we conducted repeated measures 3-factorial ANOVAs with interval (3 levels: pre-stimulus, stimulus, post-stimulus), strain (2 levels: WIK and TU), and alcohol concentration (4 levels: 0.00%, 0.25%, 0.50%, and 1.00%) and in case of significant main effects of strain or alcohol, or interaction terms, we followed up with Tukey HSD post-hoc multiple comparison tests conducted for each interval (pre-stimulus, stimulus, and post-stimulus) separately. Effects or differences were considered significant if the probability of null hypotheses was less than 5% (p < 0.05). Statistical findings for non-significant results are not detailed.
3. Results
3.1 Swimming
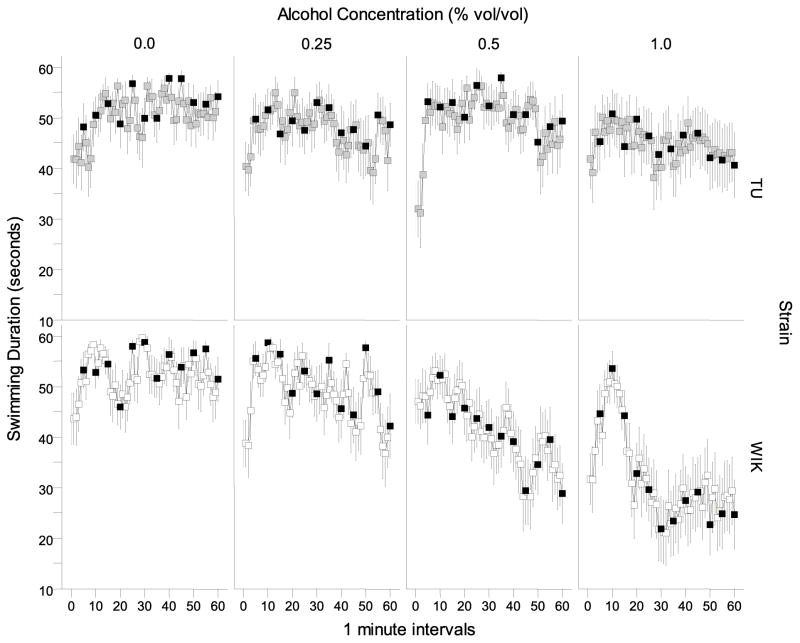
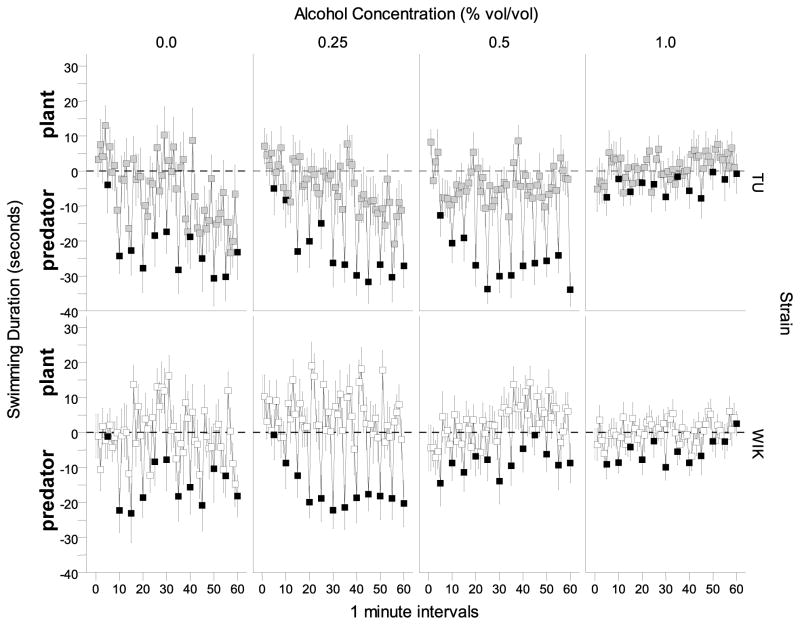
Swimming duration differed between strains and was also affected by alcohol treatment over the course of the 60 minute session (Figure 2). WIK zebrafish showed a dose- and time-dependent decrease in swimming duration compared to TU zebrafish. This observation was confirmed by a repeated measures ANOVA which showed a significant time (F(59, 8024 = 4.915, p < 0.001), strain (F(1, 136) = 4.524, p < 0.05 ) and alcohol concentration effect (F(3, 136) = 6.740, p < 0.001) as well as significant interaction terms time × strain (F(59, 8024) = 2.936, p < 0.001), time × alcohol (F(177, 8024) = 1.513, p < 0.001), and strain × alcohol (F(3, 136) = 2.877, p < 0.05). The three-way interaction (strain × alcohol × time) was non-significant.
Figure 2.
The time course of swimming duration measured over a 60 minute period is dependent on alcohol concentration and strain. Means ± S.E.M. are shown for each 1-minute interval. The values obtained for TU zebrafish during the no stimulus periods are represented by light grey filled squares and the values obtained for WIK zebrafish during the no stimulus periods are represented by the white filled squares. Values obtained for stimulus periods are shown in black.
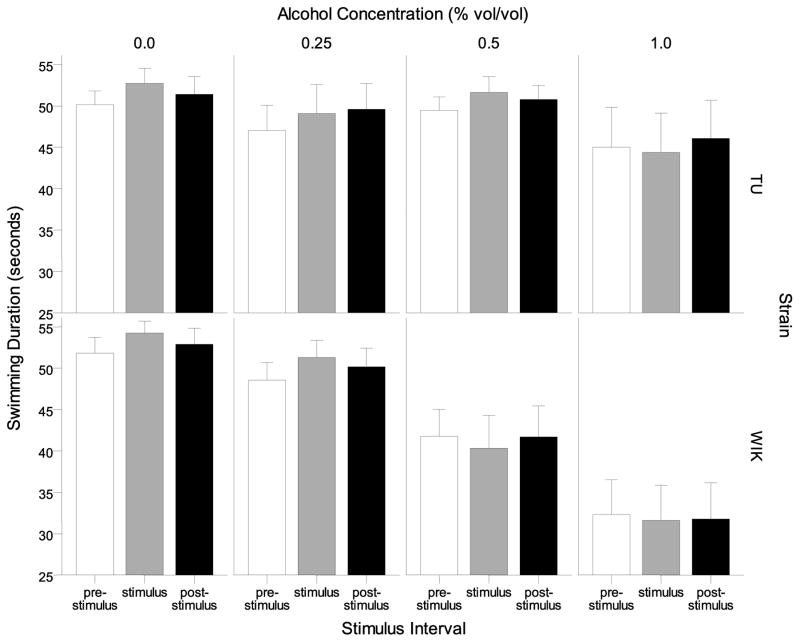
The average swimming duration was also analyzed in intervals prior to the stimulus presentation (pre-stimulus), during the stimulus presentation (stimulus) and after the stimulus presentation (post-stimulus interval) (Figure 3). Swimming duration decreased in all intervals in response to alcohol exposure in a dose-dependent manner in WIK zebrafish, but not in TU zebrafish. A repeated measures ANOVA confirmed this effect and showed a significant interval (F(2, 272) = 7.031, p < 0.01), strain (F(1, 136) = 5.052, p < 0.05), and alcohol effect (F(3, 136) = 7.288, p < 0.001), as well as significant interval × alcohol (F(6, 272) = 2.904, p < 0.01), and strain × alcohol interaction (F(3, 136) = 3.003, p < 0.05). Tukey HSD post-hoc tests conducted separately for each interval revealed that WIK zebrafish in the highest alcohol concentration group (1.00%) spent significantly less time swimming compared to WIK control (0.00%) and the lowest WIK alcohol dose group (0.25%) during all intervals (p<0.05) but no such alcohol related group differences were found in TU for any interval studied (Tukey HSD, p > 0.05).
Figure 3.
Swimming duration during one minute intervals immediately prior to the stimulus presentation (pre-stimulus), during the stimulus (stimulus), and immediately after the stimulus (post-stimulus) are shown for each alcohol concentration and strain. Means ± S.E.M. are shown.
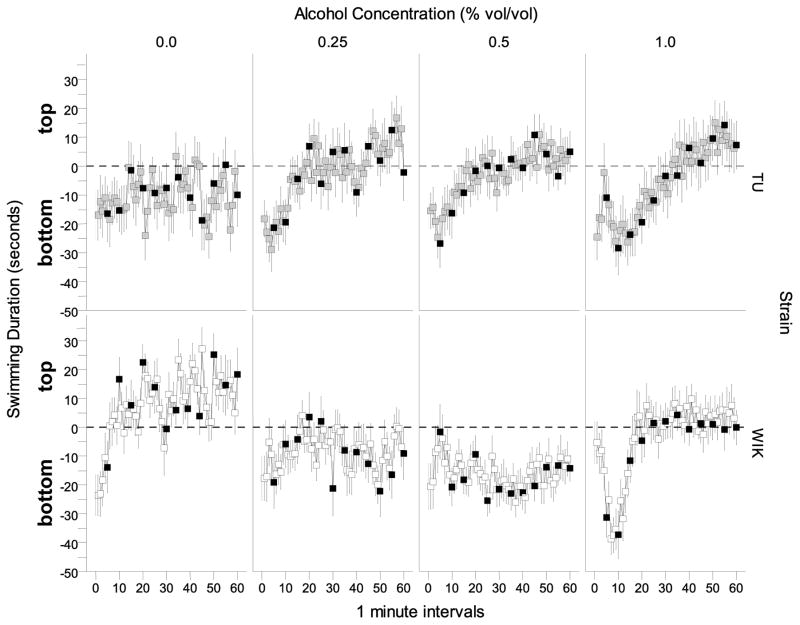
The temporal changes of where the fish swam relative to the bottom of the tank also appeared to be dependent upon the strain origin of the fish as well as on the dose of alcohol they received (Figure 4). WIK untreated with alcohol showed a classic habituation profile. These fish started swimming on the bottom and as time progressed they decreased the time near the bottom, i.e. they moved closer to the surface. In response to alcohol, WIK zebrafish showed an apparently dose-dependent reduction of time spent in the top part of the tank, with the highest alcohol concentration group showing the most interesting temporal trajectory, a rapid increase of bottom dwell time with a subsequent “recovery”. These alcohol effects, however, did not manifest in TU strain zebrafish, which seemed to perform relatively independently of how much alcohol was administered. A repeated measures ANOVA revealed a significant time effect F(59, 8024) = 7.496, p < 0.001, and no strain or alcohol effect (p > 0.05). But all interaction terms were significant (strain × alcohol, F (3, 136) = 4.855, p = 0.003; time × strain, F(59, 8024) = 1.370, p = 0.032; time × alcohol, F(177, 8024) = 1.820, p < 0.001; and time × strain × alcohol, F(177, 8024) = 1.861, p < 0.001).
Figure 4.
The difference between top vs. bottom swimming duration (top duration minus bottom duration) measured over a 60 minute period is dependent on strain and alcohol concentration. Positive values represent greater time swimming near the top compared to bottom and negative values represent greater time swimming near the bottom compared to top for each 1 minute interval. Means ± S.E.M. are shown. Values obtained for TU zebrafish during the no stimulus periods are represented by the light grey squares and values obtained for WIK zebrafish during the no stimulus periods are represented by the white squares. Values obtained for stimulus periods are shown in black. The dashed line represents the middle of the tank. Values above this line show time spent in the top half and values below this line show time spent in the bottom half of the tank.
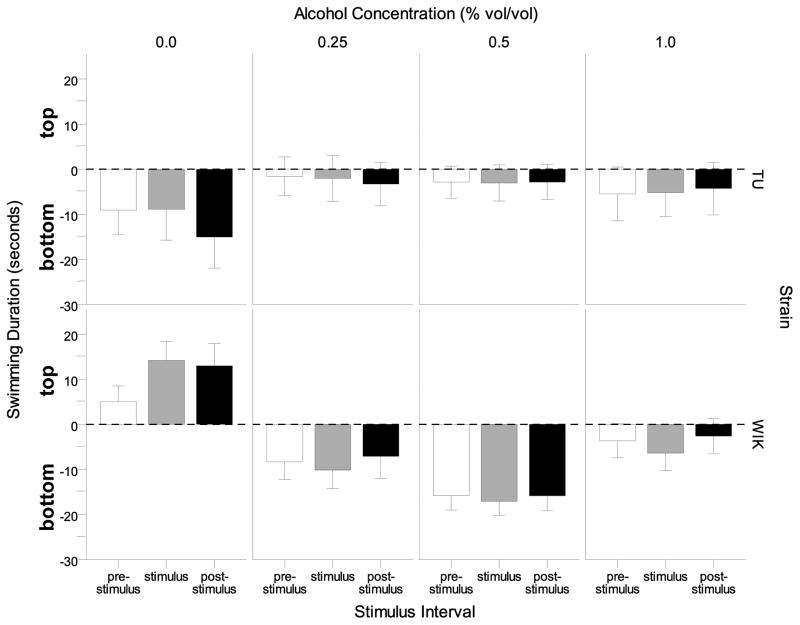
The effect of alcohol on the location of swimming (top vs. bottom) is also confirmed by the analysis of the average swim duration calculated for the pre-stimulus, stimulus and post-stimulus 1-minute long intervals (Figure 5). Acute alcohol exposure robustly reduced the time spent in the top part of the tank in WIK zebrafish with an apparently U-shaped dose response profile, whereas in TU the same alcohol doses had the opposite effect: they reduced the time these fish spent on the bottom part of the tank as compared to control. ANOVA confirmed the significance of interval × strain interaction (F(2, 272) = 4.105, p < 0.05), and also found the interval × alcohol interaction (F(6, 272) = 2.194, p < 0.05), interval × strain × alcohol interaction (F(6, 272) = 2.759, p < 0.05) and strain × alcohol interaction terms (F(3, 136) = 5.747, p < 0.01) to be significant. Tukey post-hoc tests performed for separate intervals revealed that WIK control zebrafish which preferred swimming near the top were significantly (p<0.05) different from all WIK alcohol dose groups during the stimulus period, and WIK control fish were also significantly (p < 0.05) different from the 0.5% alcohol exposed WIK zebrafish during the pre-stimulus and the post-stimulus intervals. However, no significant (Tukey HSD p> 0.05) alcohol related group differences were found in TU for any interval.
Figure 5.
The difference between top vs. bottom (top duration minus bottom duration) swimming duration during intervals prior to the stimulus presentation (pre-stimulus), during the stimulus (stimulus), and after the stimulus (post-stimulus) are shown for each alcohol concentration and strain. Positive values represent greater time swimming on the top compared to bottom and negative values represent greater time swimming on the bottom compared to top for each stimulus interval. Means ± S.E.M. are shown.
Duration of swimming in the predator vs. plant tank area (Figure 6) also varied between strains over time (time effect F(59, 8024) = 12.044, p < 0.001), and the strain origin also had a significant effect (F(1, 136) = 8.174, p < 0.01). The main effect of alcohol was found non-significant so were the interaction terms strain × alcohol and time × strain × alcohol. However, ANOVA did find a significant time × alcohol (F(177, 8024) = 1.993, p < 0.001) and time × strain interaction (F59, 8024) = 1.322, p = 0.05) suggesting that the temporal changes induced by alcohol did not follow the same temporal trajectory in the two strains.
Figure 6.
The difference between duration of swimming on the plant vs. stimulus side of the tank (plant duration minus stimulus duration) measured over a 60 minute period is dependent on strain and alcohol concentration. Positive values represent greater time swimming near the plant and negative values represent greater time swimming near the stimuli for each 1-minute interval. Means ± S.E.M. are shown. Values obtained for TU zebrafish during the no stimulus period are represented by the light grey squares and values obtained for WIK zebrafish during the no stimulus period are represented by the white squares. Values obtained for stimulus periods are shown in black. The dashed line represents the imaginary vertical center line of the tank. Values above the dashed line mean closer to plant and values below the dashed line mean closer to the predator stimulus.
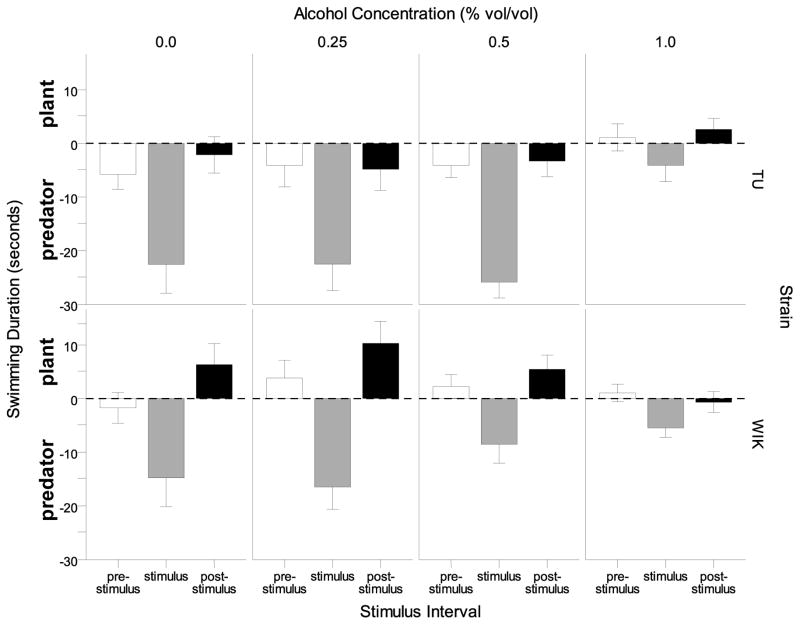
Next we analyzed the averaged intervals, i.e. the average of pre-stimulus, stimulus and post-stimulus 1-minute long intervals respectively and investigated whether fish swam near to or further away from the predator presentation side of the test tank. This analysis revealed an interesting finding (Figure 7). Both TU and WIK control zebrafish swam near the predator side of the tank during the stimulus interval, an unexpected result. Alcohol appeared to decrease preference for the predator side of the tank during stimulus interval in a dose-dependent manner in WIK zebrafish but this effect was found non-significant by a post hoc Tukey HSD test (see below). TU appeared to be unaffected by alcohol treatment, except in the highest concentration (1.00%) group. A repeated measures ANOVA showed a significant interval (F(2, 272) = 133.859, p < 0.001) and strain effect (F(1, 136) = 9.937, p < 0.01), as well as a significant interval × alcohol interaction (F(6, 272) = 6.615, p < 0.001) but the main effect of alcohol and all other interaction terms were found non-significant. Tukey HSD post-hoc tests confirmed that TU zebrafish in the 1.00% alcohol concentration group spent less time swimming near the predator image during the stimulus interval as compared to TU zebrafish at 0.00%, 0.25% and 0.50% (p<0.05) but no significant (p > 0.05) alcohol treatment effects could be found in WIK.
Figure 7.
The difference between plant vs. stimuli (plant duration minus stimuli duration) swimming duration during intervals prior to the stimulus presentation (pre-stimulus), during the stimulus (stimulus), and after the stimulus (post-stimulus) are shown for each alcohol concentration and strain. Positive values represent greater time swimming near the plant and negative values represent greater time swimming near the stimuli for each stimulus interval. Means ± S.E.M. are shown. Note the increased swimming duration near the stimulus side during the stimulus interval, and the reduction after alcohol exposure.
3.2 Freezing
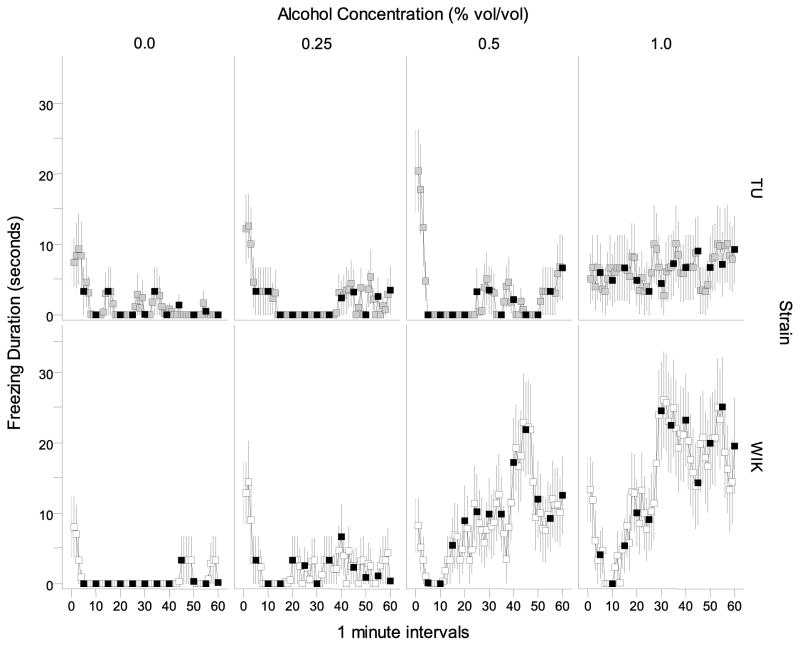
Freezing duration appeared to differ between strains and was also apparently affected by alcohol concentration (Figure 8). A repeated measures ANOVA confirmed these observations and revealed a significant interval (F(59, 8024) = 4.835, p < 0.001), strain (F(1, 136) = 5.831, p < 0.05), and alcohol effect (F(3, 136) = 8.201, p < 0.001), as well as significant interaction terms interval × strain (F(59, 8024) = 3.490, p < 0.001), interval × alcohol (F(177, 8024) = 2.007, p < 0.001), and interval × strain × alcohol (F(177, 8024) = 1.545, p < 0.001).
Figure 8.
Freezing duration measured over a 60 minute period is dependent on alcohol concentration and strain. Means ± S.E.M. are shown for each 1-minute interval. Values obtained for TU zebrafish during the no stimulus period are represented by the light grey squares and values obtained for WIK zebrafish during the no stimulus period are represented by the white squares. Values obtained for stimulus periods are shown in black.
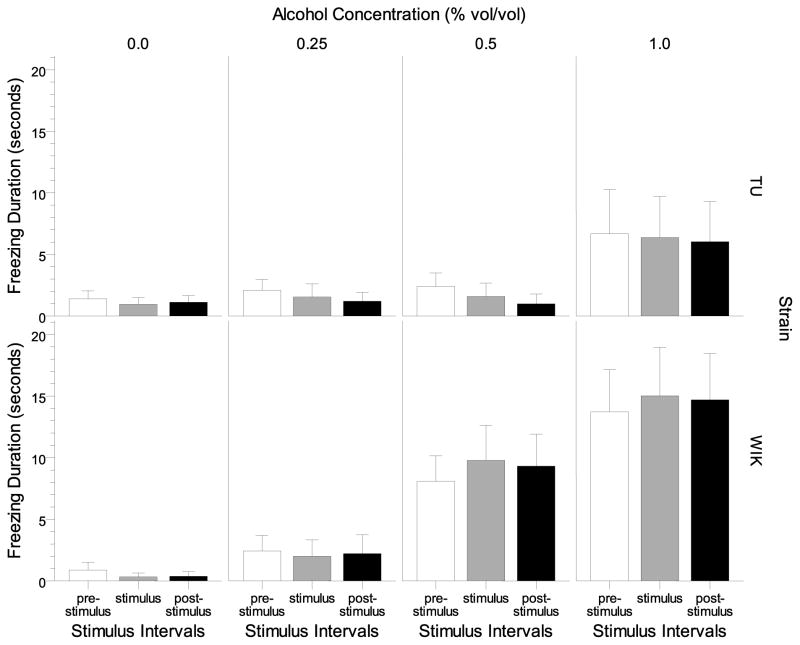
Freezing duration during the one minute pre- and post stimulus as well as stimulus intervals is shown on Figure 9. WIK zebrafish appeared to exhibit a dose-dependent increase in freezing duration in all intervals, whereas TU zebrafish increased freezing duration during all intervals only at the highest alcohol concentration (1.00%). ANOVA found a significant strain (F(1, 136) = 6.829, p < 0.010), and alcohol effect (F(3, 136) = 8.494, p < 0.001), and interval × alcohol interaction (F(2, 272) = 4.851, p = 0.009). Tukey HSD post-hoc tests revealed that WIK exposed to the highest alcohol concentration (1.00%) spent significantly more time freezing compared to WIK in the two lowest alcohol concentration groups (0.00 and 0.25%) in all intervals (p < 0.05) whereas the TU control and alcohol treatment groups did not significantly (p > 0.05) differ from each other at any interval.
Figure 9.
Freezing duration during intervals prior to the stimulus presentation (pre-stimulus), during the stimulus (stimulus), and after the stimulus (post-stimulus) are shown for each alcohol concentration and strain. Means ± S.E.M. are shown.
3.3 Jumping
The time course of the number (frequency) of jumps is shown on Figure 10. A repeated measures ANOVA revealed a dose-dependent decrease in the number of jumps over time in response to acute alcohol exposure in both strains. The effect of time (F(59, 8024) = 10.118, p < 0.001) and of alcohol (F(3, 136) = 5.278, p < 0.01) was found significant but the effect of strain was non-significant. The time × alcohol interaction was found significant (F(177, 8024) = 3.486, p < 0.001) but the other interaction terms (strain × time, strain × time × alcohol) were found non-significant.
Figure 10.
Number of jumps measured over a 60 minute period is dependent on alcohol concentration. Means ± S.E.M. are shown for 1-minute intervals over a 60 minute period. Values obtained for TU zebrafish during the no stimulus period are represented by the light grey squares and values obtained for WIK zebrafish during the no stimulus period are represented by the white squares. Values obtained for stimulus periods are shown in black.
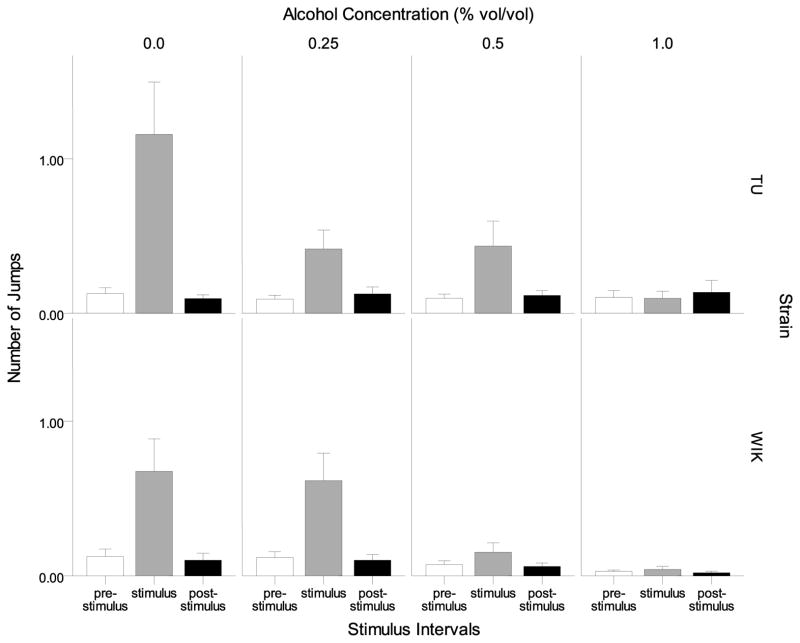
Frequency of jumps was further analyzed with a focus on the pre-stimulus, stimulus and post-stimulus 1 min intervals only (Figure 11). Both TU and WIK zebrafish increased frequency of jumps specifically during the stimulus interval and this stimulus effect appeared to be attenuated in fish exposed to acute alcohol. A repeated measures ANOVA confirmed these observations and found a significant interval effect (F(2, 272) = 40.728, p < 0.001), alcohol effect (F(3, 136) = 6.523, p < 0.001), interval × alcohol interaction (F(6, 272) = 9.626, p < 0.001) but no significant strain effect. The other interaction terms were also found non-significant. Tukey HSD post-hoc tests revealed significant differences among the alcohol groups during the stimulus interval: control zebrafish were found to perform significantly more jumps (p < 0.05) than zebrafish at highest concentration while the differences among the alcohol treatment groups did not reach statistical significance (p > 0.05).
Figure 11.
Number of jumps during intervals prior to the stimulus presentation (pre-stimulus), during the stimulus (stimulus), and after the stimulus (post-stimulus) is shown for each alcohol concentration and strain. Means ± S.E.M. are shown. Note the increased number of jumps during the stimulus interval, and the reduction after alcohol exposure.
4. Discussion
The time course of changes induced by acute exposure to alcohol differed between the two zebrafish strains (TU and WIK) in several behaviours studied here. Strain differences in behavioural responses to alcohol treatment have been demonstrated in zebrafish before (9, 10, 13, 14). However, our study is the first to provide a detailed time-course analysis of acute alcohol effects in the context of strain comparison and it is also the first study in which the TU and WIK strains were compared. Fish of these two strains were bred, raised and maintained under identical environmental conditions in the same vivarium room of our facility. The experiments with them were conducted in a blind and fully randomized manner using the exact same procedures. Therefore, we conclude that the differences between these two strains identified in the current study are due to genetic factors.
We found fish of the two strains to exhibit similar overall locomotor activity under baseline (no alcohol) conditions. However, when exposed to alcohol acutely, WIK zebrafish exhibited a dose-dependent reduction of swimming activity whereas TU remained largely unaffected. Particularly interesting is the time-course of activity changes in WIK zebrafish exposed to the highest concentration (1%) of alcohol. These fish showed an initial increase of activity followed by a rapid decline, a temporal trajectory that is in line with the known bi-phasic (stimulatory followed by depressive) effects of alcohol already replicated in zebrafish [24]. The rising phase (stimulation) of the trajectory is likely due to the rapidly increasing, but still low, levels of blood/brain alcohol [9, 15], while the declining phase may represent the motor impairing effects of the high blood/brain alcohol levels that are achieved once these reach an equilibrium with the external bath concentration of alcohol [15, 26]. Locomotor activity (quantified by swim duration here) is often used as a measure of alcohol’s stimulatory or depressive effects in mammals including humans [e.g. 1, 24 and references therein].
The detailed time-course analysis also proved to be informative in other behavioural measures. For example, the temporal changes in the location of swimming (top vs. bottom part of the tank) revealed a typical habituation response in the control WIK zebrafish. These fish started out spending more time on the bottom but after the first 10 minutes of having been in the novel tank they reached a plateau and remained closer to the top part of the tank. The initially increased bottom dwell time has been described in the literature as the “diving response”, a fear response that has been seen in novel tanks [25, 27–29]. Our time course analysis now shows that this response dissipates within as short a time as 10 min in WIK zebrafish. Exposure to alcohol changed this response. It diminished the temporal changes in the two intermediate dose groups in WIK (making these fish stay closer to the bottom at later periods of the session) and led to a biphasic response with an initial rapid increase of bottom dwell time followed by a fast return to the previous mid-layer location in the highest (1%) alcohol concentration group, a trajectory often observed in mammalian species including humans [1, 6, 21, 24 and references therein]. Interestingly, TU fish did not show these alcohol effects and exhibited a trajectory that resembled the typical habituation time course (initial closeness to the bottom, later moving closer to the top) even when these fish were exposed to alcohol.
However, we note that the temporal trajectories of behavioural responses shown here must be interpreted carefully. These trajectories are influenced by at least two main factors, both of which change with time. The first one, as already mentioned, is habituation to novelty. The second one is the actual amount of alcohol present in the brain, which depends upon the speed of absorption and the characteristics of distribution of this substance in the body of the fish (metabolism and excretion may play lesser roles in case of continuous bath application as is the case here). Briefly, alcohol levels have been shown to reach a plateau in the brain (brain tissue with blood included) after continuous immersion in the solution for 30–40 min at about 50–60% of the external concentration [9]. This finding coincides with the time course of neurochemical changes [30] and of behavioural responses [24]. The time course of behavioural responses reported here likely reflects the combination of the effects of habituation to novelty and the increasing level of alcohol in the brain during the first 30–40 min of acute exposure to this substance. For example, this combined effect is likely to be behind the temporal trajectories we obtained for freezing. Freezing is a natural (innate) response to fear and pain in multiple species including the zebrafish, and alcohol has been found to significantly affect this behavioural response in an apparently similar manner in a variety of species from fish to human arguably due to the anxiety altering effects of this substance [21 and references therein]. However, freezing may also reflect motor impairment, another known effect of alcohol. Perusal of figure 9 shows that most zebrafish groups of the current study started out with a relatively high level of freezing, which we interpret as being due to novelty induced fear. As this response dissipated due to habituation to the test tank, freezing gradually decreased and reached minimal levels after about 10 min in the test tank. However, within 15–20 min after having been immersed in the alcohol solution, a measurable amount of alcohol is expected to be in the brain of the test fish [9, 30] and motor impairment may start to manifest. This may explain the rising freezing levels obtained especially in the later parts of the session in the fish exposed to higher alcohol concentrations. It is also notable, that again TU zebrafish were less affected by alcohol as compared to WIK zebrafish.
Although no strain differences were found in distance from the predator stimulus, the findings were somewhat surprising for another reason. Fish of both strains showed a preference for, and not avoidance of, the side of the tank where the predator stimulus was shown. At this point we do not have a clear explanation for this puzzling finding. In the past, we found zebrafish of the AB strain to exhibit robust antipredatory responses towards the animated image of the Indian leaf fish, the same fear inducing stimulus employed here [3, 8, 10, 20] but an outbred wild-type zebrafish population, SF, did not show the response [15, 10]. The antipredatory responses to the predator image included increased jumping frequency as well as avoidance of, i.e. increased distance from the stimulus; although the latter was context (tank size) dependent [10, 20]. We have never observed preference for this stimulus. The explanation for this finding is speculative at this point but demonstrates potential genetic differences between TU/WIK and AB and SF in their response to predators. Nevertheless, prior studies have shown that under certain circumstances prey fish can explore their fish predator, which manifests as approach of the predator ]31, 32] This response has been termed “predator inspection” and has been argued to potentially serve important adaptive functions [31]. Although counterintuitive, predator inspection is believed to allow the prey to determine the status of the predator, and, in case of a satiated predator, such inspection may allow the prey to avoid having to perform energetically costly and time consuming antipredatory responses. It is possible that the paradoxical preference for the predator image shown by TU and WIK zebrafish of the current study reflects such predator inspection behaviour. The latter conclusion is indirectly supported by the detailed time course analysis of the horizontal location of the test fish (i.e. whether they were closer to the predator vs. plant side of the tank). This analysis revealed that zebrafish of both strains regularly shuttled between the sides and thus instead of showing a clear choice or “preference” for either side, they explored or “inspected” the predator and subsequently moved away from that side to a safer distance. It is also notable that fish in the higher alcohol concentration groups showed less of this shuttling activity, which may have been due to impaired motor function and/or reduced fear.
The last behaviour we consider is jumping frequency. This response has been found to be perhaps the most reliable reaction elicited by the sight of the sympatric predator of zebrafish, the Indian leaf fish, and thus it has been interpreted as a sign of fear [21, 25]. In the current study both WIK and TU zebrafish showed a robust increase of the number of jumps when the stimulus was being shown, a response that appeared to be attenuated in the alcohol groups in a dose dependent manner. This attenuation may be due to alcohol’s anxiolytic properties that have been identified in a variety of species including fish and humans [21 and references therein] but again, we cannot exclude motor impairing effects of higher doses of alcohol having an impact on this behaviour. We also note that the current study was not powered to investigate sex effects. Although alcohol related sex differences have not been identified in zebrafish, a previous study did detect sex differences in swimming activity and its habituation in zebrafish [33], therefore the possibility of alcohol related sex differences and potential strain × sex × alcohol interaction will be investigated in the future.
The above summary of behavioural effects of alcohol shows that not all of them revealed strain differences. This is notable. We do not know the possible mechanisms that may underlie the differences we observed between the two zebrafish strains WIK and TU. However, we suggest these differences are not likely due to simpler physiological protection from the effects of alcohol but are more likely due to central nervous system function-related processes. For example, one could argue that TU may posses more efficient alcohol-dehydrogenase enzymes and thus can detoxify alcohol faster, which then may manifest as reduced responsivity to acute alcohol treatment. Similarly, one could imagine that TU does not absorb alcohol or somehow has a more protective blood brain barrier and thus its brain tissue receives less alcohol than that of WIK. We find these speculations unlikely because continued (60 min long) immersion to alcohol solution likely would overwhelm such differences even if present. Also importantly, the fact that not all behaviours showed strain dependent alcohol responses, and in some behaviours TU was found just as responsive to alcohol as WIK, suggest that the differential sensitivity to alcohol seen in some behaviours was unlikely to be due to a general alcohol protective mechanism in which the two strains might have differed.
Irrespective of the exact mechanism, finding significant strain differences in alcohol related behavioural responses is an important first step. It may allow one to start the mechanistic analysis of the actions of alcohol in the vertebrate brain. For example, a quantitative trait locus analysis conducted using F2 segregating generations of TU × WIK hybrids could lead one to the identification of underlying genes, which in turn may allow one to unravel the biochemical mechanisms underlying the acute effects of alcohol, the ultimate goal of the current study.
Highlights.
Behavioural responses to acute alcohol exposure were compared between two strains
A predator stimulus induced unexpected inspection response
Significant alcohol dose and strain dependent effects were found
Some alcohol effects were interpreted as anxiolytic and/or sedative
TU zebrafish were tolerant to alcohol compared to WIK zebrafish
Acknowledgments
Supported by NIH/NIAAA R01 grant to RG. We would like to thank Yohaan Fernandes and Michele Taffs for their intellectual contribution and technical support throughout the duration of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sullivan E, Harris A, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Research and Health. 2011;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. World Health Statistics 2007. World Health Organization; Geneva: 2007. [Google Scholar]
- 3.Luca RM, Gerlai R. Animated bird silhouette above the tank: Acute alcohol diminishes fear responses in zebrafish. Behavioural brain Research. 2012;229:194–201. doi: 10.1016/j.bbr.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conrod P, Pihl R, Vassileva J. Differential sensitivity to alcohol reinforcement in groups of men at risk for distinct alcoholism subtypes. Alcoholism: Clinical and Experimental Research. 1998;22(3):585–597. doi: 10.1111/j.1530-0277.1998.tb04297.x. [DOI] [PubMed] [Google Scholar]
- 5.Cotton N. The familial incidence of alcoholism. Journal of Studies on Alcohol. 1979;40:89–116. doi: 10.15288/jsa.1979.40.89. [DOI] [PubMed] [Google Scholar]
- 6.Tabakoff B, Hoffman PL. Animal models in alcohol research. Alcohol Research Health. 2000;24:77–84. [PMC free article] [PubMed] [Google Scholar]
- 7.Crabbe J, Belknap J, Buck K. Genetic animal models of alcohol and drug abuse. Science. 1994;264(5166):1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- 8.Ferrnando A, Robbins T. Animal Models of Neuropsychiatric Disorders. Annual Review Clinical Psychology. 2011;7:39–61. doi: 10.1146/annurev-clinpsy-032210-104454. [DOI] [PubMed] [Google Scholar]
- 9.Dlugos C, Rabin R. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacology, Biochemistry, and Behavior. 2003;74(2):471–480. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- 10.Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and chronic alcohol dose: population differences in behaviour and neurochemistry of zebrafish. Genes, Brain, and Behaviour. 2009;8:586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saverino C, Gerlai R. The social zebrafish: behavioural responses to conspecifics, heterospecific, and computer animated fish. Behavioural Brain Research. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlai R. Using Zebrafish to Unravel the Genetics of Complex Brain Disorders. Curr Top Behavioural Neuroscience. 2012;12:3–24. doi: 10.1007/7854_2011_180. [DOI] [PubMed] [Google Scholar]
- 13.Gerlai R, Ahmad F, Prajapati S. Differences in Acute Alcohol-Induced Behavioral Responses Among Zebrafish Populations. Alcoholism: Clinical & Experimental Research. 2008;32(10):1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockwood B, Bjerke S, Kobayashi K, Guo S. Acute effects of alcohol on larval zebrafish: a genetic system for large-scale screening. Pharmacology, Biochemistry, Behaviour. 2004;77:647–654. doi: 10.1016/j.pbb.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Gerlai R, Lahav M, Guo S, Rosenthall A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacology Biochemistry and Behavior. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 16.Ryback R, Percarpio B, Vitale J. Equilibration and Metabolism of Ethanol in the Goldfish. Nature. 1969;222:1068–1070. doi: 10.1038/2221068a0. [DOI] [PubMed] [Google Scholar]
- 17.Blaser R, Gerlai R. Behavioral phenotyping in zebrafish: comparison of three behavioral quantification methods. Behavioral Research Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 18.Luca R, Gerlai R. In search of optimal fear inducing stimuli: Differential behavioral responses to computer animated images in zebrafish. Behavioural Brain Research. 2012;226:66–76. doi: 10.1016/j.bbr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed T, Fernandes Y, Gerlai R. Effects of animated images of sympatric predators and abstract shapes on fear responses in zebrafish. Behaviour. 2012;149:1125–1153. [Google Scholar]
- 20.Ahmed O, Segui D, Gerlai R. An automated predator avoidance task in zebrafish. Behavioural Brain Research. 2011;216:166–171. doi: 10.1016/j.bbr.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlai R. Zebrafish antipredatory response: A future for translational research? Behavioural Brain Research. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guryev V. Genetic variation in the zebrafish. Genome Research. 2006;4:491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cianca V, Bartolini T, Porfiri M, Macri S. A robotics-based behavioural paradigm to measure anxiety-related responses in zebrafish. PLos One. 8:e69661. doi: 10.1371/journal.pone.0069661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran S, Gerlai R. Time-course of behavioural changes induced by ethanol in zebrafish (Danio rerio) Behavioural Brain Research. 2013;252:204–213. doi: 10.1016/j.bbr.2013.05.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerlai R, Fernandes Y, Pereira T. Zebrafish ( Danio rerio) repsonds to the animated image of predator: Towards the development of an automated aversive tank. Behavioural Brain Research. 2009;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosemberg DB, Braga MM, Rico EP, Loss CM, Cordova SD, Mssulini BHM, et al. Behavioural effects of taurine pretreatment in zebrafish acutely exposed to ethanol. Neuropharmacology. 2012;63:613–623. doi: 10.1016/j.neuropharm.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 27.Levin ED, Benca Z, Ceruitti DT. Anxiolytic effects of nicotine in zebrafish. Physiology & Behaviour. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 28.Cachat J, Stewart A, Grossman L, Gaikwad S, Kadri F, Chung KM, et al. Measuring behavioural and endocrine responses to novelty stress in zebrafish. Nature Protocols. 2011;5:1786–99. doi: 10.1038/nprot.2010.140. [DOI] [PubMed] [Google Scholar]
- 29.Wong K, Elegante M, Bartels B, Elkhayat S, Tien D, Roy S, et al. Analyzing habituation responses to novelty in zebrafish (Daniorerio) Behavioural Brain Research. 2010;208:450–457. doi: 10.1016/j.bbr.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 30.Chatterjee D, Gerlai R. High precision liquid chromatography analysis of dopaminergic and serotoninergic responses to acute alcohol exposure. Behavioural Brain Research. 2009;200:208–213. doi: 10.1016/j.bbr.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dugatkin LA, McCall MA, Gregg RG, Cavanaugh A, Christensen C, Unseld M. Zebrafish (Danio rerio) exhibit individual differences in risk-taking behaviour during predator inspection. Ethology Ecology & Evolution. 2005;17:77–81. [Google Scholar]
- 32.Gerlai R. Can paradise fish (Macropodus opercularis) recognize its natural predator? An ethological analysis Ethology. 1993;94:127–136. [Google Scholar]
- 33.Tran S, Gerlai R. Individual differences in activity levels in zebrafish (Danio rerio) Behav Brain Res. 2013;257:224–229. doi: 10.1016/j.bbr.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]