Abstract
Although cancer and aging have been studied as independent diseases, mounting evidence suggest that cancer is an aging-associated disease and that cancer and aging share many molecular pathways. In particular, recent studies validated telomerase activation as a potential therapeutic target for age-related diseases, and at the same time, abnormal telomerase expression and telomerase mutations have been associated with many different types of human tumors. Here, we revisit the connection of telomerase to cancer and aging in light of recent findings supporting a role for telomerase not only in telomere elongation, but also in metabolic fitness and Wnt activation. Understanding the physiological impact of telomerase regulation is fundamental considering the therapeutic strategies that are being developed involving telomerase modulation.
Keywords: Telomerase, aging, cancer
Telomerase defects may lead to aging and cancer
Telomeres are repetitive DNA sequences at chromosome ends that are bound by a protective protein complex known as shelterin, which prevents them from eliciting a DNA damage response (DDR) 1, 2. Seminal studies have shown that telomeres shorten with each cell division due in part to the end-replication problem, an inability of the DNA replication machinery to fully replicate DNA ends 3-6. This is paralleled by the silencing of telomerase, a reverse-transcriptase responsible for de novo telomere extension in most adult tissues. Some adult cell types, such as adult stem cells, have the ability to activate telomerase, particularly in the transient amplifying compartments 6. Nevertheless, telomerase expression in stem cells is not sufficient to prevent progressive telomere shortening associated with increasing age 7.
The first connection linking telomere length to the aging process came from the observation that human primary fibroblasts had shorter telomeres with increasing donor age and that when telomeres reached a critically short length they resulted in loss of proliferative ability, a terminal condition for cells known as replicative-senescence 8. It is now thought that senescence, either triggered by telomere shortening or by other non-telomere related pathways, is a key cellular outcome which may contribute to the aging process, as well as act as a barrier for tumor progression 9. In particular, telomere shortening and increased numbers of senescent cells have been found to occur in both proliferative and non-proliferative tissues as they age 10-12. The importance of cellular senescence in the aging process was recently demonstrated by depletion of senescent cells in the context of an adult organism, the BubR1 progeroid mouse model, which rescued tissue dysfunction and increased organismal health-span (of note, BubR1 mice present an unusually high level of senescent cells and so may not be completely reflective of the natural aging process) 13. In a similar manner, telomerase activation strategies have been recently shown to prevent telomere shortening associated with aging, delay organismal aging, and increase both healthspan and longevity 14, 15.
The anti-aging role of telomerase has been demonstrated to be largely mediated by its canonical role in elongating telomeres, which prevents the accumulation of critically short telomeres and loss of tissue homeostasis 14, 15. In particular, telomere shortening in the context of adult stem cell compartments, has been previously demonstrated to cause severe impairment of stem cell mobilization and a subsequent defect in the ability to regenerate tissues 16, a situation that is similar to that of the so-called human telomere syndromes 17, 18. This is because short/unprotected chromosome ends are recognized as persistent/non-repairable DNA breaks triggering persistent DDR 18-20, as well as cellular senescence or apoptosis mediated by the p53 pathway.
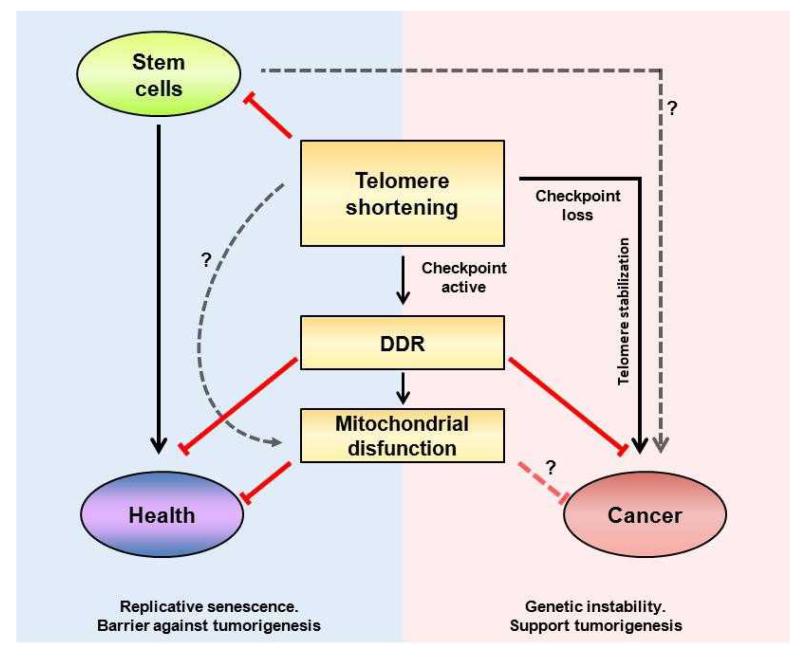
Short telomeres, and subsequent DDR activation, could occur both in cancer and aging (Fig. 1). On one hand, increased abundance of short telomeres correlates with higher genomic instability and decreased longevity in various organisms, including mice, zebrafish, and yeast 21-24. In particular, mice deficient for telomerase or for telomere binding proteins are characterized by accelerated age-related defects 14, 16, 18, 19, 21, 22, 25-32 with the load of telomere dysfunction correlating with the lifespan of mice 33. In humans, short telomeres are considered good indicators of an individual’s health status and correlate with both genetic and environmental factors 18, 34-37. Although recent findings strongly support the idea that short telomeres drive several age-related diseases 38 we cannot exclude the possibility that in some situations short telomeres may be a consequence of the disease itself.
Figure 1. Short telomeres in aging and cancer.
Major pathways affected by short telomeres and their impact on aging or cancer. DNA damage and tumor suppressor activity have been shown to impact tissue decline and aging. When DNA damage checkpoints are bypassed, cells with short telomeres could potentially progress to cancer. The role of stem cells with short telomeres in cancer and whether short telomeres could modulate other pathways independently of p53 (such as mitochondrial dysfunction) remains unknown.
Although tumors may arise from cells with short telomeres and chromosomal instability, telomerase activation and telomere maintenance are requisites for the progression of most human tumor types 39-49. Further linking TERT (telomerase reverse transcriptase, the human telomerase) to cancer are GWAS results showing correlations between particular SNP variants on the 5p15.33 bin (which includes TERT) and a higher cancer risk 21, 50-58. In particular, genetic variants in telomerase-associated genes and in the TERT-CLPTM1L locus are associated with different cancer types 50, 59-65. Although the mechanism by which these variants interfere with telomerase levels/activity is mostly unknown, there are indications that the variants may lead to an increase in the gradual shortening of telomeres over time 52, 59, but these results still need to be confirmed 66. On the other hand, two recent studies linked melanoma risk to promoter mutations in the TERT gene associated with increased transcriptional activity of the TERT promoter 67, 68, demonstrating the importance of tightly controlled telomerase expression.
Like cancer, aging encompasses a spectrum of cellular and molecular changes, but in the case of aging, these eventually result in loss of regenerative capacity and tissue dysfunction, either through loss of functional cells or through the accumulation of surviving aberrantly damaged cells, which could result in the appearance of neoplasias. In this review we focus on aging associated with telomere shortening, and on how telomerase could be an important therapeutic target for this process. To support the dual role of telomerase in aging and cancer we highlight recent studies that have demonstrated that expression of telomerase in aged organisms is a valuable tool to counteract tissue degeneration through the protection of short telomeres 69, envisioning that controlled telomerase activation under particular settings may delay age-related tumorigenesis.
Telomerase as a key factor that regulates aging
Evolution has developed different barriers against cancer amongst different species. These barriers are related to the ability to cope with DNA damage and the prevention of the accumulation of damaged cells and tissues. Irrespective of its source, damage acts as the basis for the development of dysfunctional tissues, which are a hallmark of age decline as well as the basis for cancer 69, 70.
Patients carrying mutations in genes crucial for telomere maintenance show accelerated aging phenotypes. Such is the case for patients carrying mutations in TERT, TERC or other telomere maintenance genes, which lead to an accelerated aging syndrome known as dyskeratosis congenita (DC) 71. DC encompasses a spectrum of pathologies including abnormal skin pigmentation, nail dystrophy, leukoplakia and pancytopenia 72. In patients carrying mutations in TERT and TERC, the severity of pathologies correlates with the abundance of short telomeres, so the onset of disease is anticipated with increasing generations (a phenomenon known as “genetic anticipation”) 73. Interestingly, human telomere syndromes closely recapitulate the phenotypes of previously generated mouse models for telomerase deficiency. In particular, mice genetically deficient for telomerase or some of the telomere-binding proteins present a plethora of pathologies generally characterized by the loss of tissue regeneration and organ function 32, 74. In addition to the defects in the highly proliferative tissues such as the bone marrow or the skin, mice and humans with telomerase deficiency also present pathologies in more quiescent tissues, such as cardiomyopathy, insulin resistance, and lung and liver fibrosis 75, 76. To date it remains unknown how telomerase deficiency also leads to short telomeres in tissues with a lower proliferative potential 77, 78. In this regard, mitochondrial dysfunction has been recently reported in quiescent tissues (such as the heart and liver) in the context of telomerase deficiency in mice. Several reports described that mt-TERT (TERT that localizes at mitochondria) improves mitochondrial function and protects from oxidative stress 79-81. In particular, telomerase deficient mice that have been bred for several generations and have an increased abundance of short telomeres present a marked mitochondrial compromise triggered by the suppression of the peroxisome proliferator-activated receptor gamma, coactivator 1 alpha and beta (PGC1α and PGC1β) networks which control, amongst other processes, mitochondrial function and oxidative defense 82. Interestingly, this connection between telomere dysfunction and mitochondrial dysfunction is mediated by p53, a common checkpoint to telomere syndromes 83. Additionally, mitochondrial dysfunction in quiescent tissues of telomerase-deficient mice could be initiated by pathways independent of p53 83. Of note, mitochondrial defects have been described in the first generation of TERT KO mice (G1) 82, when telomere length is still conserved, demonstrating that mitochondrial dysfunction could, at least partially, precede or parallel telomere shortening. It has also been recently demonstrated that mitochondrial dysfunction is associated with physiological mouse aging, and reverted by telomerase activation 15, 82.
With the aim of dissecting the role of telomerase activity and telomere length in cancer and aging, various mouse models for telomerase over-expression have been generated (table 1). Transgenic mice that carry the mouse TERT gene under the control of the keratin 5 promoter (K5-mTERT referred hereafter as TgTERT) show increased tissue fitness, however, owing to an increased incidence of spontaneous tumors, these mice do not show an extended median lifespan 84. To unmask the potential anti-aging role of telomerase, TgTERT mice were crossed with mice carrying extra copies of the tumor suppressors p53, p16 and Arf (Sp16/SArf/Sp53 mice), which were previously reported to be cancer resistant 14. In this context, TgTERT/Sp16/SArf/Sp53 showed improved health span and a 40% increase in median longevity compared to wild-type controls, or 26% comparing with the long-lived and healthy Sp16/SArf/Sp53 mice, demonstrating the anti-aging activity of telomerase. A similar scenario occurred when telomerase overexpression was combined with other cancer-protective conditions, such as by subjecting mice to caloric restriction (CR). In this setting, telomerase overexpression synergized with CR to significantly extend mouse lifespan 85. This synergy between telomerase and tumor resistance in extending organismal longevity seems to be a naturally occurring strategy, such as in the case of the mole rat or other small animals that are positive for telomerase, present higher tumor suppressor barriers 86, 87 and have an unusually increased longevity for their species. Although this synergy could be a strategy in some situations, there are exceptions (such as the American beaver, another long-lived rodent, which has no detectable telomerase activity 88), highlighting the complexity of aging.
Table 1. Outcomes of enforced expression of telomerase in mice.
| Ref | Model / Telomerase activation | Cancer | Aging | Comments |
|---|---|---|---|---|
| 25 |
|
Stratified epithelia histologically normal More tumors after DMBA+TPA treatment. Skin more sensitive to esters. | Increased wound-healing | High levels of telomerase activity in stratified epithelia do not alter the normal epithelium structure and are not associated with changes in p53, Ras or c-Myc levels. |
| 129 |
|
Higher incidence of breast carcinoma in all but 1 female of founder A. No differences in males. | n.d. | No susceptibility to spontaneous or DMBA-induced papillomas in mTERT Tg mice. Enforced mTERT expression did not alter the high rate of spontaneous tumor formation in Ink4a/Arf-deficient mice. |
| 130 |
|
Higher tumor incidence (spontaneous pre- neoplastic and neoplastic lesions in stratified and non- stratified epithelia) | Lower lifespan in both k5-mTERT or k5-mTERT/p53−/− | Loss of p53 results in a dramatic decrease in the life span of these mice, concomitantly with an increased incidence of tumors, in particular lymphomas. |
| 131 |
|
Higher incidence of spontaneous lymphoma. | n.d. | Lck-Tert thymocytes show greater spontaneous and IR-induced chromosomal instability. |
| 84 |
|
More hyperproliferative lesions | Increased maximal lifespan Decreased degenerative lesions (kidney, male germ line) |
|
| 132 |
|
n.d. | Enhancing of hair growth through stem cell mobilization (independently of the TERC component) | |
| 14 |
|
Higher tumor incidence (mainly lymphomas) and similar lifespan (K5-mTERT/Sp53 vs K5-mTERT) | Lower tumor incidence and higher lifespan and health-span in K5-mTERT/Sp53/SArf/Sp16 vs K5-mTERT/Sp53 or WT controls | |
| 89 |
|
Telomerase activation was not sufficient to promote tumorigenesis. | Extended life and health span | Chromosomal instability was referred. |
| 120 |
|
No increase in tumor incidence | Extended health. No differences in lifespan | Activation of telomerase is not direct Other studies have described similar telomerase activators in mice and humans (see references: 127, 133) |
| 82 |
|
n.d. | Ad-mTERT injection partial rescue PGC-1α/β, Glc-6-P and Pepck expression, accompanied by a 30% increase in glucose levels relative to Ad-GFP controls, in G4-TERT−/− mice | |
| 134 |
|
n.d. | Ad-mTERT-GFP led to neurogenesis upregulation, produced antidepressant-like behaviors, and prevented the CMS-induced behavioral modifications | |
| 128 |
|
n.d. | Extended health (neuroprotective effects in NMDA-injected CD-1 mice) | No mechanism of telomerase activation |
| 15 |
|
No increased tumor incidence | Extended life and health span |
More recently, two independent studies demonstrated that telomerase activation either in a mouse model of accelerated-aging (late generation TERT-ER mouse model) or in natural-aged mice (1 and 2 year old wild-type mice) is sufficient to delay aging without increasing cancer incidence 15, 89. These studies support the idea that telomere shortening is one of the molecular mechanisms of cellular aging and lifespan modulation, and more notably, they demonstrate that telomerase reactivation in adult (or aged) organisms has a positive impact in delaying aging, which can be separated from its role in cancer when its aberrantly expressed. Future work should focus on understanding the molecular mechanisms by which telomerase delays aging and disease in different organs and tissues. Below we discuss novel pathways and telomerase partners which could be also involved in these processes.
Telomerase regulation in cancer
The role of telomerase in cancer has been extensively studied. Almost all human cancers present activation of telomerase as a hallmark, most likely as a mechanism to allow unlimited cell proliferation of tumor cells 90. Although telomerase activation can be an early event in cancer, it is not necessary for cancer initiation 91. However, telomerase can stimulate tumor progression by ensuring maintenance of telomeres above a critically short length, thus preventing induction of cellular senescence or apoptosis. Several mechanisms have been reported to activate telomerase in cancer, such as different oncogenes including Myc and Wnt 92-94 which act as transcriptional regulators of telomerase. Additional telomerase activation mechanisms involving alternative splicing or epigenetic alterations have also been described 95. Recently, mutations increasing transcriptional activity of the TERT promoter from generation of de novo consensus binding motifs for E-twenty-six (ETS) transcription factors have been described in human melanomas 67, 68. In addition to the canonical role of telomerase in maintaining telomeres above a critical length, telomerase has also been proposed to regulate other pathways, which could have an impact on cancer growth, such as regulation of Wnt targets and metabolism (82, 96). Getting rid of telomerase can also be problematic; the lack of telomerase could lead to increased chromosomal instability, which in turn could be at the basis for cancer initiation when tumor suppressor barriers are bypassed 97. Indeed, recent evidence demonstrated that short telomeres alone could lead to genomic instability and cancer 98. Thus, the current view is that telomerase deficiency may contribute to the early steps of cancer development by fueling chromosomal instability, while subsequent activation of telomerase may be necessary to allow tumor growth and tumor progression towards more malignant states 99.
Loss of function and gain of function mouse models for telomerase have been instrumental in understanding the role of telomerase in cancer. On one hand, telomerase deficient mice (mTR−/−) are resistant to both induced and spontaneous tumorigenesis 100, except when telomerase deficient mice were crossed with p53+/− or p53−/− 101, 102. In this scenario a switch to epithelial carcinogenesis was observed, consistent with the role of telomere shortening in the pathophysiology of human cancers 103. Short telomeres could be recognized as DNA double strand (dsDNA) breaks, a deleterious DNA aberration that results in a strong activation of DNA damage repair (DDR) pathways. With an intact DDR and active checkpoints, cells with dsDNA breaks activate a multitude of signaling cascades which conclude in p53 and tumor suppressor activation. This cascade of events culminates in activation of anti-proliferation signals. On the other hand, if tumor suppressors or p53 are bypassed, a common characteristic of tumors, chromosome fusions and genomic instability could converge to give rise to cancer. This potential of telomerase to sustain the growth of tumor cells illustrates the importance of telomerase regulation in adult tissues, and probably explains why most adult cells silence telomerase expression.
Given the importance of telomerase to sustain cancer growth, telomerase inhibitors were considered as potential therapies against tumor malignancy. Recent evidence demonstrates, however, that tumors in which telomerase are lost may well activate different pathways to overcome this situation, such as alternative telomere lengthening 104-106.
In addition to the canonical role of telomerase in maintaining telomeres, telomerase overexpression has also been shown to influence the regulation of the Wnt pathway, although the physiological relevance and mechanism of this regulation is still debated 15, 93, 94, 96, 107. Nevertheless, given that telomerase activity is aberrantly overexpressed in some cancers, it is possible that Wnt modulation through higher levels of telomerase could contribute to the phenotype of some neoplasias 108.
Metabolic defects are an important link between cancer and aging. Interestingly, metabolically relevant genes that have been shown to be down-regulated in the presence of short telomeres, such as PGC1α/β, and potentially activated by telomerase re-expression, are also linked to tumor progression 109, 110. Thus, telomerase activation in tumors may also alter cellular metabolism. Further work will be required to refine these complex relationships been telomeres, telomerase and metabolism.
In this regard, transgenic mouse models (e.g., TgTERT mice 14) have shown that constitutive telomerase over-expression throughout mouse development results in a slightly higher incidence of cancer. Interestingly, telomerase over-expression to similar levels but in the context of the adult organism using a gene therapy strategy, showed beneficial effects delaying aging and extending longevity without increased cancer incidence 15. This could be related to the fact that the gene-therapy vectors employed (AAV) lead to a loss of TERT expression in highly proliferating cells or tissues. Another explanation could be that AAV preferentially targets post-mitotic cells, which are potentially more resistant to cancer initiation. Alternatively, although the TgTERT mice are the product of single germline integration, they constitutively express telomerase, independently of the replicative potential of a tissue, most likely facilitating proliferation and expansion of cells carrying pathogenic mutations.
Telomerase in stem cells
Stem cells play an important role in the aging process. Stem cell depletion seems to be at the basis of some diseases and could account for accelerated aging syndromes 111-115. Moreover, conditions that trigger premature aging, such as telomere shortening, also impair the ability of stem cells to regenerate tissues 16. Indeed, cells with the longest telomeres are enriched at adult stem cell niches both in mice and humans, most likely owing to the fact that these cells have the ability to activate telomerase 7, 116. However, physiological telomerase activation in stem cell compartments is not sufficient to maintain overall telomere length with aging, and telomere shortening and DNA damage accumulation is also a characteristic of aged stem cells 117.
Tumors are thought to be sustained by a subpopulation of cells with stem cell-like properties, the so called cancer initiating cells 118, 119. It will be of interest to address whether these cancer-initiating populations also have the ability to maintain telomeres and activate telomerase activity.
Therapies based on telomerase: therapeutic value and future perspectives
As discussed above, telomerase activation is a potential therapeutic strategy for the treatment of age-related diseases 14, 120. In particular, telomerase activation in adult or old mice by means of a gene therapy strategy was shown to be sufficient to improve metabolic fitness, neuromuscular capacity, and prevent bone loss, as well as significantly increase both median and maximum longevity, without increased cancer incidence. The finding that this strategy of telomerase activation does not lead to cancer could be due to the fact that the vectors used (AAV)9121 are non-integrative, thus preventing the expansion of clones with telomerase overexpression 122. Similarly, telomerase expression in an accelerated model of ageing owing to telomere loss (G4TERT-ER model) rescued several age phenotypes 89, and although higher genomic instability was detected, it did not lead to an increase in tumorigenesis. These studies suggest that telomerase expression could be considered a feasible approach to reverse tissue dysfunction and extend healthy lifespan without increasing cancer incidence. Dedicated studies should be performed in the future, using mice at different ages and comparisons at the same age, to assess the safety potential of these strategies. The actual value of these new therapies will reside in their safety, and a detailed understanding of the telomeric and non-telomeric roles of telomerase in tissue-specific healing and cancer will be crucial for considering telomerase for anti-aging therapies.
Whether these promising results could be translated to humans is unknown. It seems hazardous to use the lack of tumorigenesis in mice as evidence for the safety of pro-telomerase therapies in humans, as it is known that telomerase is differentially regulated in these organisms 123, 124. The fact that human longevity is much longer than that of mice could increase the probability of cancer formation favored by an external telomerase treatment. The opposite argument can be made, however, in that humans are much more resistant to cancer than mice and therefore it is less likely that telomerase activation could lead to cancer in humans compared to mice. Even though the peak of telomerase activity in humans occurs at early stages, as it does in mice,, humans almost completely lose telomerase activity from somatic tissues in the adulthood, contrary to mice where telomerase is found in some somatic tissues 125, 126. As a starting point for translating these findings to the clinic, telomerase activation is likely to be first tested for treatment of the so-called telomere syndromes 17. In this scenario the use of tissue specific gene-therapy vectors expressing telomerase could be envisaged as a potential solution. Based on those outcomes, it will be easier to assess the feasibility of expanding telomerase activation as a strategy for combating cancer.
Concluding Remarks
The finding that telomerase plays roles in distinct and complementary circuitries have helped reveal its function in cancer and aging. Indeed, a change of paradigm seems to be occurring in telomerase biology, with a switch from viewing telomerase as fueling cancer to reversing aging. Telomerase expression in a background of high levels of tumor suppressors or in aged organisms seems to prevent its expected pro-cancer activity and yet it still functions as an anti-aging factor. Supporting this notion are novel telomerase activators 120, 127, 128, some of which are commercially available, used as anti-aging supplements. Although much of the recent work provides only proof-of-principle that telomerase works for tissue healing, we cannot dismiss that in the future telomerase expression could be used as a safe approach for certain telomere-diseases 17 or other accelerated aging syndromes.
Acknowledgments
Funding Work at the Blasco lab was funded by Spanish Ministry of Science and Innovation Projects SAF2008-05384 and CSD2007-00017, European Union FP7 Projects 2007-A-201630 (GENICA) and 2007-A-200950 (TELOMARKER), European Research Council Advanced Grant GA#232854, Körber Foundation, Fundación Botín and Fundación Lilly.
Footnotes
Disclosures: MAB is a co-founder of Life Length, S.L. a biotechnology company that commercializes telomere length tests.
References
- 1.McClintock B. The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proc Natl Acad Sci U S A. 1939;25:405–416. doi: 10.1073/pnas.25.8.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muller HJ. The remaking of chromosomes. The Collecting Net. 1938;8:182–195. [Google Scholar]
- 3.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 4.Levy MZ, et al. Telomere end-replication problem and cell aging. J Mol Biol. 1992;225:951–960. doi: 10.1016/0022-2836(92)90096-3. [DOI] [PubMed] [Google Scholar]
- 5.Olovnikov AM. Telomeres, telomerase, and aging: origin of the theory. Exp Gerontol. 1996;31:443–448. doi: 10.1016/0531-5565(96)00005-8. [DOI] [PubMed] [Google Scholar]
- 6.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 7.Flores I, et al. The longest telomeres: a general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jesus BB, Blasco MA. Assessing cell and organ senescence biomarkers. Circ Res. 2012;111:97–109. doi: 10.1161/CIRCRESAHA.111.247866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collado M, Serrano M. The power and the promise of oncogene-induced senescence markers. Nat Rev Cancer. 2006;6:472–476. doi: 10.1038/nrc1884. [DOI] [PubMed] [Google Scholar]
- 10.Jiang H, et al. Telomere shortening and ageing. Z Gerontol Geriatr. 2007;40:314–324. doi: 10.1007/s00391-007-0480-0. [DOI] [PubMed] [Google Scholar]
- 11.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101:17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canela A, et al. High-throughput telomere length quantification by FISH and its application to human population studies. Proc Natl Acad Sci U S A. 2007;104:5300–5305. doi: 10.1073/pnas.0609367104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomas-Loba A, et al. Telomerase reverse transcriptase delays aging in cancer-resistant mice. Cell. 2008;135:609–622. doi: 10.1016/j.cell.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Bernardes de Jesus B, et al. Telomerase gene therapy in adult and old mice delays aging and increases longevity without increasing cancer. EMBO Mol Med. 2012;4:1–14. doi: 10.1002/emmm.201200245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flores I, et al. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 17.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armanios M, et al. Short telomeres are sufficient to cause the degenerative defects associated with aging. Am J Hum Genet. 2009;85:823–832. doi: 10.1016/j.ajhg.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao LY, et al. Short telomeres, even in the presence of telomerase, limit tissue renewal capacity. Cell. 2005;123:1121–1131. doi: 10.1016/j.cell.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 20.Morrish TA, Greider CW. Short telomeres initiate telomere recombination in primary and tumor cells. PLoS Genet. 2009;5:e1000357. doi: 10.1371/journal.pgen.1000357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blasco MA, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 22.Hemann MT, et al. The shortest telomere, not average telomere length, is critical for cell viability and chromosome stability. Cell. 2001;107:67–77. doi: 10.1016/s0092-8674(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 23.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57:633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 24.Henriques CM, et al. Telomerase is required for zebrafish lifespan. PLoS Genet. 2013;9:e1003214. doi: 10.1371/journal.pgen.1003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gonzalez-Suarez E, et al. Increased epidermal tumors and increased skin wound healing in transgenic mice overexpressing the catalytic subunit of telomerase, mTERT, in basal keratinocytes. EMBO J. 2001;20:2619–2630. doi: 10.1093/emboj/20.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martinez P, Blasco MA. Role of shelterin in cancer and aging. Aging Cell. 2010;9:653–666. doi: 10.1111/j.1474-9726.2010.00596.x. [DOI] [PubMed] [Google Scholar]
- 27.Armanios MY, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell JR, et al. A telomerase component is defective in the human disease dyskeratosis congenita. Nature. 1999;402:551–555. doi: 10.1038/990141. [DOI] [PubMed] [Google Scholar]
- 29.Tsakiri KD, et al. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci U S A. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vulliamy T, et al. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature. 2001;413:432–435. doi: 10.1038/35096585. [DOI] [PubMed] [Google Scholar]
- 31.Yamaguchi H, et al. Mutations in TERT, the gene for telomerase reverse transcriptase, in aplastic anemia. N Engl J Med. 2005;352:1413–1424. doi: 10.1056/NEJMoa042980. [DOI] [PubMed] [Google Scholar]
- 32.Martinez P, Blasco MA. Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nat Rev Cancer. 2011;11:161–176. doi: 10.1038/nrc3025. [DOI] [PubMed] [Google Scholar]
- 33.Vera E, et al. The rate of increase of short telomeres predicts longevity in mammals. Cell Rep. 2012;2:732–737. doi: 10.1016/j.celrep.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Alder JK, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Benetos A, et al. Short telomeres are associated with increased carotid atherosclerosis in hypertensive subjects. Hypertension. 2004;43:182–185. doi: 10.1161/01.HYP.0000113081.42868.f4. [DOI] [PubMed] [Google Scholar]
- 36.Cipriano C, et al. Accumulation of cells with short telomeres is associated with impaired zinc homeostasis and inflammation in old hypertensive participants. J Gerontol A Biol Sci Med Sci. 2009;64:745–751. doi: 10.1093/gerona/glp048. [DOI] [PubMed] [Google Scholar]
- 37.Elvsashagen T, et al. The load of short telomeres is increased and associated with lifetime number of depressive episodes in bipolar II disorder. J Affect Disord. 2011;135:43–50. doi: 10.1016/j.jad.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Codd V, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45:422–427. 427e421–422. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chadeneau C, et al. Telomerase activity associated with acquisition of malignancy in human colorectal cancer. Cancer Res. 1995;55:2533–2536. [PubMed] [Google Scholar]
- 40.Tang R, et al. Close correlation between telomerase expression and adenomatous polyp progression in multistep colorectal carcinogenesis. Cancer Res. 1998;58:4052–4054. [PubMed] [Google Scholar]
- 41.Fang DC, et al. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522–526. doi: 10.3748/wjg.v7.i4.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engelhardt M, et al. Telomerase and telomere length in the development and progression of premalignant lesions to colorectal cancer. Clin Cancer Res. 1997;3:1931–1941. [PubMed] [Google Scholar]
- 43.Maruyama Y, et al. Telomere length and telomerase activity in carcinogenesis of the stomach. Jpn J Clin Oncol. 1997;27:216–220. doi: 10.1093/jjco/27.4.216. [DOI] [PubMed] [Google Scholar]
- 44.Lantuejoul S, et al. Telomere shortening and telomerase reverse transcriptase expression in preinvasive bronchial lesions. Clin Cancer Res. 2005;11:2074–2082. doi: 10.1158/1078-0432.CCR-04-1376. [DOI] [PubMed] [Google Scholar]
- 45.Meeker AK, et al. Telomere shortening occurs in subsets of normal breast epithelium as well as in situ and invasive carcinoma. Am J Pathol. 2004;164:925–935. doi: 10.1016/S0002-9440(10)63180-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Heek NT, et al. Telomere shortening is nearly universal in pancreatic intraepithelial neoplasia. Am J Pathol. 2002;161:1541–1547. doi: 10.1016/S0002-9440(10)64432-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meeker AK, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- 48.Finley JC, et al. Chromosomal instability in Barrett’s esophagus is related to telomere shortening. Cancer Epidemiol Biomarkers Prev. 2006;15:1451–1457. doi: 10.1158/1055-9965.EPI-05-0837. [DOI] [PubMed] [Google Scholar]
- 49.Zheng YL, et al. Telomere attrition in cancer cells and telomere length in tumor stroma cells predict chromosome instability in esophageal squamous cell carcinoma: a genome-wide analysis. Cancer Res. 2009;69:1604–1614. doi: 10.1158/0008-5472.CAN-08-3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKay JD, et al. Lung cancer susceptibility locus at 5p15.33. Nat Genet. 2008;40:1404–1406. doi: 10.1038/ng.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y, et al. Common 5p15.33 and 6p21.33 variants influence lung cancer risk. Nat Genet. 2008;40:1407–1409. doi: 10.1038/ng.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hills M, Lansdorp PM. Short telomeres resulting from heritable mutations in the telomerase reverse transcriptase gene predispose for a variety of malignancies. Ann N Y Acad Sci. 2009;1176:178–190. doi: 10.1111/j.1749-6632.2009.04565.x. [DOI] [PubMed] [Google Scholar]
- 53.Willeit P, et al. Telomere length and risk of incident cancer and cancer mortality. JAMA. 2010;304:69–75. doi: 10.1001/jama.2010.897. [DOI] [PubMed] [Google Scholar]
- 54.Londono-Vallejo JA. Telomere length heterogeneity and chromosome instability. Cancer Lett. 2004;212:135–144. doi: 10.1016/j.canlet.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 55.Meeker AK, et al. Telomere length abnormalities occur early in the initiation of epithelial carcinogenesis. Clin Cancer Res. 2004;10:3317–3326. doi: 10.1158/1078-0432.CCR-0984-03. [DOI] [PubMed] [Google Scholar]
- 56.Calado RT, Chen J. Telomerase: not just for the elongation of telomeres. Bioessays. 2006;28:109–112. doi: 10.1002/bies.20365. [DOI] [PubMed] [Google Scholar]
- 57.Hosgood HD, 3rd, et al. Genetic variation in telomere maintenance genes, telomere length, and lung cancer susceptibility. Lung Cancer. 2009;66:157–161. doi: 10.1016/j.lungcan.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van Dyke AL, et al. Chromosome 5p Region SNPs Are Associated with Risk of NSCLC among Women. J Cancer Epidemiol. 2009;2009:242151. doi: 10.1155/2009/242151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rafnar T, et al. Sequence variants at the TERT-CLPTM1L locus associate with many cancer types. Nat Genet. 2009;41:221–227. doi: 10.1038/ng.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shete S, et al. Genome-wide association study identifies five susceptibility loci for glioma. Nat Genet. 2009;41:899–904. doi: 10.1038/ng.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wrensch M, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41:905–908. doi: 10.1038/ng.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin G, et al. Common genetic variants on 5p15.33 contribute to risk of lung adenocarcinoma in a Chinese population. Carcinogenesis. 2009;30:987–990. doi: 10.1093/carcin/bgp090. [DOI] [PubMed] [Google Scholar]
- 63.Landi MT, et al. A genome-wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. Am J Hum Genet. 2009;85:679–691. doi: 10.1016/j.ajhg.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Petersen GM, et al. A genome-wide association study identifies pancreatic cancer susceptibility loci on chromosomes 13q22.1, 1q32.1 and 5p15.33. Nat Genet. 2010;42:224–228. doi: 10.1038/ng.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hofer P, et al. MNS16A tandem repeats minisatellite of human telomerase gene: a risk factor for colorectal cancer. Carcinogenesis. 2011;32:866–871. doi: 10.1093/carcin/bgr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pooley KA, et al. No association between TERT-CLPTM1L single nucleotide polymorphism rs401681 and mean telomere length or cancer risk. Cancer Epidemiol Biomarkers Prev. 2010;19:1862–1865. doi: 10.1158/1055-9965.EPI-10-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang FW, et al. Highly recurrent TERT promoter mutations in human melanoma. Science. 2013;339:957–959. doi: 10.1126/science.1229259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Horn S, et al. TERT promoter mutations in familial and sporadic melanoma. Science. 2013;339:959–961. doi: 10.1126/science.1230062. [DOI] [PubMed] [Google Scholar]
- 69.de Jesus BB, Blasco MA. Potential of telomerase activation in extending health span and longevity. Curr Opin Cell Biol. 2012 doi: 10.1016/j.ceb.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 71.Calado RT, Young NS. Telomere diseases. N Engl J Med. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dokal I. Dyskeratosis congenita. Hematology Am Soc Hematol Educ Program. 2011;2011:480–486. doi: 10.1182/asheducation-2011.1.480. [DOI] [PubMed] [Google Scholar]
- 73.Armanios M. Syndromes of telomere shortening. Annu Rev Genomics Hum Genet. 2009;10:45–61. doi: 10.1146/annurev-genom-082908-150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beier F, Foronda M, Martinez P, Blasco MA. Conditional TRF1 knockout in the hematopoietic compartment leads to bone marrow failure and recapitulates clinical features of Dyskeratosis congenita. Blood. 2012;120:2990–3000. doi: 10.1182/blood-2012-03-418038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leri A, et al. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basel-Vanagaite L, et al. Expanding the clinical phenotype of autosomal dominant dyskeratosis congenita caused by TERT mutations. Haematologica. 2008;93:943–944. doi: 10.3324/haematol.12317. [DOI] [PubMed] [Google Scholar]
- 77.Aikata H, et al. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. doi: 10.1006/excr.2000.4862. [DOI] [PubMed] [Google Scholar]
- 78.Chimenti C, et al. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 79.Chiodi I, Mondello C. Telomere-independent functions of telomerase in nuclei, cytoplasm, and mitochondria. Front Oncol. 2012;2:133. doi: 10.3389/fonc.2012.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Saretzki G. Telomerase, mitochondria and oxidative stress. Exp Gerontol. 2009;44:485–492. doi: 10.1016/j.exger.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 81.Gordon DM, Santos JH. The emerging role of telomerase reverse transcriptase in mitochondrial DNA metabolism. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/390791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sahin E, et al. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sahin E, DePinho RA. Axis of ageing: telomeres, p53 and mitochondria. Nat Rev Mol Cell Biol. 2012;13:397–404. doi: 10.1038/nrm3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Suarez E, et al. Antagonistic effects of telomerase on cancer and aging in K5-mTert transgenic mice. Oncogene. 2005;24:2256–2270. doi: 10.1038/sj.onc.1208413. [DOI] [PubMed] [Google Scholar]
- 85.Vera E, et al. Telomerase Reverse Transcriptase Synergizes with Calorie Restriction to Increase Health Span and Extend Mouse Longevity. PLoS ONE. 2013;8:e53760. doi: 10.1371/journal.pone.0053760. doi: 53710.51371/journal.pone.0053760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gorbunova V, Seluanov A. Coevolution of telomerase activity and body mass in mammals: from mice to beavers. Mech Ageing Dev. 2009;130:3–9. doi: 10.1016/j.mad.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seluanov A, et al. Hypersensitivity to contact inhibition provides a clue to cancer resistance of naked mole-rat. Proc Natl Acad Sci U S A. 2009;106:19352–19357. doi: 10.1073/pnas.0905252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gorbunova V, Bozzella MJ, Seluanov A. Rodents for comparative aging studies: from mice to beavers. Age. 2008;30:111–119. doi: 10.1007/s11357-008-9053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jaskelioff M, et al. Telomerase reactivation reverses tissue degeneration in aged telomerase-deficient mice. Nature. 2011;469:102–106. doi: 10.1038/nature09603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 91.Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21:619–626. doi: 10.1038/sj.onc.1205061. [DOI] [PubMed] [Google Scholar]
- 92.Wu KJ, et al. Direct activation of TERT transcription by c-MYC. Nat Genet. 1999;21:220–224. doi: 10.1038/6010. [DOI] [PubMed] [Google Scholar]
- 93.Hoffmeyer K, et al. Wnt/beta-catenin signaling regulates telomerase in stem cells and cancer cells. Science. 2012;336:1549–1554. doi: 10.1126/science.1218370. [DOI] [PubMed] [Google Scholar]
- 94.Greider CW. Molecular biology. Wnt regulates TERT--putting the horse before the cart. Science. 2012;336:1519–1520. doi: 10.1126/science.1223785. [DOI] [PubMed] [Google Scholar]
- 95.Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy? Oncogene. 2002;21:688–697. doi: 10.1038/sj.onc.1205163. [DOI] [PubMed] [Google Scholar]
- 96.Park JI, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Feldser DM, Greider CW. Short telomeres limit tumor progression in vivo by inducing senescence. Cancer Cell. 2007;11:461–469. doi: 10.1016/j.ccr.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Begus-Nahrmann Y, et al. Transient telomere dysfunction induces chromosomal instability and promotes carcinogenesis. J Clin Invest. 2012;122:2283–2288. doi: 10.1172/JCI61745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding Z, et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell. 2012;148:896–907. doi: 10.1016/j.cell.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gonzalez-Suarez E, et al. Telomerase-deficient mice with short telomeres are resistant to skin tumorigenesis. Nat Genet. 2000;26:114–117. doi: 10.1038/79089. [DOI] [PubMed] [Google Scholar]
- 101.Chin L, et al. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 102.Artandi SE, et al. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- 103.Chang S, et al. Modeling chromosomal instability and epithelial carcinogenesis in the telomerase-deficient mouse. Semin Cancer Biol. 2001;11:227–239. doi: 10.1006/scbi.2000.0374. [DOI] [PubMed] [Google Scholar]
- 104.Hu J, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148:651–663. doi: 10.1016/j.cell.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sachsinger J, et al. Telomerase inhibition in RenCa, a murine tumor cell line with short telomeres, by overexpression of a dominant negative mTERT mutant, reveals fundamental differences in telomerase regulation between human and murine cells. Cancer Res. 2001;61:5580–5586. [PubMed] [Google Scholar]
- 106.Herrera E, et al. Impaired germinal center reaction in mice with short telomeres. EMBO J. 2000;19:472–481. doi: 10.1093/emboj/19.3.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Strong MA, et al. Phenotypes in mTERT+/− and mTERT−/− Mice Are Due to Short Telomeres, Not Telomere-Independent Functions of Telomerase Reverse Transcriptase. Mol Cell Biol. 2011;31:2369–2379. doi: 10.1128/MCB.05312-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liang Y, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu Y, et al. Amplification and overexpression of peroxisome proliferator-activated receptor binding protein (PBP/PPARBP) gene in breast cancer. Proc Natl Acad Sci U S A. 1999;96:10848–10853. doi: 10.1073/pnas.96.19.10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhalla K, et al. PGC1alpha promotes tumor growth by inducing gene expression programs supporting lipogenesis. Cancer Res. 2011;71:6888–6898. doi: 10.1158/0008-5472.CAN-11-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosengardten Y, et al. Stem cell depletion in Hutchinson-Gilford progeria syndrome. Aging Cell. 2011;10:1011–1020. doi: 10.1111/j.1474-9726.2011.00743.x. [DOI] [PubMed] [Google Scholar]
- 112.Schlessinger D, Van Zant G. Does functional depletion of stem cells drive aging? Mech Ageing Dev. 2001;122:1537–1553. doi: 10.1016/s0047-6374(01)00299-8. [DOI] [PubMed] [Google Scholar]
- 113.Ruzankina Y, et al. Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell. 2007;1:113–126. doi: 10.1016/j.stem.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rossi DJ, et al. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 115.Rando TA. Stem cells, ageing and the quest for immortality. Nature. 2006;441:1080–1086. doi: 10.1038/nature04958. [DOI] [PubMed] [Google Scholar]
- 116.Jung P, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–1227. doi: 10.1038/nm.2470. [DOI] [PubMed] [Google Scholar]
- 117.Sperka T, et al. DNA damage checkpoints in stem cells, ageing and cancer. Nat Rev Mol Cell Biol. 2012;13:579–590. doi: 10.1038/nrm3420. [DOI] [PubMed] [Google Scholar]
- 118.Jordan CT, et al. Cancer stem cells. N Engl J Med. 2006;355:1253–1261. doi: 10.1056/NEJMra061808. [DOI] [PubMed] [Google Scholar]
- 119.Reya T, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 120.de Jesus BB, et al. The telomerase activator TA-65 elongates short telomeres and increases health span of adult/old mice without increasing cancer incidence. Aging Cell. 2011;10:604–621. doi: 10.1111/j.1474-9726.2011.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kaplitt MG, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 122.Buning H, et al. Recent developments in adeno-associated virus vector technology. J Gene Med. 2008;10:717–733. doi: 10.1002/jgm.1205. [DOI] [PubMed] [Google Scholar]
- 123.Fujiki T, et al. Regulatory mechanisms of human and mouse telomerase reverse transcriptase gene transcription: distinct dependency on c-Myc. Cytotechnology. 2010;62:333–339. doi: 10.1007/s10616-010-9276-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Weise JM, Gunes C. Differential regulation of human and mouse telomerase reverse transcriptase (TERT) promoter activity during testis development. Mol Reprod Dev. 2009;76:309–317. doi: 10.1002/mrd.20954. [DOI] [PubMed] [Google Scholar]
- 125.Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- 126.Prowse KR, Greider CW. Developmental and tissue-specific regulation of mouse telomerase and telomere length. Proc Natl Acad Sci U S A. 1995;92:4818–4822. doi: 10.1073/pnas.92.11.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harley CB, et al. A natural product telomerase activator as part of a health maintenance program. Rejuvenation Res. 2011;14:45–56. doi: 10.1089/rej.2010.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Eitan E, et al. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4:313–329. doi: 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Artandi SE, et al. Constitutive telomerase expression promotes mammary carcinomas in aging mice. Proc Natl Acad Sci U S A. 2002;99:8191–8196. doi: 10.1073/pnas.112515399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gonzalez-Suarez E, et al. Cooperation between p53 mutation and high telomerase transgenic expression in spontaneous cancer development. Mol Cell Biol. 2002;22:7291–7301. doi: 10.1128/MCB.22.20.7291-7301.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Canela A, et al. Constitutive expression of tert in thymocytes leads to increased incidence and dissemination of T-cell lymphoma in Lck-Tert mice. Mol Cell Biol. 2004;24:4275–4293. doi: 10.1128/MCB.24.10.4275-4293.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sarin KY, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fauce SR, et al. Telomerase-based pharmacologic enhancement of antiviral function of human CD8+ T lymphocytes. J Immunol. 2008;181:7400–7406. doi: 10.4049/jimmunol.181.10.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou QG, et al. Hippocampal telomerase is involved in the modulation of depressive behaviors. J Neurosci. 2011;31:12258–12269. doi: 10.1523/JNEUROSCI.0805-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]