Abstract
Phosphate is an essential nutrient required for important biological reactions that maintain the normal homoeostatic control of the cell. The adverse effects of phosphate metabolism in obesity have not been studied in detail, chiefly because such an association is thought to be uncommon. However, in some animal models of obesity, serum phosphate levels were noted to be higher than the nonobese controls. For example, leptin-deficient (ob/ob) mice become severely obese and have high serum phosphate levels. In this study, we analyzed the phosphate content in saliva collected from children (n = 77; 10.5 ± 1.8) to evaluate association with body mass index; there is a significant increase of salivary phosphate content in obese compared to normal-weight children (ANOVA p < 0.001). The correlation coefficient (r) between BMI and phosphate was 0.33 (p = 0.0032). Our results suggest that the human salivary phosphate level may be an early biomarker of the genesis of obesity in children. The diagnostic importance lies in the fact that the salivary phosphate level could provide a noninvasive predictive marker in the development of obesity. Further studies will be required to understand the underlying mechanism of increased salivary phosphate accumulation in obese and overweight children. Nevertheless, its occurrence without systemic changes could be of diagnostic value, particularly in monitoring evolvement of obesity.
The increasing occurrence of obesity and its related complications, including type 2 diabetes mellitus and cardiovascular anomalies, is alarming and becoming a major public health problem. Despite significant improvement of our understanding of underlying molecular mechanisms of adipose tissue remodeling and eventual development of obesity, potential factors that might serve as a biomarker to monitor evolvement of obesity is not yet clearly identified. Earlier studies have found that the composition of salivary bacteria changes in overweight women [1]. In particular, the percentage of the bacterium Selenomonas noxia was capable of identifying 98.4% of studied 313 overweight women from a group of generally healthy individuals, with a remarkably high degree of diagnostic power [1]. Whether change in the composition of salivary content could serve as biological marker of a developing overweight condition is an area that needs further studies. Of even greater interest, and the subject of future research, is the possibility of using easily available saliva as a diagnostic and prognostic marker of systemic diseases, including obesity.
A clinical association between phosphate imbalance and metabolic diseases has been reported elsewhere. For instance, serum phosphate levels were positively correlated with insulin sensitivity (but not with insulin secretion) in a study conducted in 881 individuals without diabetes mellitus [2]; such a correlation was independent of age, sex, proportion of body adipose content, serum calcium and serum creatinine levels [2]. In a separate study of 298 children and adolescents aged 6–12 years old, in which 190 individuals with obesity and 108 controls without obesity were compared, reduced phosphate serum levels were significantly associated with the development of insulin resistance in children with obesity [3]. Moreover, in a leptin-deficient animal model of obesity, higher serum phosphate levels were noted, as compared to the nonobese controls [4–6]. Summarizing the above-mentioned evidence, it appears likely there might be an association between phosphate metabolism and adipose tissue turnover. This study was designed to study whether the phosphate content of saliva might be clinically useful in monitoring the development of obesity.
The physiological phosphate balance is mostly accomplished by cross-organ talk among kidney, intestine, and bone, where sodium-dependent phosphate transporters (Na/Pi) play a key role in the absorption and reabsorption of consumed phosphate [7–12]. The Na/Pi-2b is mostly present in the luminal side of the intestinal cells where it helps in intestinal phosphate absorption according to the body's need of the phosphate [13]. Almost 80% of the filtered phosphate in the kidney is reabsorbed in the proximal tubular epithelial cells and is also partly accomplished by sodium-dependent phosphate uptake through Na/Pi-2a and Na/Pi-2c transporters. Of relevance, Na/Pi-2b transporters are also expressed in the salivary gland; studies have identified the expression of Na/Pi-2b mRNA and protein in the salivary duct cells [14, 15]. In fact, the ductal cells mostly demonstrated apical Na/Pi-2b expression in salivary glands, whereas the acinar cells showed mainly basolateral expression, suggesting that there might be phosphate secretion by the acini and phosphate reabsorption by the ducts [15]. Moreover, klotho, a cofactor for circulating fibroblast growth factor 23 (FGF23), a master regulator of phosphate metabolism [11, 16–18], is also present in the salivary gland [19], and is therefore providing the necessary machinery for a local salivary regulation of phosphate metabolism, dependent or independent of systemic regulation. This study provides the data to show that the phosphate content in saliva collected from children has a close association with BMI.
Materials and Methods
Saliva and Blood Collection from Children
Saliva and blood samples were collected from 77 children (59% male), aged 10.5 ± 1.8 years, under fasting conditions. Height, weight, blood pressure, heart rate and fitness were measured and recorded. Fitness was measured by heart rate elevation to a Queens College step test [20]. BMI percentile was used to define the children into body weight categories. This study was reviewed and approved by the Forsyth Institute institutional review board. Informed consent was obtained from parents or guardians and assent was obtained from each child. The study was conducted in Portland, Me., USA.
Measurement of Phosphate Contents
Paired 100-μl samples of saliva and plasma from each child were prepared and maintained frozen at −80 °C until assayed. Phosphate was measured on a nontargeted metabolic profiling platform (Metabolon, Durham, N.C, USA) employing gas chromatography/mass spectrometry (GC/MS) [21, 22]. For each sample, 100 μl of plasma or saliva was used for analyses. Using an automated liquid handler (Hamilton LabStar, Salt Lake City, Utah, USA), protein was precipitated with methanol that contained four standards to report on extraction efficiency. The resulting supernatant was split into equal aliquots for analysis. One aliquot, dried under nitrogen and vacuum-desiccated, was subsequently derivatized to a final volume of 50 μl for GC/MS analysis using equal parts of bis(trimethylsilyl)trifluoroacetamide and the solvent mixtureacetonitrile:dichloromethane:cyclohexane (5:4:1) with 5% triethylamine at 60°C for 1 h. Mass spectrometric salivary phosphate data were tested and found non-normal using the Shapiro-Wilk statistics and were also both skewed and kurtotic. Saliva phosphate levels were also determined by colorimetric measurements using the Stanbio Phosphorus Liqui-UV Test (Stanbio Laboratory, Boerne, Tex., USA) as detailed earlier [18, 23, 24]. Statistical evaluations of these data were performed by the Mann-Whitney test. BMI percentile was computed from software from the Center for Disease Control [25]. Body weight categories were assigned using their percentile recommendations (normal healthy weight <85%, overweight ≥85–95% and obese ≥95%). Two children were classified as underweight (percentile <5%). These were included in the normal healthy weight group for analysis.
Leptin-Deficient Obese Mice
Heterozygous leptin-deficient obese [C57B1/6J lepob (+/−)] mutants were obtained from Jackson Laboratory, Me., USA and maintained in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Mouse breeding was performed according to the protocols approved by the institution's subcommittee on animal care (IACUC). Routine PCR using genomic DNA extracted from tail clips was performed for genotyping of the various groups of mice, as detailed earlier [23, 26, 27]. The total body weight of wild-type and ob/ob mice were taken weekly starting at 3 weeks of age until 30 weeks. At least three or more mice from each genotype were sacrificed at 9 weeks and retroperitoneal, mesenteric and epididymal fat tissue weights were recorded. In addition, liver weights of the wild-type and ob/ob mice were taken before fixing part of the liver for histological analysis. Fasting blood glucose levels were determined in all four genotypes. In addition, serum isolated from blood obtained by cheek-pouch bleeding of wild-type and ob/ob mice was isolated and stored at −80°C. Serum phosphate levels were determined by colorimetric measurements using the Stanbio Phosphorus Liqui-UV Test [26, 28, 29]. Statistically significant differences between groups were evaluated either by the Student's t test or the Mann-Whitney U test for a comparison between two groups. All values are expressed as mean ± SE. p < 0.05 was considered to be statistically significant.
Results
Animal Studies
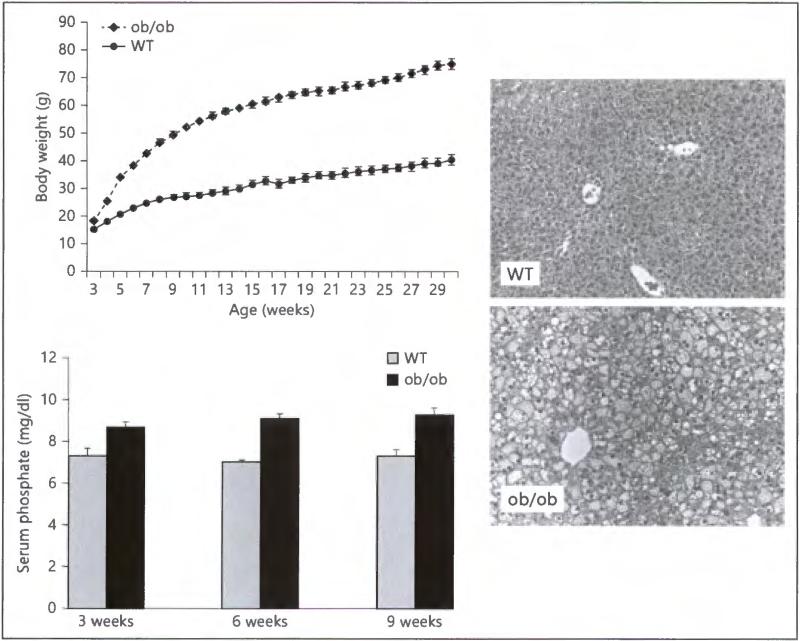
Leptin-deficient obese (ob/ob) mice develop hyperglycemia, glucose intolerance, and obesity. Since the ob/ob mouse could not produce leptin, its food intake is uncontrolled due to the lack of feedback signaling [30]. Mutant ob/ob mice are indistinguishable from their unaffected wild-type littermates at birth, but gain weight rapidly throughout their lives, reaching a weight three times that of unaffected wild-type mice (fig. 1, upper left panel). Compared to the wild-type controls, the increased body size of ob/ob mice is also associated with signifi-candy increased fat accumulation, as determined by analysis of retroperitoneal, mesenteric and epididymal fat tissue weights. Increased accumulation of fat is also obvious in the livers obtained from ob/ob mice (fig. 1, right panel). The ob/ob mice also develop hyperglycemia [31] with increased serum levels of phosphate (fig. 1, lower left panel). Although increased serum levels of phosphate in ob/ob mice could pardybe related to the increased food consumption, it is, however, an intriguing association that needs further exploration.
Fig. 1.
Upper left panel: The body weight chart of wild-type and ob/ob animals. Note that compared to the wild-type control animals, the ob/ob mice are significantly heavier due to an excessive accumulation of fat tissues. Right panel: In accord with the relatively increased liver weight in ob/ob mice over wild-type controls, the histological analysis of the liver shows increased fat tissue accumulation in ob/ob mice. Lower left panel: Biochemical analysis of serum phosphate levels. Note that the serum phosphate levels are significantly higher in ob/ob mice compared with the wild-type (WT) mice at 3, 6 and 9 weeks of age. * p < 0.05 vs. WT. Serum samples collected from at least 4 or more mice were used for the measurements.
Human Study
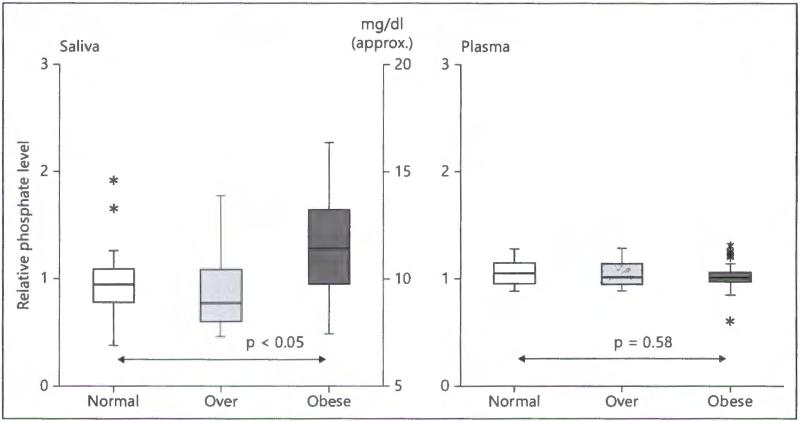
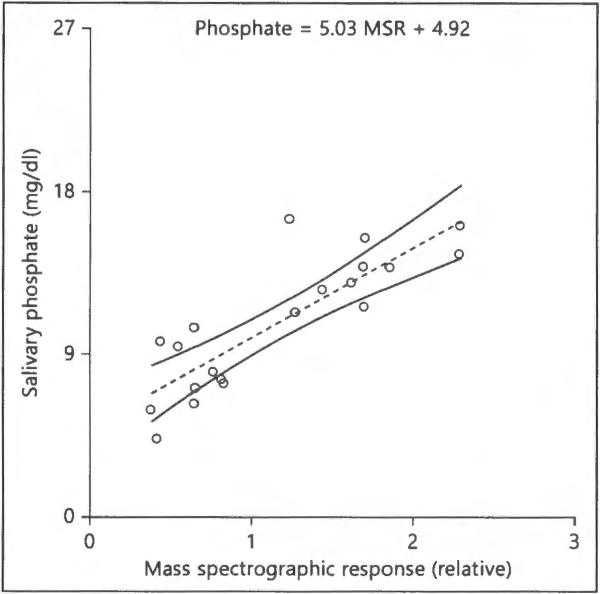
Biometric characteristics of the 77 children admitted into the study are shown in table 1. Differences between normal and obese children (p 1:3) were all statistically significant except for diastolic blood pressure and heart rate. The acceptance criteria (10 ± 1 year) was expanded (10 ± 2 years) for overweight and obese volunteers to obtain a sufficient number willing to provide both saliva and blood samples. This resulted in small but significant differences in age for heavier weight groups. The correlation and statistical significance between salivary phosphate and other parameters are presented in table 2. We have found that different obesity parameters, including BMI, waist circumference, fitness and weight, along with cardiovascular parameter (systolic blood pressure) were significantly associated with salivary phosphate content. Saliva determination by nontargeted mass spectrometric analysis revealed that phosphate was significantly higher in the saliva of obese children. By comparison, however, plasma phosphate was not associated with obesity (fig. 2), suggesting that changes in salivary phosphate levels could occur even before serum changes become apparent, and thereby could be used as a biomarker for monitoring evolvement of obesity. Of relevance, an r2 of 0.70 and a p of 0.003 for prediction of phosphate from the spectrographic response by the quantitative phosphate levels (mg/dl) = 5.03 MSR + 4.92. Thus, the median mass spectrographic value for saliva of 1.0 would roughly equivalent to 9.95 mg/dl (fig. 3).
Table 1.
Characteristics of children investigated in this study
| Normal weight (n = 36) |
Overweight (n = 15) |
Obese (n = 26) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| median | intraquartile range | p = 1:3 | median | intraquartile range | p = 1:2 | median | intraquartile range | p = 2:3 | |
| Weight, lb | 74.4 | 24.4 | <0.0001 | 106.1 | 22.4 | <0.0001 | 151.5 | 50.1 | 0.002 |
| Height, inches | 56.5 | 5.0 | 0.004 | 58.5 | 4.9 | 0.07 | 60.1 | 9.5 | 0.3 |
| Age, years | 9.6 | 2.4 | 0.02 | 10.5 | 1.9 | 0.02 | 10.8 | 2.5 | 0.8 |
| BMI | 17.2 | 2.2 | <0.0001 | 21.1 | 2.4 | <0.0001 | 27.4 | 7.6 | <0.0001 |
| Waist, inches | 24.0 | 3.5 | <0.0001 | 27.5 | 5.0 | <0.0001 | 34.8 | 9.0 | 0.004 |
| Systolic BP, mm Hg | 117.5 | 15.2 | 0.02 | 122.0 | 12.0 | 0.03 | 121.2 | 14.7 | 0.9 |
| Diastolic BP, mm Hg | 67.3 | 8.5 | 0.1 | 69.0 | 6.7 | 0.2 | 71.2 | 8.7 | 0.9 |
| Heart rate, BPM | 77.3 | 15.5 | 0.7 | 73.3 | 23.3 | 0.3 | 81.8 | 20.3 | 0.6 |
| Fitness, BPM | 24.7 | 16.7 | 0.002 | 37.2 | 25.5 | 0.02 | 35.1 | 15.3 | 0.9 |
| Salivary flow, ml/h | 37.3 | 27.4 | 0.1 | 40.3 | 25.3 | 0.9 | 32.7 | 27.5 | 0.3 |
Values listed include median, intraquartile range and the significance p value of differences between body weight categories (1 = normal, 2 = overweight, 3 = obese) by Mann-Whitney analysis.
Table 2.
Correlation (r) and statistical significance (p) between salivary phosphate and each measure
| Measure | r | p |
|---|---|---|
| BMI | 0.33 | 0.003 |
| Waist, inches | 0.31 | 0.007 |
| Fitness, BPM | 0.29 | 0.011 |
| Weight, lb | 0.27 | 0.018 |
| Systolic BP, mm Hg | 0.22 | 0.051 |
| Heart rate, BPM | 0.21 | 0.067 |
| Diastolic BP, mm Hg | 0.17 | 0.139 |
| Height, inches | 0.09 | 0.433 |
| Age, years | 0.01 | 0.913 |
| Salivary flow, ml/h | –0.24 | 0.035 |
Results indicate that measures of obesity (BMI, waist circumference, fitness and weight), one cardiovascular parameter (systolic blood pressure) and salivary flow were significantly associated with salivary phosphate.
Fig. 2.
Relative phosphate levels in saliva and plasma by mass spectroscopy. Central bars are the median, rectangles are the intraquartile range, whiskers are ×1.5 intraquartile range, asterisks are outside values and p is computed using the Mann-Whitney test. Relative phosphate values were obtained by dividing the spectrograph response by the median of all values. This comparison indicates that salivary phosphate levels in obese children were significantly greater than in normal healthy weight children whereas plasma levels were not. Approximate concentrations (mg/dl) were computed from colorometric determinations of aliquots.
Fig. 3.
Least-squares fit linear function and 95% CIs. The relationship has an r2 of 0.72 and a p < 0.0001 for prediction of phosphate from the spectrographic response by the function phosphate (mg/dl) = 5.03 MSR + 4.92. Thus, the median mass spectrographic value for saliva of 1.0 would be 9.95 mg/dl.
Discussion
Child obesity has accelerated over the last decade [32]. As a result, investigation of biomarkers that can serve as early warning signs has become increasingly important. Salivary analysis ranks high in consideration due to ease of collection in a population of children that are understandably reticent to provide blood samples. In this report, we provide evidence that salivary phosphate is elevated in obese children. By contrast, plasma phosphate was not found to be elevated in these children. Taken together, these observations suggest that phosphate metabolism might be associated with fat cell turnover, and that salivary phosphate could be an early marker of metabolic disease associated with development of obesity.
In this study, we presented values for phosphate that were obtained by colorimetric phosphate test from the supernatant of unstimulated saliva (fig. 2). The similar phosphate values in our study are in the similar range compared to other published studies; for instance, studies that were done on healthy children presenting a range of salivary phosphate concentration of 10.5–17 mg/dl [33–35]. No data on BMI were presented in these published studies, except for one study that stated that children with metabolic disorder were excluded [34]. It is, therefore, not possible to estimate the accurate phosphate concentration from these studies, as some children might have been overweight. In a similar line of study that was done on children, adolescents and young adults who have chronic kidney disease showed salivary phosphate values for the healthy subjects of 15.8 mg/dl and patients (on predialytic) of 20.77 mg/dl [36].
Why salivary phosphate levels are high in obese children is not yet clear, and might be related to the altered functions of phosphate-regulating machineries present in the salivary glands. It is important to note that type II sodium-phosphate cotransporters (Na/Pi-2b) are present in the salivary gland [14, 15]. In addition, the type III sodium phosphate cotransporters, PiT1 and PiT2, have been detected in human salivary glands [37]. The possible role of PiT1 and PiT2 in the salivary gland has not been well studied, but a number of studies demonstrated the role for both PiT1 and PiT2 in phosphate reabsorption in the kidney. Depending on the sodium phosphate cotransporter activities in the salivary gland, it is likely that there might be a local regulation of phosphate metabolism that is independent of systemic phosphate regulation. In fact, such possibilities are raised from our observations of increased salivary phosphate contents in obese children, despite their normal plasma phosphate levels. It is important to note that despite recognition that serum phosphate level is the gold standard to estimate the overall phosphate status of the body, the amount of intracellular phosphate or phosphate storage is not taken into consideration in such traditional methods of phosphate measurement. Both human and experimental studies have shown clearly that certain features of phosphate toxicity might appear even in normophosphatemic conditions [38], thereby exposing the limitation of serum phosphate measurements to detect early events of phosphate toxicity. In that context, our result of increased salivary phosphate content without any change in plasma levels provides a meaningful early marker for eventual obesity development.
Since consumption of soda drinks and fast foods are well connected with childhood obesity [39], it will be interesting to know whether such phosphate-rich foods and drinks, by local regulation, might increase the salivary phosphate content that we found in our obese cohorts. Moreover, obese children exhibit more bone resorption than healthy normal-weight children [40], and thereby could induce altered mineral ion metabolism. Further studies will explain the underlying mechanism of increased salivary phosphate accumulation in obese and overweight children, with its biological and clinical significances. This particular study, although limited in numbers of subjects involved, provides the evidence of potential clinical importance of salivary phosphate in monitoring the evolvement of obesity.
Acknowledgements
This work was funded by the grants from the Dasman Institute, Kuwait (to J.M.G), the US National Institutes of Health grant No. DK077276 (to M.S.R.), and the institutional support from the Harvard School of Dental Medicine (to M.S.R).
References
- 1.Goodson JM, Groppo D, Halem S, Carpino E. Is obesity an oral bacterial disease? J Dent Res. 2009;88:519–523. doi: 10.1177/0022034509338353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haap M, Heller E, Thamer C, Tschritter O, Stefan N, Fritsche A. Association of serum phosphate levels with glucose tolerance, insulin sensitivity and insulin secretion in non-diabetic subjects. Eur J Clin Nutr. 2006;60:734–739. doi: 10.1038/sj.ejcn.1602375. [DOI] [PubMed] [Google Scholar]
- 3.Celik N, Andiran N. The relationship between serum phosphate levels with childhood obesity and insulin resistance. J Pediatr Endocrinol Metab. 2011;24:81–83. doi: 10.1515/jpem.2011.116. [DOI] [PubMed] [Google Scholar]
- 4.Razzaque MS. The role of klotho in energy metabolism. Nat Rev Endocrinol. 2012;8:579–587. doi: 10.1038/nrendo.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsuji K, Maeda T, Kawane T, Matsunuma A, Horiuchi N. Leptin stimulates fibroblast growth factor 23 expression in bone and suppresses renal 1alpha,25-dihydroxyvitamin d3 synthesis in leptin-deficient mice. J Bone Miner Res. 2010;25:1711–1723. doi: 10.1002/jbmr.65. [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi M, Kato S, Akiyoshi J, Atfi A, Razzaque MS. Dietary and genetic evidence for enhancing glucose metabolism and reducing obesity by inhibiting klotho functions. FASEB J. 2011;25:2031–2039. doi: 10.1096/fj.10-167056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Razzaque MS. Phosphate toxicity: new insights into an old problem. Clin Sci (Lond) 2011;120:91–97. doi: 10.1042/CS20100377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razzaque MS. Osteo-renal regulation of systemic phosphate metabolism. IUBMB Life. 2011;63:240–247. doi: 10.1002/iub.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gattineni J, Baum M. Regulation of phosphate transport by fibroblast growth factor 23 (fgf23): implications for disorders of phosphate metabolism. Pediatr Nephrol. 2010;25:591–601. doi: 10.1007/s00467-009-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Razzaque MS. Fgf23-mediated regulation of systemic phosphate homeostasis: 1s klotho an essential player? Am J Physiol Renal Physiol. 2009;296:F470–F476. doi: 10.1152/ajprenal.90538.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Razzaque MS. The fgf23-klotho axis: endocrine regulation of phosphate homeostasis. Nat Rev Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y, Feng JQ. Fgf23 in skeletal modeling and remodeling. Curr Osteoporos Rep. 2011;9:103–108. doi: 10.1007/s11914-011-0053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tenenhouse HS. Regulation of phosphorus homeostasis by the type IIa Na/phosphate cotransporter. Annu Rev Nutr. 2005;25:197–214. doi: 10.1146/annurev.nutr.25.050304.092642. [DOI] [PubMed] [Google Scholar]
- 14.Homann V, Rosin-Steiner S, Stratmann T, Arnold WH, Gaengler P, Kinne RK. Sodium-phosphate cotransporter in human salivary glands: molecular evidence for the involvement of NPT2b in acinar phosphate secretion and ductal phosphate reabsorption. Arch Oral Biol. 2005;50:759–768. doi: 10.1016/j.archoralbio.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Lederer E, Miyamoto K. Clinical consequences of mutations in sodium phosphate cotransporters. Clin J Am Soc Nephrol. 2012;7:1179–1187. doi: 10.2215/CJN.09090911. [DOI] [PubMed] [Google Scholar]
- 16.Goetz R, Ohnishi M, Kir S, Kurosu H, Wang L, Pastor J, Ma J, Gai W, Kuro-o M, Razzaque MS, Mohammadi M. Conversion of a paracrine fibroblast growth factor into an endocrine fibroblast growth factor. J Biol Chem. 2012;287:29134–29146. doi: 10.1074/jbc.M112.342980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goetz R, Ohnishi M, Ding X, Kurosu H, Wang L, Akiyoshi J, Ma J, Gai W, Sidis Y, Pitteloud N, Kuro OM, Razzaque MS, Mohammadi M. Klotho coreceptors inhibit signaling by paracrine fibroblast growth factor 8 subfamily ligands. Mol Cell Biol. 2012;32:1944–1954. doi: 10.1128/MCB.06603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (FGF23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amano I, Imaizumi Y, Kaji C, Kojima H, Sawa Y. Expression of podoplanin and classical cadherins in salivary gland epithelial cells of klotho-deficient mice. Acta Histochem Cytochem. 2011;44:267–276. doi: 10.1267/ahc.11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suriano K, Curran J, Byrne SM, Jones TW, Davis EA. Fatness, fitness, and increased cardiovascular risk in young children. J Pediatr. 2010;157:552–558. doi: 10.1016/j.jpeds.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 21.Evans AM, DeHaven CD, Barrett T, Mitchell M, Milgram E. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;81:6656–6667. doi: 10.1021/ac901536h. [DOI] [PubMed] [Google Scholar]
- 22.Ohta T, Masutomi N, Tsutsui N, Sakairi T, Mitchell M, Milburn MV, Ryals JA, Beebe KD, Guo L. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;37:521–535. doi: 10.1177/0192623309336152. [DOI] [PubMed] [Google Scholar]
- 23.Nakatani T, Ohnishi M, Razzaque MS. Inactivation of klotho function induces hyperphosphatemia even in presence of high serum fibroblast growth factor 23 levels in a genetically engineered hypophosphatemic (Hyp) mouse model. FASEB J. 2009;23:3702–3711. doi: 10.1096/fj.08-123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohnishi M, Kato S, Razzaque MS. Genetic induction of phosphate toxicity significantly reduces the survival of hypercholesterolemic obese mice. Biochem Biophys Res Commun. 2011;415:434–438. doi: 10.1016/j.bbrc.2011.10.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC Child and teen BMI calculator. 2012 http://apps.nccd.cdc.gov/dnpabmi/
- 26.Ohnishi M, Razzaque MS. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010;24:3562–3571. doi: 10.1096/fj.09-152488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakatani T, Bara S, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS. In vivo genetic evidence of klotho-dependent functions of fgf23 in regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohnishi M, Nakatani T, Lanske B, Razzaque MS. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin d 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- 31.Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- 32.Hsia DS, Fallon SC, Brandt ML. Adolescent bariatric surgery. Arch Pediatr Adolesc Med. 2012;166:757–766. doi: 10.1001/archpediatrics.2012.1011. [DOI] [PubMed] [Google Scholar]
- 33.Damle SG, VI, Yadav R, Bhattal H, Loomba A. Quantitative determination of inorganic constituents in saliva and their relationship with dental caries experience in children. Dentistry. 2012;2:131. [Google Scholar]
- 34.Shahrabi M, Nikfarjam J, Alikhani A, Akhoundi N, Ashtiani M, Seraj B. A comparison of salivary calcium, phosphate, and alkaline phosphatase in children with severe, moderate caries, and caries free in Tehran's kindergartens. J Indian Soc Pedod Prev Dent. 2008;26:74–77. doi: 10.4103/0970-4388.41621. [DOI] [PubMed] [Google Scholar]
- 35.Wu KP, Ke JY, Chung CY, Chen CL, Hwang TL, Chou MY, Wong AM, Hu CF, Lee YC. Relationship between unstimulated salivary flow rate and saliva composition of healthy children in Taiwan. Chang Gung Med J. 2008;31:281–286. [PubMed] [Google Scholar]
- 36.Davidovich E, Davidovits M, Peretz B, Shapira J, Aframian DJ. The correlation between dental calculus and disturbed mineral metabolism in paediatric patients with chronic kidney disease. Nephrol Dial Transplant. 2009;24:2439–2445. doi: 10.1093/ndt/gfp101. [DOI] [PubMed] [Google Scholar]
- 37.Corut A, Senyigit A, Ugur SA, Altin S, Ozcelik U, Calisir H, Yildirim Z, Gocmen A, Tolun A. Mutations in slc34a2 cause pulmonary alveolar microlithiasis and are possibly associated with testicular microlithiasis. Am J Hum Genet. 2006;79:650–656. doi: 10.1086/508263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osuka S, Razzaque MS. Can features of phosphate toxicity appear in normophosphatemia? J Bone Miner Metab. 2012;30:10–18. doi: 10.1007/s00774-011-0343-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D'Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation. 2007;116:480–488. doi: 10.1161/CIRCULATIONAHA.107.689935. [DOI] [PubMed] [Google Scholar]
- 40.Dimitri P, Wales JK, Bishop N. Adipokines, bone-derived factors and bone turnover in obese children: evidence for altered fat-bone signalling resulting in reduced bone mass. Bone. 2011;48:189–196. doi: 10.1016/j.bone.2010.09.034. [DOI] [PubMed] [Google Scholar]