Abstract
Psychological and physical stress can suppress the immune system in both humans and animals. The mechanism by which stress affects immune responses, however, remains poorly defined. Toll-like receptors (TLRs) play a key role in modulating immune responses and cell survival. The mechanisms by which TLRs modulates chronic stress are largely unexplored. In current study, we revealed that a deficiency of TLR9 is resistant to chronic stress-induced lymphocyte apoptosis. In addition, TLR9 knockout (KO) mice significantly diminish the chronic stress-induced up-regulation of corticosterone levels. Notably, we found that lymphocytes from both TLR9 KO mice and wild type mice were similarly sensitive to corticosteroid-induced cell apoptosis. Moreover, we demonstrated that a deficiency of TLR9 blocks chronic stress-induced imbalance of Th1 and Th2 cytokine levels. Taken together, our findings reveal that TLR9 plays an essential role in chronic stress-induced immune suppression.
Keywords: TLR9, Chronic stress, Corticosteroid, Immune suppression, Apoptosis
1. Introduction
Different studies with various model systems have revealed that depending on the mood and duration, physical and psychological stress could either increase or decrease immune functions in both humans and animals [1–4]. An interaction between stress and immune responses has been studied in various experimental paradigms [4,5]. It is well documented that exhausting physical activity and psychological stress leads to immunosuppression of the immune system [4–6]. Such suppression of the immune system has strong implications for infectious disease susceptibility and progression [1,7,8], which is at least in part due to lymphocyte apoptosis [4,9]. Investigations have shown that stress could promote autoimmunity and infectious diseases by influencing the course and outcome of the pathological processes [9–11]. However, acute stress modulates immunoprotection via cell-mediated immunity [12,13]. It is well established that acute restraint stress could significantly increase delayed-type hypersensitivity (DTH) reaction 8, while chronic stress could decrease immune function and enhance susceptibility to diseases [7,14]. Stress hormones, including cortisol, play a fundamental role in regulating immune responses and the balance of T help 1 (Th1) and Th2 cytokines, thereby modulating the susceptibility of various immune-related disorders 4. We observed that chronic restraint stress caused a dramatic decrease in Th1 cytokine IFN-gamma and IL-2 levels but increase in Th2 cytokine IL-4. However, the mechanisms by which TLR9 participate in chronic stress-induced immune suppression are unexplored. To define the role of immune responses and stress hormones in mice, chronic restraint stress murine model has been widely employed [5,15]. We utilized this model to investigate TLR9-mediated immune responses as evaluated by alterations in the number of apoptosis, corticosterone production, and the balance of Th1 and Th2 cytokines.
TLRs play an essential role in modulating innate immunity and inflammation as well as cell apoptosis and cell survival [16–18]. There are at least 11 TLRs in mammals. TLR2 detects a broad range of Gram-positive bacterial products, including peptidoglycan, and TLR4 functions as a signal transducer for LPS. TLR4 modulates cell apoptotic signaling via the interaction of the death domain of myeloid differentiation factor 88 (MyD88) with Fas associated death domain (FADD) [19]. TLR3, TLR7, and TLR9 are distinct from other TLRs in that they are not expressed on the plasma membrane [18,20,21]. TLR9 is identified as a key immune receptor in TLRs family that can recognize bacterial DNA as well as oligodeoxynucleotides (ODN) containing the CpG motifs responsible for the activating capacity of bacterial DNA (CpG s-ODN) [20,21]. Recent studies from ours and others have revealed that activation of TLR9 signaling triggers activation of pro-apoptotic signaling pathways, and causes cell apoptosis in various systems [17,18,20]. Whether TLR9 participates in chronic stress-induced immune dysfunction is not clear. We have performed TLR9 KO mice and wild type (WT) mice to investigate the consequence of TLR9 deficiency to the effect of animals on chronic stress. Our findings demonstrated a novel role of TLR9 in regulating immune suppression by modulating stress-induced the production of corticosteroid.
2. Materials and Methods
2.1. Experimental animals
Breeding pairs of TLR9 KO (not a functional KO) mice on a Balb/c background were kindly provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan) via Dr. Dennis Klinman (National Cancer Institute, Frederick, MD). WT Balb/c male mice were purchased from the Hallan (Indianapolis, Indiana) and maintained in the Division of Laboratory Animal Resources at East Tennessee State University (ETSU). The physical restraint procedure was approved by the Institutional Animal Care and Use Committee of ETSU. All male mice used in the experiments were age- and weight-matched.
2.2. Physical restraint stress
Six- to eight-week-old male mice were subjected to an established chronic physical restraint protocol used in our laboratory as well as others [4,5,9,14,22]. They were placed in a 50-ml conical centrifuge tube filled with multiple punctures to allow ventilation. Mice were held horizontally in the tubes for 12 h followed by a 12-h rest. During the rest period food and water were provided ad libitum. Control littermates were kept in their original cage and food and water were provided only during the 12 h rest. Mice were physically restrained for one to three cycles as specified. After physical restraint, mice were sacrificed by CO2 asphyxiation and the spleens were harvested.
2.3. Detection of apoptosis by TUNEL assay
The frozen spleen sections from unstress and stressed WT and TLR9 KO mice were harvested for terminal deoxynucleotidyl transferase biotin-dUTP nick end labeling (TUNEL) assay using an in situ cell death detection kit (Roche Diagnostic, Indianapolis, IN) according to the manufacturer's instructions and our previous publications [23,24]. Splenocytes isolated from WT and TLR9 KO mice were culture in 96-well plates at 5 × 105 cells/ ml with or without dexamethasone treatment at different concentrations. After 12 hours treatment, apoptotic cells were determined by TUNEL assay [21,23,24]. The percentage of apoptotic cells was calculated by counting approximately 300 cells.
2.4. Measurement of cytokines by enzyme linked immunosorbent assay (ELISA)
The culture supernatants were harvested after 36 h of cultivation. The presence of cytokines in the supernatants was determined using cytokine-specific sandwich ELISA kits (R&D Systems, Minneapolis, MN) according to manufactures’s instruction oras described in our previous publication [5].
2.5. Measurement of corticosterone levels
Experimental mice were sacrificed immediately after stress and blood was harvested from each of mice. Serum was collected and stored at −80°C until determination of corticosterone. The serum corticosterone levels were determined using a corticosterone ELISA kit (IBL America, Minneapolis, MN) according to the manufacturer's instruction.
2.6. Isolation of splenic CD4+ T cells and determination of CYP11A1 expression by quantitative real-time RT-PCR
Splenic CD4+ T cells were negatively selected by using the MagCellect™ Mouse CD4+ T Cell Isolation Kit (R&D Systems, Minneapolis, MN). Total RNA was isolated from CD4+ T cells by the VERSAGENE™ RNA Tissue Kit (Gentra SYSTEMS; Minnesota), as described previously [22,25]. Quantitative real-time RT-PCR was carried out to quantify the expression levels of CYP11A1 on Bio-Rad iCycler iQ Multicolor Real-Time PCR Detection System (Bio-Rad Life Science Research, Hercules, CA) as described in our previous studies 22,25. The primers specific for CYP11A1 were described previously [26,27]. PCR assays were performed in triplicate. The reaction conditions were: 95 °C for 12 min; followed by 40 cycles at 95 °C for 15 s, 55 °C for 30 s, and 72 °C for 30 s. Threshold cycle numbers (CT) were determined with Bio-Rad iCycler iQ Multicolor Real- Time PCR Detection System (version 1.1 software) and transformed using the ΔCT comparative method. Gene-specific expression values were normalized to expression values of GAPDH and/or β-actin (endogenous control) within each sample. The amount of CYP11A1, normalized to an endogenous reference and relative to a calibrator, was determined by the comparative Ct method (ΔΔCT).
2.7. Statistical analysis
All data were represented as mean ± SEM. The data were analyzed using one-way analysis of variance (ANOVA) followed by Bonferroni tests to determine where differences among groups existed. Differences were considered statistically significant for values of p ≤ 0.05.
3. Results
3.1. Chronic stress-induced lymphocyte reduction depends on TLR9
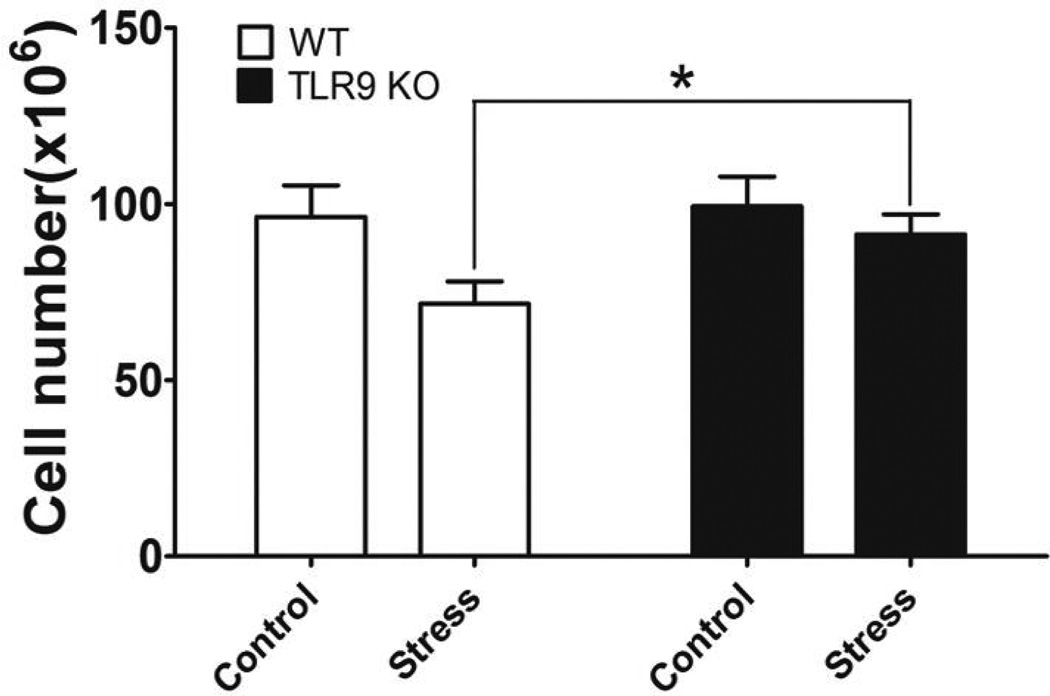
Our previous studies have shown that chronic stress induced splenocyte reduction [5,14]. However, the mechanisms by which TLRs play a critical role in immune suppression induced by chronic stress are not known yet. To determine the role of TLR9 in chronic stress-induced lymphocyte reduction, we subjected TLR9 KO male mice and their WT control Balb/c male mice to a 12-h physical restraint daily for 2 days to determined the number of splenocytes [5,14]. We found that chronic stress-induced lymphocyte reduction in the WT mice, however, a deficiency of TLR9 blocked stress-induced reduction in lymphocyte numbers (Fig. 1). Therefore, TLR9 KO mice lose their sensitivity to restraint stress-induced reduction in lymphocyte numbers, supporting an important role of TLR9 in stress-induce immune response.
Fig. 1.
A deficiency of TLR9 blocks chronic restraint stress-induced lymphocyte reduction. TLR9 knockout (KO) mice or wild type (WT) Balb/c mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily. After 2 d stress, mice were sacrificed by CO2 asphyxiation, and total splenocytes were enumerated. Means and SEs were calculated from five to seven mice each group. * p < 0.01 compared with indicated groups.
3.2. TLR9 is required for chronic stress-induced lymphocyte apoptosis
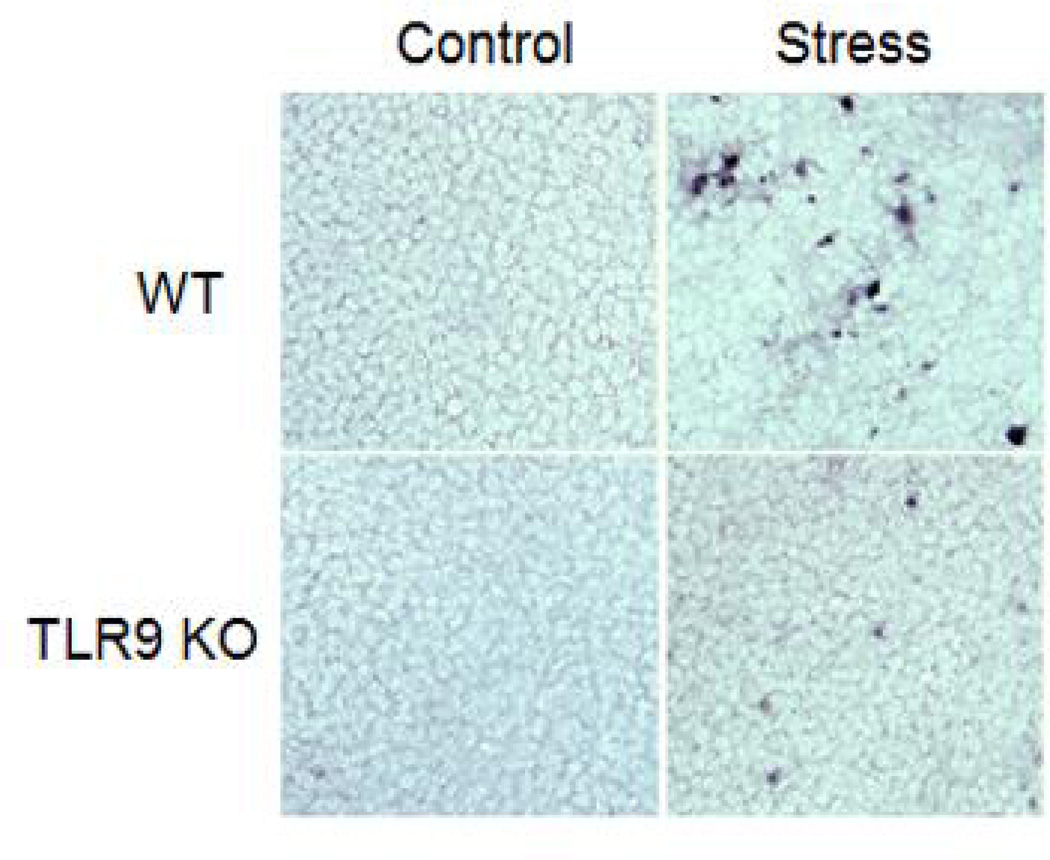
To investigate whether a deficiency of TLR9 inhibits stress-induced lymphocyte reduction is due to the induction of apoptosis, we assessed apoptotic cells by the TUNEL assay using histological spleen sections. Our data showed that the number of apoptotic cells was dramatically increased in the spleen of stressed WT mice compared with unstressed control WT mice, whereas no significant apoptotic cells was observed in the spleen of stressed TLR9 KO mice (Fig. 2). Therefore, chronic stress induces lymphocyte apoptosis through a TLR9-dependent manner.
Fig. 2.
Chronic stress induces lymphocyte apoptosis through TLR9. WT mice or TLR9 KO mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily for 2 d. Apoptotic cells in frozen spleen sections were determined by TUNEL assay. The data are representative of three experiments.
3.3. A deficiency of TLR9 attenuates stress-enhanced corticosterone levels
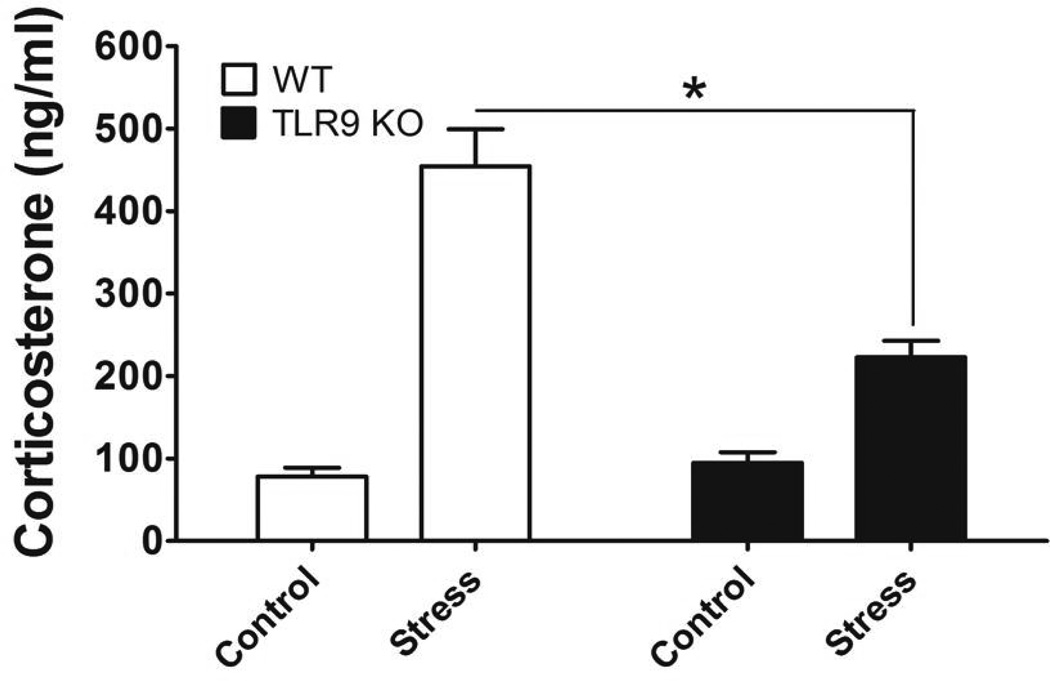
It has been shown that chronic stress increases corticosterone levels, one of major stress hormone [4,28]. Enhanced corticosterone levels induces the induction of lymphocyte apoptosis [4,28]. We next examined whether TLR9 participates in modulating the glucocorticoid following chronic stress, corticosterone level in serum was determined. We showed that chronic stress significantly enhanced corticosterone levels in the WT mice. Interestingly, a deficiency of TLR9 attenuates stress-enhanced corticosterone levels (Fig. 3). Taken together, our results suggest that TLR9 is necessary for up-regulation of the steroid hormone in response to chronic stress.
Fig. 3.
A deficiency of TLR9 inhibits chronic stress-induced corticosterone production. We subjected WT mice or TLR9 KO mice aged 6 to 8 weeks to a 12 h physical restraint daily. After 2 d stress, blood was harvested immediately and serum was collected for determination of corticosterone production. The levels of corticosterone were examined by a corticosterone ELISA kit. Means and SEs were calculated from four to six mice per group. * p < 0.01 compared with indicated groups.
3.4. A deficiency of TLR9 does not impair exogenous dexamethasone-induced lymphocyte apoptosis
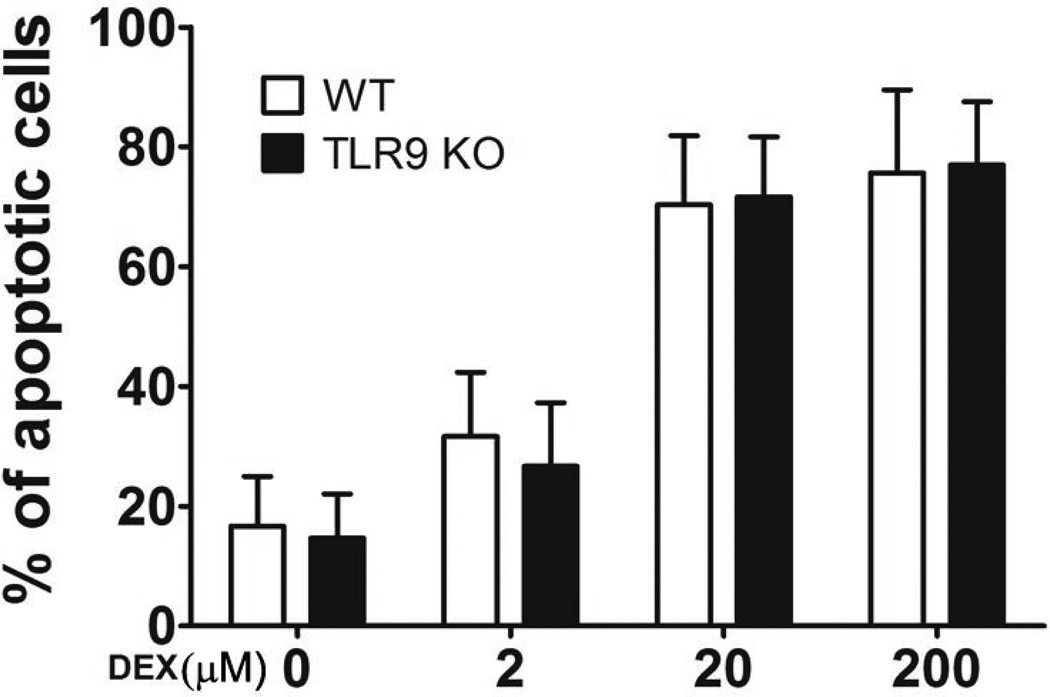
TLR9 plays a key role in up-regulation of the steroid hormone induced by stress (Fig. 3). We next defined whether TLR9 acts upstream of the corticosterone hormone. Splenocytes from WT mice and TLR9 KO mice were incubated in the presence or absence of exogenous dexamethasone and then determined the number of cell apoptosis. Our results showed that there are no significant differences in the apoptotic cell numbers from WT mice and TLR9 KO mice (Fig. 4). These results suggest that steroid-induced cell death is not impaired in TLR9 KO cells, indicating that endogenous TLR9 acts upstream of the corticosterone hormone.
Fig. 4.
TLR9 is not necessary for exogenous dexamethasone-induced lymphocyte apoptosis. Single-cell suspensions of splenocytes isolated from TLR9-deficienct mice or WT mice aged 6 to 8 weeks were incubated in the absence or presence of dexamethasone at different concentrations for 12 h. The apoptotic cells were determined by TUNEL assay. The percentage of apoptotic cells was calculated by counting approximately 300 cells. Means and SEs were calculated from three independent experiments.
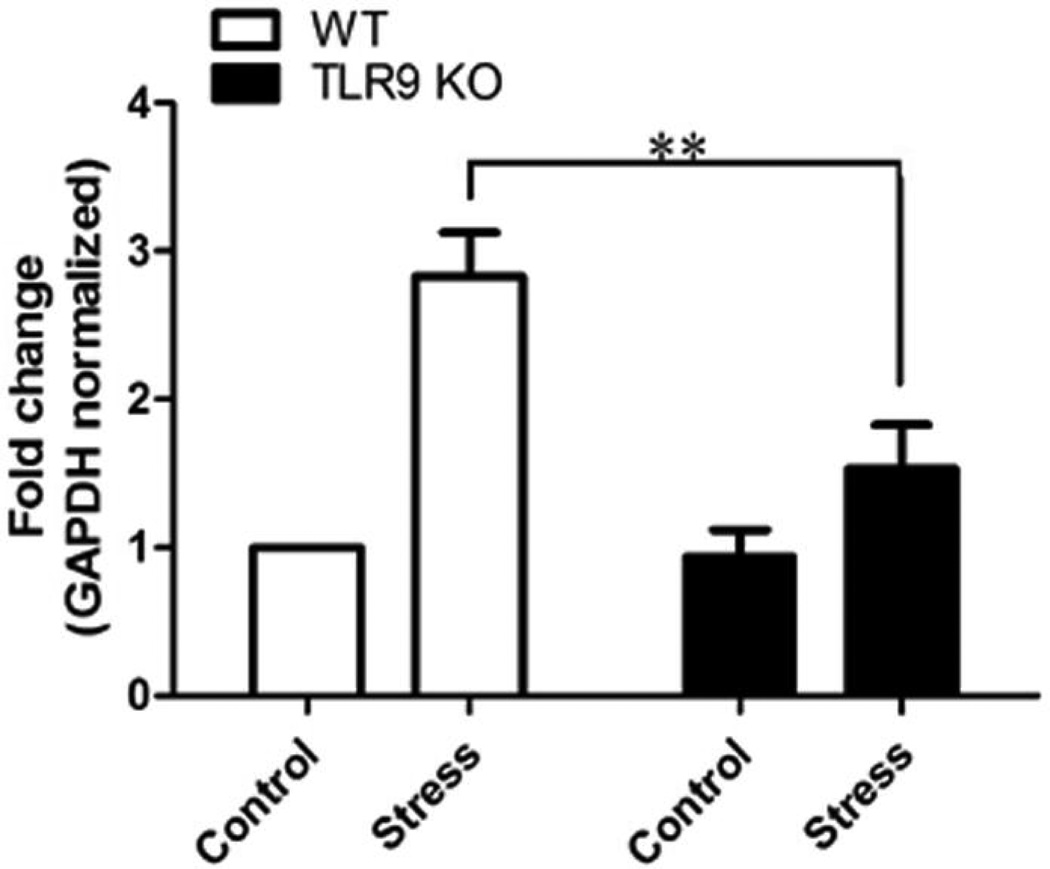
3.5. A deficiency of TLR9 diminishes stress-increased the expression of CYP11A1
CYP11A1 (cytochrome P450scc, cholesterol side-chain cleavage enzyme) plays the first and rate-limiting step in the synthesis of the steroid hormones. CYP11A1 is a definitive marker for corticosterone synthesis [26,27,29]. To investigate the effect of TLR9 on regulating CYP11A1 expression during chronic stress, we examined the expression of CYP11A1 in splenic CD4+ T cells in the stressed and unstressed (control) mice. Our studies showed that chronic stress enhanced the expression of CYP11A1 in the WT mice (Fig. 5). Interestingly, chronic stress-enhanced CYP11A1 expression was dramatically attenuated in the TLR9 KO mice. These results suggest that TLR9 plays an important role in corticosterone synthesis during chronic stress.
Fig. 5.
TLR9 deficient diminishes chronic stress-induced the expression of CYP11A1. WT mice or TLR9 KO mice aged 6 to 8 weeks were subjected to a 12 h physical restraint daily for 2 days. Splenic CD4+ T cells were isolated for examination of CYP11A1 expression by real time PCR. Means and SEs were calculated from four to six mice per group. ** p < 0.01 compared with indicated groups.
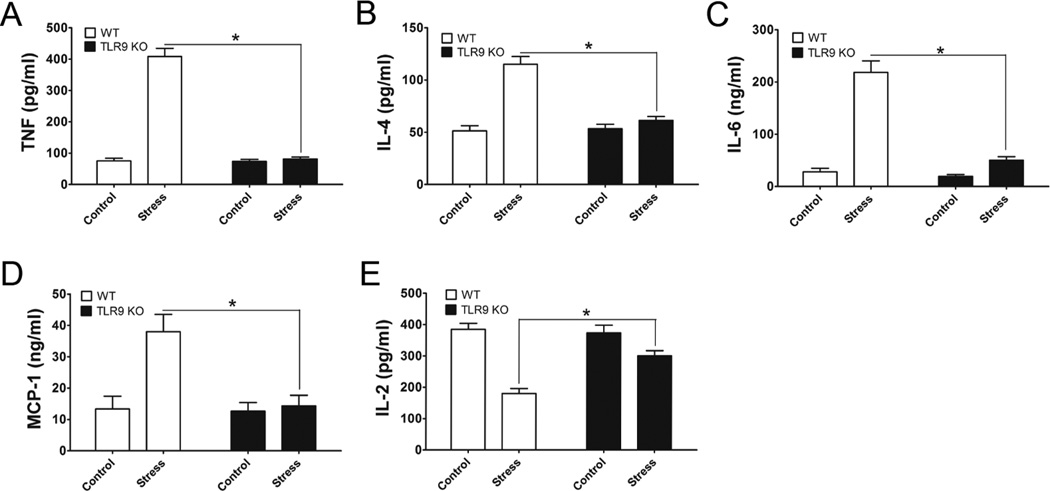
3.6. TLR9 KO mice are resistant to stress-induced changes of Th1 and Th2 cytokine levels
Since TLR9 plays an important role in modulating inflammatory responses [30,31], we then examined the effects TLR9 on the T helper 1 (Th1) and Th2 cytokine production in response to chronic stress. At 2 days after stress of WT mice and TLR9 KO mice, we assessed the levels of Th1 and Th2 cytokines from culture supernatants from Con A-stimulated lymphocytes by ELISA. Our data showed that chronic stress significantly enhances the production of TNF-α (Fig. 6A), IL-4 (Fig. 6B), IL-6 (Fig. 6C), and MCP-1 (Fig. 6D) but dramatically inhibits IL-2 production (Fig. 6E) in lymphocytes from stressed WT mice than lymphocytes from unstressed WT mice. Surprisingly, chronic stress failed to induce these cytokine changes in TLR9 KO mice (Fig. 6). Thus, our studies suggest that TLR9 is required for chronic stress-induce immune responses.
Fig. 6.
Effect of TLR9 on chronic stress-induced changes of Th1 and Th2 cytokines. TLR9 KO mice and WT mice aged 6 to 8 weeks were subjected to a 12-h physical restraint stress for 2 d. Splenic lymphocytes were treated with Con A (5 µg/ml). After 36 h incubation, the culture supernatants were harvested. Cytokine production in the supernatants, TNF (A), IL-4 (B), IL-6 (C), MCP-1 (D), and IL-2 (E) was assessed using cytokine-specific sandwich ELISA kits. Means and SEs were calculated from three to five mice per group. * p < 0.01 compared with indicated groups.
4. Discussion
It is well established that various stress model systems of physical stress can either enhance or inhibit immune function depending on the type and duration of the stressors [2,6,7,14]. It has been shown that chronic stress-induced alterations of immune responses could result from increased cell death and apoptosis or decreased cell proliferation [4,5,14]. Physical or psychological stress promotes stress hormone and neuronal transmitters [15,32,33]. However, the role of TLR9 in stress-mediated immune responses has remained unexplored. In the present study, we showed that TLR9 is resistant to chronic restraint-induced reduction in the number of lymphocytes. We also showed that chronic stress-induced lymphocyte apoptosis requires TLR9.
Recent studies indicate that steroid hormones play an important role in modulating immune functions under chronic stress conditions [4]. It is important to point out that some studies have challenged the general effect of steroids on stress situations [34]. As an example, recent researchers have shown that chronic stress significantly enhances corticosterone production and induces lymphocyte apoptosis is, at least partially, mediated by corticosterioids [4,28]. We first evaluated whether TLR9 plays a role in modulating glucocorticoid levels under chronic stress conditions, TLR9 KO mice and WT mice were subjected to chronic stress. Corticosterone levels were enhanced more than a 4-fold in the serum of WT mice following chronic stress. Surprisingly, TLR9 KO mice are resistant to chronic stress-induced up-regulation of corticosterone production, suggesting that TLR9 is required for chronic stress-caused up-regulation of steroid hormone. We next defined whether TLR9 participates in CYP11A1 regulation, a definitive marker for corticosterone synthesis [26,27,29]. Our studies showed that isolated splenic CD4+ T cells from the stressed WT mice increased the expression of CYP11A1 compared with unstressed control mice. Importantly, we found that a deficiency of TLR9 significantly attenuated the chronic stress-induced CYP11A1 expression.
We then determined whether TLR9 involves in exogenous dexamethasone-induced lymphocyte apoptosis. Our data showed that lymphocytes isolated from TLR9 KO mice and WT mice did not show a significant difference in the percentage of dexamethasone-induced apoptosis, indicating that TLR9 is an upstream of the corticosterone. These findings suggest that TLR9 plays an important role in the interaction between the neuronal and the immune systems. We speculated that TLR9 performs upstream of the corticosterone, and might be responsible for its enhanced levels under chronic stress conditions. We observed that chronic stress of mice caused in an increase in Th2 cytokine production, whereas a decrease of Th1 cytokine production was observed. In our current study, we subjected TLR9-KO mice and WT mice to chronic stress and found that a deficiency of TLR9 attenuates chronic stress-induced alterations in Th1 and Th2 cytokine levels. Therefore, chronic stress-caused the imbalance of Th1 and Th2 cytokines appears to require TLR9.
In summary, to our best knowledge our study is the first to reveal that TLR9 is necessary for chronic stress-induced immune suppression by modulating corticosteroid levels. Our findings suggest that further identification of the TLR9-mediated signaling in response to chronic stress may provide a new insight into the prevention and/or treatment of stress induced-immune suppression and infectious diseases.
Acknowledgments
This work was supported in part by research grants NIGMS094740 and NIDA020120 from NIH to D. Yin. The authors wish to express their appreciation to Dr. Shizuo Akira, Osaka University, Osaka, Japan and Dr. Dennis Klinman, National Cancer Institute, Frederick of MD, for providing breeding pairs of TLR9 KO mice.
References
- 1.Shi Y, Devadas S, Greeneltch KM, Yin D, Allan MR, Zhou JN. Stressed to death: implication of lymphocyte apoptosis for psychoneuroimmunology. Brain Behav.Immun. 2003;17(Suppl 1):S18–S26. doi: 10.1016/s0889-1591(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 2.Stone AA, Bovbjerg DH. Stress and humoral immunity: a review of the human studies. Adv.Neuroimmunol. 1994;4:49–56. doi: 10.1016/s0960-5428(06)80189-0. [DOI] [PubMed] [Google Scholar]
- 3.Wilder RL. Neuroendocrine-immune system interactions and autoimmunity. Annu.Rev.Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- 4.Wang KX, Shi Y, Denhardt DT. Osteopontin regulates hindlimb-unloading-induced lymphoid organ atrophy and weight loss by modulating corticosteroid production. Proc.Natl.Acad.Sci.U.S.A. 2007;104:14777–14782. doi: 10.1073/pnas.0703236104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Chen L, Zhang Y, LeSage G, Zhang Y, Wu Y, Hanley G, Sun S, Yin D. Chronic stress promotes lymphocyte reduction through TLR2 mediated PI3K signaling in a beta-arrestin 2 dependent manner. J.Neuroimmunol. 2011;233:73–79. doi: 10.1016/j.jneuroim.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu.Rev.Psychol. 1993;44:53–85. doi: 10.1146/annurev.ps.44.020193.000413. [DOI] [PubMed] [Google Scholar]
- 7.Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav.Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- 8.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc.Natl.Acad.Sci.U.S.A. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Foster R, Sun X, Yin Q, Li Y, Hanley G, Stuart C, Gan Y, Li C, Zhang Z, Yin D. Restraint stress induces lymphocyte reduction through p53 and PI3K/NF-kappaB pathways. J.Neuroimmunol. 2008;200:71–76. doi: 10.1016/j.jneuroim.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheridan JF, Dobbs C, Jung J, Chu X, Konstantinos A, Padgett D, Glaser R. Stress-induced neuroendocrine modulation of viral pathogenesis and immunity. Ann.N.Y.Acad.Sci. 1998;840:803–808. doi: 10.1111/j.1749-6632.1998.tb09618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shanks N, Kusnecov AW. Differential immune reactivity to stress in BALB/cByJ and C57BL/6J mice: in vivo dependence on macrophages. Physiol Behav. 1998;65:95–103. doi: 10.1016/s0031-9384(98)00149-8. [DOI] [PubMed] [Google Scholar]
- 12.Dhabhar FS, McEwen BS. Stress-induced enhancement of antigen-specific cell-mediated immunity. J.Immunol. 1996;156:2608–2615. [PubMed] [Google Scholar]
- 13.Nash MS. Exercise and immunology. Med.Sci.Sports Exerc. 1994;26:125–127. doi: 10.1249/00005768-199402000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Li H, Smalligan DA, Xie N, Javer A, Zhang Y, Hanley G, Yin D. beta-Arrestin 2-Mediated Immune Suppression Induced by Chronic Stress. Neuroimmunomodulation. 2011;18:142–149. doi: 10.1159/000322868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang KX, Shi YF, Ron Y, Kazanecki CC, Denhardt DT. Plasma osteopontin modulates chronic restraint stress-induced thymus atrophy by regulating stress hormones: inhibition by an anti-osteopontin monoclonal antibody. J.Immunol. 2009;182:2485–2491. doi: 10.4049/jimmunol.0803023. [DOI] [PubMed] [Google Scholar]
- 16.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 17.He L, Li H, Chen L, Miao J, Jiang Y, Zhang Y, Xiao Z, Hanley G, Li Y, Zhang X, LeSage G, Peng Y, Yin D. Toll-like receptor 9 is required for opioid-induced microglia apoptosis. PLoS.One. 2011;6:e18190. doi: 10.1371/journal.pone.0018190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Shi W, Li H, Sun X, Fan X, LeSage G, Li H, Li Y, Zhang Y, Zhang X, Zhang Y, Yin D. Critical role of toll-like receptor 9 in morphine and Mycobacterium tuberculosis-Induced apoptosis in mice. PLoS.One. 2010;5:e9205. doi: 10.1371/journal.pone.0009205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haase R, Kirschning CJ, Sing A, Schrottner P, Fukase K, Kusumoto S, Wagner H, Heesemann J, Ruckdeschel K. A dominant role of Toll-like receptor 4 in the signaling of apoptosis in bacteria-faced macrophages. J.Immunol. 2003;171:4294–4303. doi: 10.4049/jimmunol.171.8.4294. [DOI] [PubMed] [Google Scholar]
- 20.Liang X, Moseman EA, Farrar MA, Bachanova V, Weisdorf DJ, Blazar BR, Chen W. Toll-like receptor 9 signaling by CpG-B oligodeoxynucleotides induces an apoptotic pathway in human chronic lymphocytic leukemia B cells. Blood. 2010;115:5041–5052. doi: 10.1182/blood-2009-03-213363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plitas G, Burt BM, Nguyen HM, Bamboat ZM, DeMatteo RP. Toll-like receptor 9 inhibition reduces mortality in polymicrobial sepsis. J.Exp.Med. 2008;205:1277–1283. doi: 10.1084/jem.20080162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin D, Zhang Y, Stuart C, Miao J, Zhang Y, Li C, Zeng X, Hanley G, Moorman J, Yao Z, Woodruff M. Chronic restraint stress modulates expression of genes in murine spleen. J.Neuroimmunol. 2006;177:11–17. doi: 10.1016/j.jneuroim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Yang X, Zhou G, Ren T, Li H, Zhang Y, Yin D, Qian H, Li Q. beta-Arrestin prevents cell apoptosis through pro-apoptotic ERK1/2 and p38 MAPKs and anti-apoptotic Akt pathways. Apoptosis. 2012;17:1019–1026. doi: 10.1007/s10495-012-0741-2. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Sun X, LeSage G, Zhang Y, Liang Z, Chen J, Hanley G, He L, Sun S, Yin D. beta-Arrestin 2 regulates toll-like receptor 4-mediated apoptotic signalling through glycogen synthase kinase-3beta. Immunology. 2010;130:556–563. doi: 10.1111/j.1365-2567.2010.03256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Li H, Zhang Y, Sun X, Hanley GA, LeSage G, Zhang Y, Sun S, Peng Y, Yin D. Toll-like receptor 2 is required for opioids-induced neuronal apoptosis. Biochem.Biophys.Res.Commun. 2010;391:426–430. doi: 10.1016/j.bbrc.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oka H, Emori Y, Hayashi Y, Nomoto K. Breakdown of Th cell immune responses and steroidogenic CYP11A1 expression in CD4+ T cells in a murine model implanted with B16 melanoma. Cell Immunol. 2000;206:7–15. doi: 10.1006/cimm.2000.1715. [DOI] [PubMed] [Google Scholar]
- 27.Qiao S, Chen L, Okret S, Jondal M. Age-related synthesis of glucocorticoids in thymocytes. Exp.Cell Res. 2008;314:3027–3035. doi: 10.1016/j.yexcr.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann.N.Y.Acad.Sci. 2006;1069:62–76. doi: 10.1196/annals.1351.006. [DOI] [PubMed] [Google Scholar]
- 29.Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J.Exp.Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Tang Y, Teng L, Wu Y, Zhao X, Pei G. Association of beta-arrestin and TRAF6 negatively regulates Toll-like receptor-interleukin 1 receptor signaling. Nat.Immunol. 2006;7:139–147. doi: 10.1038/ni1294. [DOI] [PubMed] [Google Scholar]
- 32.Dhabhar FS. Stress-induced augmentation of immune function--the role of stress hormones, leukocyte trafficking, and cytokines. Brain Behav.Immun. 2002;16:785–798. doi: 10.1016/s0889-1591(02)00036-3. [DOI] [PubMed] [Google Scholar]
- 33.Dhabhar FS, Satoskar AR, Bluethmann H, David JR, McEwen BS. Stress-induced enhancement of skin immune function: A role for gamma interferon. Proc.Natl.Acad.Sci.U.S.A. 2000;97:2846–2851. doi: 10.1073/pnas.050569397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Minton JE. Function of the hypothalamic-pituitary-adrenal axis and the sympathetic nervous system in models of acute stress in domestic farm animals. J.Anim Sci. 1994;72:1891–1898. doi: 10.2527/1994.7271891x. [DOI] [PubMed] [Google Scholar]