Abstract
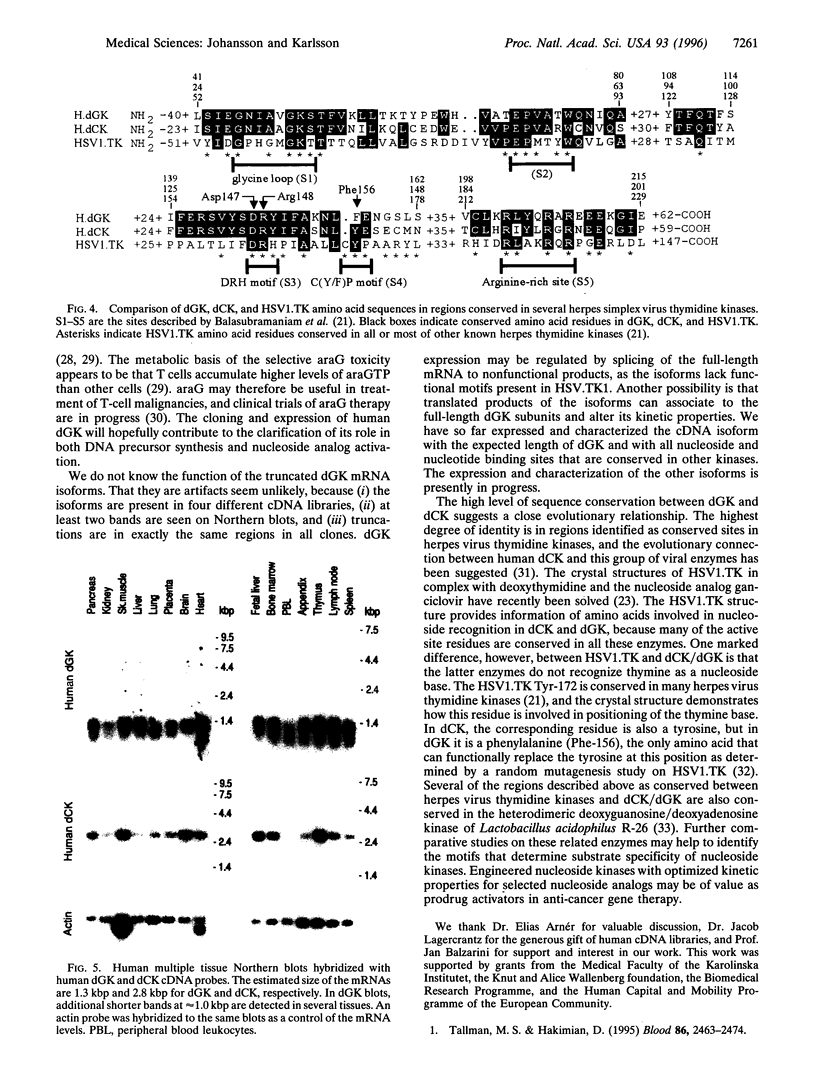
A human cDNA sequence homologous to human deoxycytidine kinase (dCK; EC 2.7.1.74) was identified in the GenBank sequence data base. The longest open reading frame encoded a protein that was 48% identical to dCK at the amino acid level. The cDNA was expressed in Escherichia coli and shown to encode a protein with the same substrate specificity as described for the mitochondrial deoxyguanosine kinase (dGK; EC 2.7.1.113). The N terminus of the deduced amino acid sequence had properties characteristic for a mitochondrial translocation signal, and cleavage at a putative mitochondrial peptidase cleavage site would give a mature protein size of 28 kDa. Northern blot analysis determined the length of dGK mRNA to 1.3 kbp with no cross-hybridization to the 2.8-kbp dCK mRNA. dGK mRNA was detected in all tissues investigated with the highest expression levels in muscle, brain, liver, and lymphoid tissues. Alignment of the dGK and herpes simplex virus type 1 thymidine kinase amino acid sequences showed that five regions, including the substrate-binding pocket and the ATP-binding glycine loop, were also conserved in dGK. To our knowledge, this is the first report of a cloned mitochondrial nucleoside kinase and the first demonstration of a general sequence homology between two mammalian deoxyribonucleoside kinases. Our findings suggest that dCK and dGK are evolutionarily related, as well as related to the family of herpes virus thymidine kinases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arnér E. S., Eriksson S. Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 1995;67(2):155–186. doi: 10.1016/0163-7258(95)00015-9. [DOI] [PubMed] [Google Scholar]
- Arnér E. S., Flygar M., Bohman C., Wallström B., Eriksson S. Deoxycytidine kinase is constitutively expressed in human lymphocytes: consequences for compartmentation effects, unscheduled DNA synthesis, and viral replication in resting cells. Exp Cell Res. 1988 Oct;178(2):335–342. doi: 10.1016/0014-4827(88)90403-x. [DOI] [PubMed] [Google Scholar]
- Arnér E. S. On the phosphorylation of 2-chlorodeoxyadenosine (CdA) and its correlation with clinical response in leukemia treatment. Leuk Lymphoma. 1996 Apr;21(3-4):225–231. doi: 10.3109/10428199209067604. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam N. K., Veerisetty V., Gentry G. A. Herpesviral deoxythymidine kinases contain a site analogous to the phosphoryl-binding arginine-rich region of porcine adenylate kinase; comparison of secondary structure predictions and conservation. J Gen Virol. 1990 Dec;71(Pt 12):2979–2987. doi: 10.1099/0022-1317-71-12-2979. [DOI] [PubMed] [Google Scholar]
- Bohman C., Eriksson S. Deoxycytidine kinase from human leukemic spleen: preparation and characteristics of homogeneous enzyme. Biochemistry. 1988 Jun 14;27(12):4258–4265. doi: 10.1021/bi00412a009. [DOI] [PubMed] [Google Scholar]
- Brown D. G., Visse R., Sandhu G., Davies A., Rizkallah P. J., Melitz C., Summers W. C., Sanderson M. R. Crystal structures of the thymidine kinase from herpes simplex virus type-1 in complex with deoxythymidine and ganciclovir. Nat Struct Biol. 1995 Oct;2(10):876–881. doi: 10.1038/nsb1095-876. [DOI] [PubMed] [Google Scholar]
- Chottiner E. G., Shewach D. S., Datta N. S., Ashcraft E., Gribbin D., Ginsburg D., Fox I. H., Mitchell B. S. Cloning and expression of human deoxycytidine kinase cDNA. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1531–1535. doi: 10.1073/pnas.88.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Q., Russell M., Birch D. E., Raymond J., Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992 Apr 11;20(7):1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A., Lee J. W., Gelfand E. W. Selective toxicity of deoxyguanosine and arabinosyl guanine for T-leukemic cells. Blood. 1983 Apr;61(4):660–666. [PubMed] [Google Scholar]
- Datta N. S., Shewach D. S., Mitchell B. S., Fox I. H. Kinetic properties and inhibition of human T lymphoblast deoxycytidine kinase. J Biol Chem. 1989 Jun 5;264(16):9359–9364. [PubMed] [Google Scholar]
- Eriksson S., Kierdaszuk B., Munch-Petersen B., Oberg B., Johansson N. G. Comparison of the substrate specificities of human thymidine kinase 1 and 2 and deoxycytidine kinase toward antiviral and cytostatic nucleoside analogs. Biochem Biophys Res Commun. 1991 Apr 30;176(2):586–592. doi: 10.1016/s0006-291x(05)80224-4. [DOI] [PubMed] [Google Scholar]
- Gower W. R., Jr, Carr M. C., Ives D. H. Deoxyguanosine kinase. Distinct molecular forms in mitochondria and cytosol. J Biol Chem. 1979 Apr 10;254(7):2180–2183. [PubMed] [Google Scholar]
- Habteyesus A., Nordenskjöld A., Bohman C., Eriksson S. Deoxynucleoside phosphorylating enzymes in monkey and human tissues show great similarities, while mouse deoxycytidine kinase has a different substrate specificity. Biochem Pharmacol. 1991 Oct 9;42(9):1829–1836. doi: 10.1016/0006-2952(91)90522-7. [DOI] [PubMed] [Google Scholar]
- Harrison P. T., Thompson R., Davison A. J. Evolution of herpesvirus thymidine kinases from cellular deoxycytidine kinase. J Gen Virol. 1991 Oct;72(Pt 10):2583–2586. doi: 10.1099/0022-1317-72-10-2583. [DOI] [PubMed] [Google Scholar]
- Hendrick J. P., Hodges P. E., Rosenberg L. E. Survey of amino-terminal proteolytic cleavage sites in mitochondrial precursor proteins: leader peptides cleaved by two matrix proteases share a three-amino acid motif. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4056–4060. doi: 10.1073/pnas.86.11.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Karlsson A. Differences in kinetic properties of pure recombinant human and mouse deoxycytidine kinase. Biochem Pharmacol. 1995 Jul 17;50(2):163–168. doi: 10.1016/0006-2952(95)00129-n. [DOI] [PubMed] [Google Scholar]
- Lennon G., Auffray C., Polymeropoulos M., Soares M. B. The I.M.A.G.E. Consortium: an integrated molecular analysis of genomes and their expression. Genomics. 1996 Apr 1;33(1):151–152. doi: 10.1006/geno.1996.0177. [DOI] [PubMed] [Google Scholar]
- Lewis R. A., Link L. Phosphorylation of arabinosyl guanine by a mitochondrial enzyme of bovine liver. Biochem Pharmacol. 1989 Jun 15;38(12):2001–2006. doi: 10.1016/0006-2952(89)90500-5. [DOI] [PubMed] [Google Scholar]
- Ma G. T., Hong Y. S., Ives D. H. Cloning and expression of the heterodimeric deoxyguanosine kinase/deoxyadenosine kinase of Lactobacillus acidophilus R-26. J Biol Chem. 1995 Mar 24;270(12):6595–6601. [PubMed] [Google Scholar]
- Munir K. M., French D. C., Dube D. K., Loeb L. A. Permissible amino acid substitutions within the putative nucleoside binding site of herpes simplex virus type 1 encoded thymidine kinase established by random sequence mutagenesis [corrected]. J Biol Chem. 1992 Apr 5;267(10):6584–6589. [PubMed] [Google Scholar]
- Nakai K., Kanehisa M. A knowledge base for predicting protein localization sites in eukaryotic cells. Genomics. 1992 Dec;14(4):897–911. doi: 10.1016/S0888-7543(05)80111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W. Heinrich Wieland--prize lecture. Transport of proteins across mitochondrial membranes. Clin Investig. 1994 Mar;72(4):251–261. doi: 10.1007/BF00180036. [DOI] [PubMed] [Google Scholar]
- Park I., Ives D. H. Kinetic mechanism and end-product regulation of deoxyguanosine kinase from beef liver mitochondria. J Biochem. 1995 May;117(5):1058–1061. doi: 10.1093/oxfordjournals.jbchem.a124806. [DOI] [PubMed] [Google Scholar]
- Park I., Ives D. H. Properties of a highly purified mitochondrial deoxyguanosine kinase. Arch Biochem Biophys. 1988 Oct;266(1):51–60. doi: 10.1016/0003-9861(88)90235-4. [DOI] [PubMed] [Google Scholar]
- Snyder F. F., Jenuth J. P., Dilay J. E., Fung E., Lightfoot T., Mably E. R. Secondary loss of deoxyguanosine kinase activity in purine nucleoside phosphorylase deficient mice. Biochim Biophys Acta. 1994 Oct 21;1227(1-2):33–40. doi: 10.1016/0925-4439(94)90103-1. [DOI] [PubMed] [Google Scholar]
- Spasokoukotskaja T., Arnér E. S., Brosjö O., Gunvén P., Juliusson G., Liliemark J., Eriksson S. Expression of deoxycytidine kinase and phosphorylation of 2-chlorodeoxyadenosine in human normal and tumour cells and tissues. Eur J Cancer. 1995;31A(2):202–208. doi: 10.1016/0959-8049(94)00435-8. [DOI] [PubMed] [Google Scholar]
- Tallman M. S., Hakimian D. Purine nucleoside analogs: emerging roles in indolent lymphoproliferative disorders. Blood. 1995 Oct 1;86(7):2463–2474. [PubMed] [Google Scholar]
- Traut T. W. The functions and consensus motifs of nine types of peptide segments that form different types of nucleotide-binding sites. Eur J Biochem. 1994 May 15;222(1):9–19. doi: 10.1111/j.1432-1033.1994.tb18835.x. [DOI] [PubMed] [Google Scholar]
- Ullman B., Martin D. W., Jr Specific cytotoxicity of arabinosylguanine toward cultured T lymphoblasts. J Clin Invest. 1984 Sep;74(3):951–955. doi: 10.1172/JCI111514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Karlsson A., Arnér E. S., Eriksson S. Substrate specificity of mitochondrial 2'-deoxyguanosine kinase. Efficient phosphorylation of 2-chlorodeoxyadenosine. J Biol Chem. 1993 Oct 25;268(30):22847–22852. [PubMed] [Google Scholar]
- Yamada Y., Goto H., Ogasawara N. Deoxyguanosine kinase from human placenta. Biochim Biophys Acta. 1982 Dec 20;709(2):265–272. doi: 10.1016/0167-4838(82)90469-1. [DOI] [PubMed] [Google Scholar]