Abstract
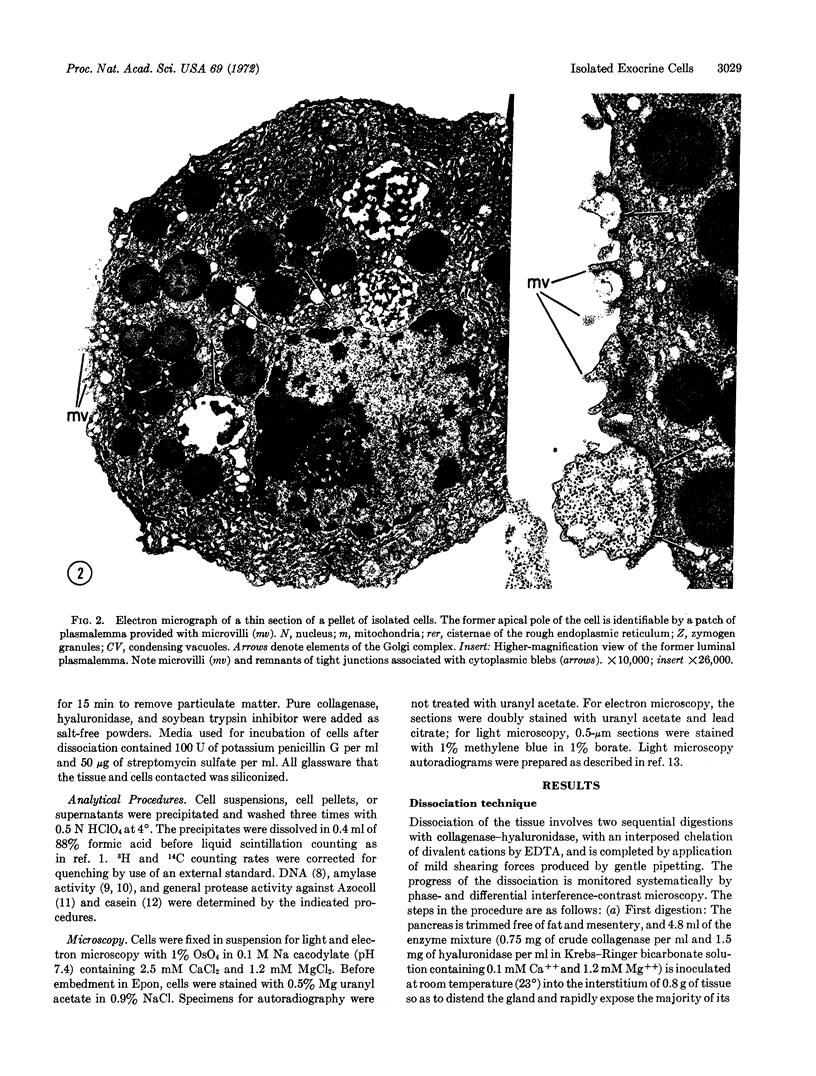
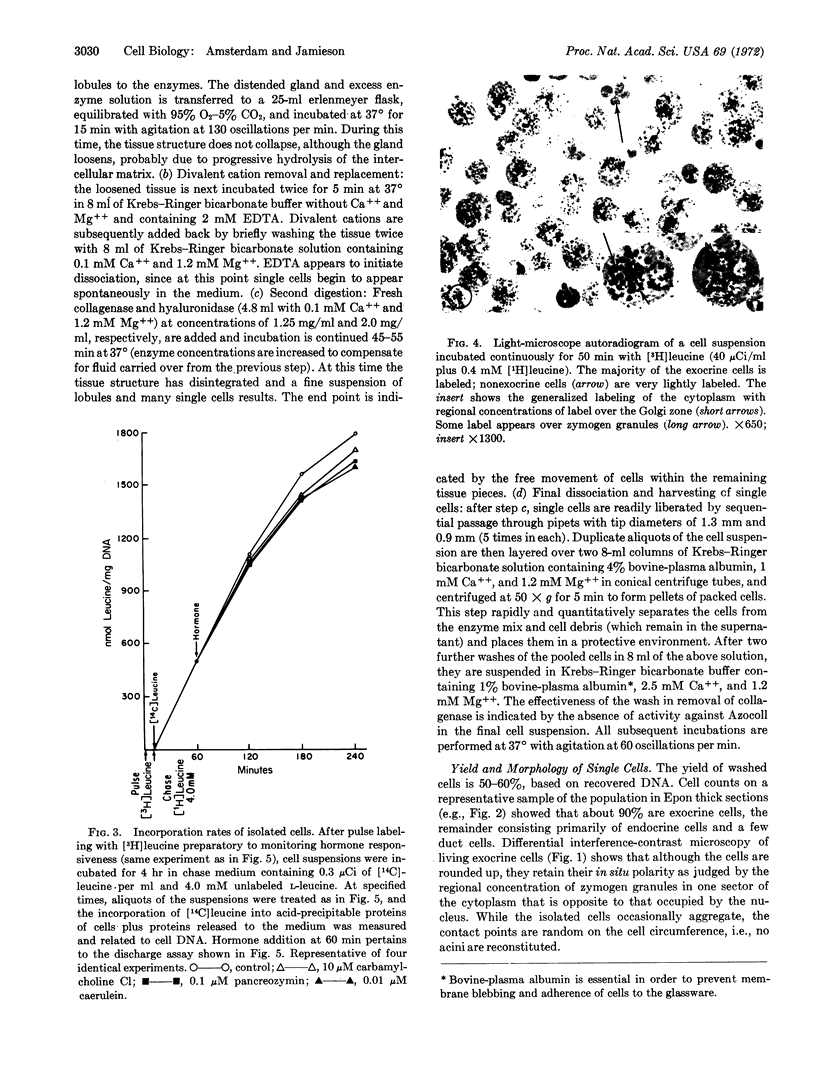
Viable isolated exocrine cells have been obtained from guinea-pig pancreas by a tissue dissociation procedure using crude collagenase (EC 3.4.4.19) and hyaluronidase (EC 3.2.1.35), chelation of divalent cations, and mild shearing forces. Cell yields are 50-60%, based on recovered DNA, and about 90% of the population consists of exocrine cells which, although rounded up, retain their in situ polarity with regard to regional distribution of zymogen granules and specialization of the former luminal plasmalemma. The isolated cells incorporate labeled amino acids into proteins at linear rates for at least 4 hr at levels comparable to pancreatic slices in vitro; more than 95% of the exocrine cell population is active in this process, as shown by autoradiography. In response to secretogogues at optimal doses (100 μM carbamylcholine; 0.1 μM pancreozymin, or 0.01 μM caerulein), the cells discharge up to 30% of their content of pulse-labeled secretory proteins to the medium over a 3-hr period; in the same time, the controls release about 5% of their content. The results indicate that isolated exocrine cells are capable of synthesizing the processing secretory proteins, and of responding directly to cholinergic and peptidic secretogogues.
Keywords: tissue dissociation, collagenase, hyaluronidase, protein synthesis, secretogogue response
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARO L. G., VAN TUBERGEN R. P., KOLB J. A. High-resolution autoradiography. I. Methods. J Cell Biol. 1962 Nov;15:173–188. doi: 10.1083/jcb.15.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle J. D., Jamieson J. D., Palade G. E. Radioautographic analysis of the secretory process in the parotid acinar cell of the rabbit. J Cell Biol. 1972 May;53(2):290–311. doi: 10.1083/jcb.53.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. B., Christensen A. K., Gibbs F. A., Pesch L. A. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967 Dec;35(3):675–684. doi: 10.1083/jcb.35.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Synthesis, intracellular transport, and discharge of secretory proteins in stimulated pancreatic exocrine cells. J Cell Biol. 1971 Jul;50(1):135–158. doi: 10.1083/jcb.50.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono T. Destruction and restoration of the insulin effector system of isolated fat cells. J Biol Chem. 1969 Nov 10;244(21):5777–5784. [PubMed] [Google Scholar]
- MANDL I., MACLENNAN J. D., HOWES E. L. Isolation and characterization of proteinase and collagenase from Cl. histolyticum. J Clin Invest. 1953 Dec;32(12):1323–1329. doi: 10.1172/JCI102861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir A. R. The effects of divalent cations on the ultrastructure of the perfused rat heart. J Anat. 1967 Apr;101(Pt 2):239–261. [PMC free article] [PubMed] [Google Scholar]
- Pessac B., Defendi V. Cell aggregation: role of acid mucopolysaccharides. Science. 1972 Feb 25;175(4024):898–900. doi: 10.1126/science.175.4024.898. [DOI] [PubMed] [Google Scholar]






