Abstract
Traditional stem cell differentiation protocols make use of a variety of cytokines including growth factors (GFs) and inhibitors in an effort to provide appropriate signals for tissue specific differentiation. In this study, iPSC-derived type II pneumocytes (iPSC-ATII) as well as native isolated human type II pneumocytes (hATII) were differentiated toward a type I phenotype using a unique air–liquid interface (ALI) system that relies on a rotating apparatus that mimics in vivo respiratory conditions. A relatively homogenous population of alveolar type II-like cells from iPSC was first generated (iPSC-ATII cells), which had phenotypic properties similar to mature human alveolar type II cells. iPSC-ATII cells were then cultured in a specially designed rotating culture apparatus. The effectiveness of the ALI bioreactor was compared with the effectiveness of small molecule-based differentiation of type II pneumocytes toward type 1 pneumocytes. The dynamics of differentiation were examined by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), flow cytometry and immunocytochemistry. iPSC-ATII and hATII cells cultured in the ALI bioreactor had higher levels of type I markers, including aquaporin-5(AQ5), caveolin-1, and T1α, at both the RNA and protein levels as compared with the flask-grown iPSC-ATII and hATII that had been treated with small molecules to induce differentiation. In summary, this study demonstrates that a rotating bioreactor culture system that provides an air–liquid interface is a potent inducer of type I epithelial differentiation for both iPS-ATII cells and hATII cells, and provides a method for large-scale production of alveolar epithelium for tissue engineering and drug discovery.
Keywords: Induced pluripotent stem (iPS), Alveolar epithelium, Bioreactor, Air—liquid interface
1. Introduction
The lung has a complex three-dimensional structure that features major differences in the composition of the epithelium along its proximo-distal axis. The most distal region of the lung is organized into a complex system of alveoli that are lined by two primary epithelial cells types: Type I (ATI) and Type II (ATII) pneumocytes. ATI cells line the majority of the alveoli in lung (covering up to 95% of the alveolar surface area) and are primarily responsible for gas-exchange, while ATII pneumocytes secrete alveolar surfactants and are primarily cuboidal in shape [1–3].
Primary isolations of ATII and ATI pneumocytes have been performed using various protocols [4,5], but their yield and proliferation rate is low. Typically, freshly isolated pneumocytes are exceedingly difficult to grow and maintain. These primary cells lose many of their characteristics within a few days of standard cell culture [5]. Therefore, a significant emphasis is being placed on identifying a reliable source of functional lung epithelial cells and designing a suitable cell culture system to maintain proper epithelial cell phenotypes [6,7]. One approach consists of using stem cells that can be manipulated to become alveolar epithelial cells. Induced pluripotent stem cells (iPSC) are the product of adult somatic cell reprogramming to an embryonic-like state by inducing a “forced” expression of specific pluripotent genes [7–12]. Given that iPS cells can be derived from the patient to be treated, they could provide a cell source that is genetically identical to the patient, allowing tissue that is generated from these cells to avoid immune rejection [9,13].
Lung epithelia remain among the least studied lineages derived from ESCs and iPSCs in vitro [2,10–12,14–16]. In our previous study, we showed the feasibility of producing ATII cells with a very high purity from iPSCs [17]. These iPSC-derived ATII cells, referred to as iPSC-ATII, display as a typical cuboidal appearance and express markers associated with ATII cells (Fig. 1) [17]. In this study we specifically focused on the differentiation of the iPSC-ATII cells toward the type I (ATI) alveolar phenotype.
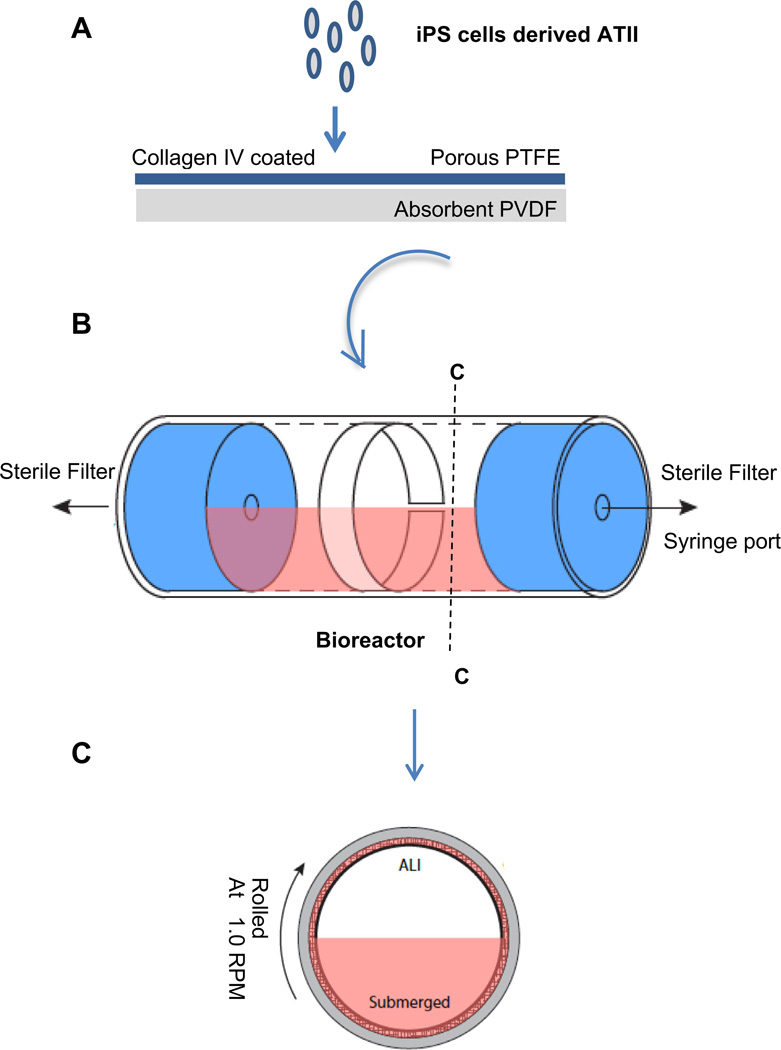
Fig. 1.
(A) Schematic summarizing the iPSC-derived ATII transfer onto membrane and differentiation to ATI in rolling ALI bioreactor system. (B) Schematic figure of assembled bioreactor. (C) Cross sectional view of cells cultured in air–liquid interface condition in bioreactor.
Although ATII cells are differentiated, these cells nonetheless retain a level of plasticity. In peripheral lung injury, ATII undergo proliferation and differentiation toward the ATI phenotype [18– 20]. In fact, ATII are considered to be putative alveolar stem cells and are crucial to the natural regenerative process of the alveoli [3,18,19,21,22]. Several studies have shown that exposure to air or inhibition of the Wnt/b-catenin signaling pathway can change the marker expression profile of the differentiated ATII-like phenotype to a predominantly ATI-like phenotype [2,19,21,23–26].
Based upon these facts, following the generation of iPSC-ATII, we examined the impact of air—liquid interface culture, as well as Wnt/β-catenin inhibitors, on the differentiation of iPSC-ATII to the ATI phenotype. iPSC-ATII cells were cultured on a non-cytotoxic mesh, and then transferred to a dynamic rolling system to recapitulate the air–liquid interface. This in vitro biological environment in some ways mimics in vitro respiratory conditions, as the system is continuously rolled to allow cells to spend an equal amount of time in air and liquid. Herein we investigated the impact that the rolling bioreactor culture system (ALI system) has on the differentiation of iPSC-ATII and hATII cells into cells that are ATI-like, either in the absence of or with the addition of soluble factors.
2. Materials and methods
2.1. Cultivation of human iPS cells
The human iPSC line IMR90 (which is denoted as “C1” here), and a line derived from neonatal foreskin, denoted as “C2” here, were utilized. Both lines were provided by Prof. James A Thomson, Department of Anatomy, University of Wisconsin–Madison, Madison, WI [9]. Both human iPSC lines were generated by lentiviral infection of isolated human skin fibroblasts with OCT-4, SOX2, Nanog and lin28 genes. These cells have normal karyotypes, express telomerase activity, express cell surface markers and genes that characterize human ES cells, and maintain the developmental potential to differentiate into advanced derivatives of all three primary germ layers. Both human iPS cells were cultured and maintained as described previously [8,9]. Briefly, iPSCs were propagated on irradiated mouse embryonic fibroblast (MEF) feeder layers in DMEM-F12 media and 20% of knock out serum replacement supplemented with 4 ng/ml bFGF, 1 mm Glutamine, 1% mm nonessential amino acids and 0.1 mm β-mercapthoethanol at 37 °C, 5% CO2 and 90– 95% humidity, with medium changes every day. Undifferentiated iPS cells were passaged every 4–5 days onto fresh feeders by mechanical dissociation using a Stem Cell Cutting Tool (VWR).
2.2. In vitro differentiation of iPS cells to alveolar type II
The human iPSCs were differentiated to alveolar epithelium in a directed differentiation protocol via definitive endoderm (DE) and anterior foregut endoderm (AFE) as previously described [17]. iPSCs were first differentiated toward definitive endoderm (DE) under conditions described previously [27–29]. Briefly, iPSCs were cultured in RPMI 1640 medium supplemented with 100 ng/ml activin A, 2 mm l-glutamine and 1% antibiotic-antimycotic for 48 h. Then, 1 × B27 supplement, 0.5 mm sodium butyrate and 0.1% FBS were added into the same medium and iPSCs were cultured in this medium for another 4 days with daily medium changes.
Following the generation of a DE population, cells were then split with trypsin and reseeded at a ratio of 1:1–2 on human ECM protein coated plates for anterior foregut endoderm (AFE) differentiation in the culture medium consisting of IMDM with 5% FBS, 2 mm l-glutamine, 1 mm nonessential amino acids, 1% antibiotic-antimycotic supplemented with 100 ng/ml NOGGIN and 10 mm SB-431542 for 2 days [12].
Subsequently, the AFE cells were maintained in the differentiation medium consisting of IMDM with 10% FBS, 2 mm l-glutamine, 1 mm nonessential amino acids, 1% antibiotic-antimycotic, retinoic acid (0.5 µm), FGF-10 (10 ng/ml), EGF (10 ng/ml), Wnt3a (100 ng/ml), and KGF (10 ng/ml each) for 10–14 days. Then the cells were further differentiated and maintained in Celprogen’s Human type II alveolar primary cell culture complete growths medium until use. Cells resulting from this differentiation protocol from clones C1 and C2 express many type II markers and are termed ‘iPSC-ATII cells’ [17].
2.3. Native human alveolar type II cell isolation
Human alveolar type II (hATII) cells were isolated from human lungs that were rejected for transplant, following protocols approved by the University of North Carolina Institutional Review Board and as previously described with slight modifications [30]. Briefly, the right middle lobe was cannulated through the main stem bronchus, followed by instillation of Elastase (13 units/ml) and a 45 min period at 37 °C. The tissue was then minced in the presence of 5% FBS. The cells were filtered through a series of 150 and 50 um filters, followed by incubation in pan-mouse igG Dynabeads to deplete the fibroblast population. The final suspension (hATII cells) was cultured in DMEM plus 10% FBS supplemented with amphotericin B, ceftazidime, tobramycin, and vancomycin.
2.4. Bioreactor design, fabrication and assembly
The bioreactor in this study is designed to simulate the air–liquid interface, as previously described [31]. It consists of a borosilicate glass tube, 38 mm OD, 32.3 mm ID, capped with two silicone stoppers (Cole-Parmer R-06298-14). A hole is bored through the center of each stopper to accommodate the insertion of Master-Flex L/S 16 tubing (Cole-Parmer ZW-06508-16). A rotating, sealing luer-lock (Cole-Parmer ZW-06464-90) is then attached to one end of the vial to allow the chamber to rotate freely, even with a stopcock attached (Fig. 1A–C). Sterile filters are attached to each end for periodic airflow, and a syringe port is attached to allow daily culture medium exchanges. A custom system of rollers was designed and manufactured (Raredon Resources Inc.) to rotate the reactor chambers at speeds ranging from 0 to 100 rpm. The rollers were designed to be modular, accommodating the simultaneous culture of up to 10 rolling chambers, with units easily added or removed as needed [31].
Millipore’s Biopore Membrane (BGCM00010) is used as a cell support inside the reactor chamber [31]. This membrane contains two layers that adhere to one another. The upper layer, upon which cells were seeded, is polytetrafluorethylene (PTFE), 50 µm thick, with 0.4 µm pores. The lower layer is hydrophilic polyvinyl (PVDF), 200 µm thick, with 0.22 µm pores. The PVDF layer acts as an absorbent sponge for culture medium during ALI culture, and supplies the above PTFE layer and attached cells with a medium reservoir for nutrient transfer (Fig. 1A).
2.5. Culturing iPSC-ATII on different matrix proteins
iPSC-ATII was cultured on different extracellular proteins including fibronectin (50 µg/ml) (Sigma–Aldrich), collagen I (100 µg/ml)(Sigma–Aldrich), collagen IV (50 µg/ml)(Sigma–Aldrich), Matrigel(1:80)(BD Bioscience), and a mixture of fibronectin, collagen I, collagen IV (mix at the ratio of 1:1:1), human ECM proteins (1:100) (consisting of collagens, laminin, fibronectin, tenascin, elastin, and a number of proteoglycans and glycosaminoglycans; Sigma) for 7 days. qRT-PCR for expression of epithelial type II markers, SPC and SPB and SPA was performed on iPSC-ATII cultured on different extracellular proteins.
2.6. Differentiation of iPS-derived alveolar type II to type I cells in bioreactor culture system
Prior to cell seeding, the membranes were sterilized by immersion in 70% ethanol for 30 min. Then the membranes were coated with collagen IV (Sigma– Aldrich, 0.05 mg/ml) overnight at 37 °C. The iPSC-ATII cells that had been differentiated for 22 days were removed by trypsinization and reseeded onto the collagen IV-coated membrane at a concentration of 2 × 105 cells/cm2 (Fig. 1A). The constructs were incubated for 6 h at 37 °C to allow the cells to attach, and then were completely submerged in the culture medium consisting of DMEM with 10% FBS, 2 mm l-glutamine, 1 mm nonessential amino acids, 1% penicillin/streptomycin and incubated for another 24 h. The membranes were then lifted and carefully rolled and inserted into pre-sterilized reactor chambers under sterile conditions. The reactor was sealed with the silicone caps, including the already assembled rotating ports, stopcock, and attached filters and syringe port (Fig. 1A–C). The vials were filled with 15 ml of DMEM-10% FBS with medium changes every day, corresponding to 1.5 ml of medium for every square centimeter of construct. The assembled reactors were then placed on the custom roller and set to roll at approximately 1 rpm/min. All rolled constructs had a matched control, which was grown under static conditions submerged in a tissue culture dish rather than in a rolled vial. In additional experiments, native human type II alveolar epithelial cells (hATII cells) were seeded at a density of ~ 2 × 105 per cm2 and cultured in the reactors for 7 days under either static or rolling conditions in DMEM with 10% FBS and daily medium changes. Samples were taken for analysis immediately prior to insertion into the reactors, and after 7 days of culture under either static or rolling conditions.
2.7. In vitro differentiation of iPSC-AETII cells to type I-like cells using small molecules
We also examined the type I cell phenotype induction of iPSC-ATII cells in the presence of Wnt/β-catenin inhibitors. To differentiate iPSC-ATII cells toward type I cells, at day 22 of differentiation cells were switched to DMEM medium with 10% FBS, 2 mm l-glutamine, 1 mm nonessential amino acids, 1% antibiotic-antimycotic and 100 mm IWR-1 (Sigma–Aldrich) (a Wnt/b-catenin inhibitor) for 7 days. In parallel experiments, native human hATII cells were cultured under the same conditions for 7 days. In another set of experiments, the effect of another Wnt/β-catenin inhibitor, ICG-001 (Sigma–Aldrich), on the differentiation of iPSC-ATII cells toward type I cells was examined and compared to IWR-1. iPSC-ATII were cultured in the same medium supplemented with 5 mm of ICG-001 for 7 days.
2.8. Flow cytometry and immunohistochemistry analysis
The differentiation of iPSC-ATII and hATII to ATI cells was assessed by immunofluorescence staining and/or flow cytometry at day 7, after cultivation in the rolling bioreactor or in static culture with soluble factors. Constructs were extracted from the bioreactor and a portion of the constructs was cut into 1 cm × 0.5 cm pieces. For immunostaining, cells in static culture and those from the rolling bioreactor were washed twice with PBS, fixed in 4% paraformaldehyde in PBS for 20 min at room temperature (RT), permeabilized with 0.1% Triton X-100 in PBS for 15 min at RT, and then blocked in 3% BSA in PBS for 60 min at RT. The cells were incubated with primary antibody overnight at 4 °C. The next day, cells were washed three times with PBS, cell were then incubated with corresponding secondary antibody for 2 h at RT. After washing with 1 × PBS, the cells were incubated with 1 (µg/ml 4, 6-diamidino-2-phenylindole (DAPI) to stain the nuclei. The PTFE membranes from the bioreactors were carefully removed from the PVDF sponge layer and mounted with PVA-DABCO cover slipping solution. The PTFE layer is hydrophilic and is optically clear under fluorescent channels, allowing planar staining and imaging. Cell preparations were imaged with a Zeiss Axiovert 200M inverted microscope and a Hamamatsu camera.
For flow cytometry, cells were fixed with the fixation solution from the Fixation/Permeabilization kit (BD Biosciences), and stained with primary and detection antibodies as described by the manufacturer. Briefly, cells cultured in either tissue culture flasks or on bioreactor membranes were dissociated into single-cell suspensions by incubation with 0.25% trypsin for 2 min. The dissociated cells were resuspended (0.5 × 106 cells) in 250 µl of fixation/permeabilization solution, kept on ice for 20 min, and washed twice with Perm/Wash buffer. After blocking with blocking solution for 30 min on ice, the cells were incubated with the corresponding primary antibody in blocking solution for 30 min on ice. The cells were then resuspended in 350 µl of Perm/Wash buffer after incubation with corresponding conjugated secondary for 30 min on ice, washed twice, and analyzed by flow cytometry. The primary and detection antibodies used are listed in Table 1.
Table 1.
List of antibodies used in staining, flow cytometry and western blot for various experiments.
| Primary antibodies | |||
|---|---|---|---|
| Antigen | Type | Provider (Cat#) (lot#) | Application |
| Nkx2.1 | Rabbit polyclonal | Abcam (Cat# ab76013, Lot# GR76790-2) | ICC, IHC, FC |
| proSPB | Rabbit polyclonal | Millipore (Cat# ab3430, Lot# NG1820771) | ICC, FC |
| proSPC | Rabbit monoclonal | Millipore (Cat# ab3786, Lot# 2117989) | ICC, IHC, FC |
| SPA | Rabbit polyclonal | Millipore (Cat# ab3420, Lot# NG1888873) | ICC |
| SPC | Rabbit polyclonal | Santa Cruz (Cat# sc-13979, Lot# L1710) | ICC, IHC, FC |
| T1α | Monoclonal | Abcam (Cat# ab10288, Lot# GR47830-3) | IHC |
| AQ5 | Rabbit polyclonal | Abcam(Cat# ab18530) | ICC |
| Caveolin-1 | Rabbit monoclonal | Millipore(Cat#MAB4381, Lot# LV1512392) | ICC |
| CD54-PE | Mouse monoclonal | BD Pharmingen (Cat# 560971, Lot# 10609) | FC |
| Detection antibodies | |||
| Type | Provider (Cat#) (lot#) | Application | |
| Alexa Fluor® 555 goat anti-rabbit IgG (H+L), highly cross-absorbed | Invitrogen (Cat# A21429, Lot# 1010124) | ICC, IHC | |
| Alexa Fluor® 488 goat anti-rabbit IgG (H+L), highly cross-adsorbed | Invitrogen (Cat# A11034, Lot# 1008720) | ICC, IHC | |
| Alexa Fluor® 555 goat anti-mouse IgG (H+L), highly cross-adsorbed | Invitrogen (Cat# A21424, Lot# 1214852) | ICC, IHC | |
| APC Mouse IgG2a, κ isotype control | BD Pharmingen (Cat# 555576, Lot# 33828) | Isotype control | |
| PE Mouse IgG1, κ isotype control | eBiosciences (Cat# 12-4714-82, Lot# E01672-1630) | Isotype control | |
| Mouse IgG2a, κ isotype control FTIC– | eBiosciences (Cat# 11-4724-81, Lot# E00590-1630) | Isotype control | |
| Isotype FITC goat anti mouse Ig | (Cat# 611233, Lot# 039557) | ||
| PE mouse IgG κ isotype control | (Cat# 555749, Lot# 38193) | ||
Abbreviations: IHC, immunohistochemistry; ICC, immunocytochemistry; FC, flow cytometry; PE, phycoerythrin, APC, allophycocyanin; FITC, fluorescein isothiocyanate; SPA, surfactant protein A; SPB, surfactant protein B; SPC, surfactant protein C; Nkx2.1, NK2 homeobox 1.
2.9. Real time quantitative RT-PCR
Total RNA was extracted using the RNeasy Mini Kit from Qiagen, following the manufacturer’s instructions. First-strand complementary DNA (cDNA) was synthesized with random hexamers as primers, using the SuperScript First-Strand Synthesis System according to the manufacturer’s protocol (Invitrogen). An equal volume mixture of the products was used as templates for PCR amplification. Reactions were performed in a 25 µl volume with iQ™ SYBR Green Supermix (Bio-Rad) and 200 nM each of forward and reverse primers using iCyler and iQ software (Bio-Rad). Each sample was run in triplicate. PCR conditions included an initial denaturation step of 4 min at 95 °C, followed by 40 cycles of PCR consisting of 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. Average threshold cycle (Ct) values from the triplicate PCR reactions for a gene of interest (GOI) were normalized against the average Ct values for GAPDH from the same cDNA sample. Fold change of GOI transcript levels between sample A and sample B equals 2−ΔΔct , where ΔCt = Ct(GOI) – Ct(GAPDH), and ΔΔCt = ΔCt(A) – ΔCt(B). The primers used are listed in Table 2.
Table 2.
Sequences of primers used in qRT-PCR for various experiments.
| Gene | Length (bp) | Primer sequences |
|---|---|---|
| hSPA | 180 | Forward: TCCAAGCCACACTCCACGA |
| Reverse: TTCCTCTGGATTCCTTGGG | ||
| hSPB | 69 | Forward: TGGGAGCCGATGACCTATG |
| Reverse: GCCTCCTTGGCCATCTTGT | ||
| hSPC | 94 | Forward: CCTTCTTATCGTGGTGGTGGT |
| Reverse: TCTCCGTGTGTTTCTGGCTCAT | ||
| hCaveolin-1 | 122 | Forward: CTACAAGCCCAACAACAAGG |
| Reverse: CATCGTTGAGGTGTTTAGGGT | ||
| hAQ5 | 79 | Forward: ACTGGGTTTTCTGGGTAGGG |
| Reverse: ATGGTCTTCTTCCGCTCTTC | ||
| hT1α | 110 | Forward: TCCAGGAACCAGCGAAGAC |
| Reverse: CGTGGACTGTGCTTTCTGA | ||
| hGAPDH | 122 | Forward: GACAACAGCCTCAAGATCATCAG |
| Reverse: ATGGCATGGACTGTGGTCATGAG |
2.10. Statistical analyses
All experiments were repeated at least three times and each condition was analyzed in triplicate. Data are presented as the means ± SEM for quantitative variables. An unpaired Student t test was performed to evaluate whether the two groups were significantly different from each other. One-way variance (ANOVA) analysis was used to determine whether there was a significant difference in the gene expression among different test groups. p ≤ 0.05 (two-tailed) was considered statistically significant.
3. Results
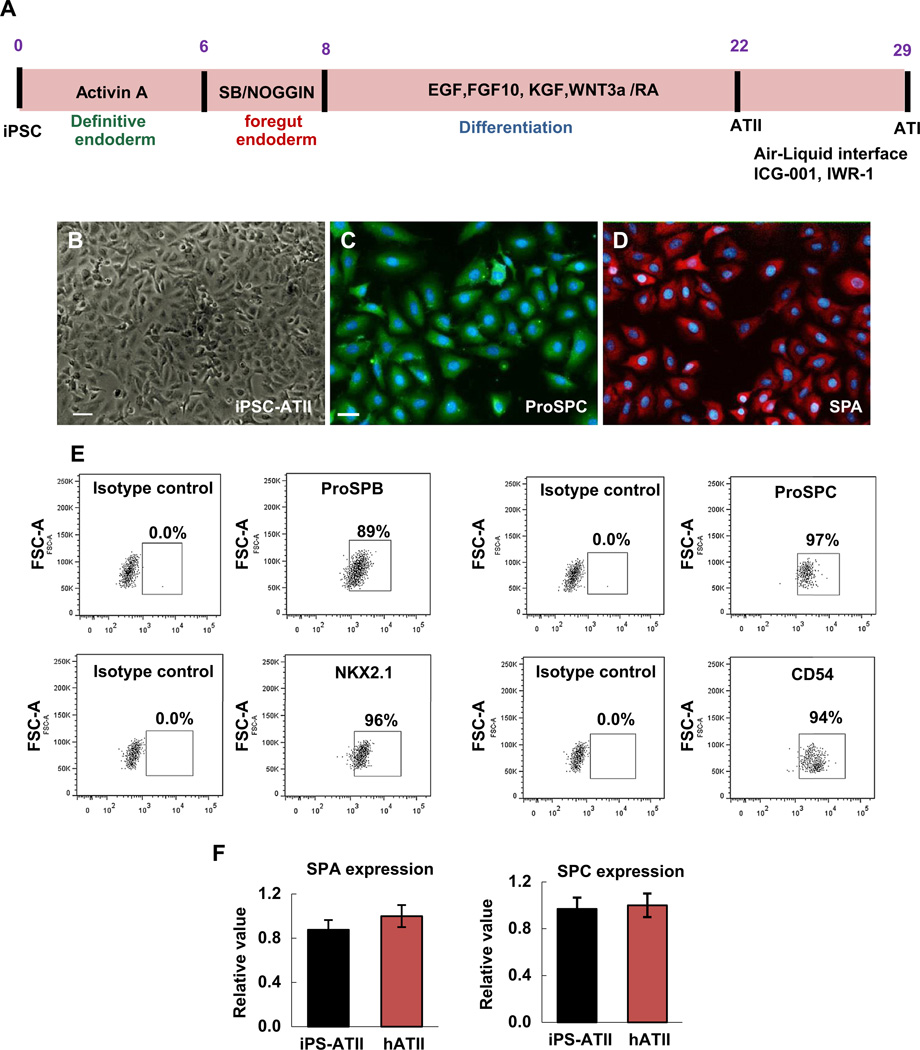
In our previous studies, we reported the step-wise differentiation method to generate definitive endoderm (DE), anterior foregut endoderm (AFE), and subsequently, a relatively homogeneous population of human alveolar type II cells from human iPSCs [17]. These iPSC-ATII cells not only demonstrate the phenotype of mature human alveolar type II cells, but also express a high percentage of type II cell markers when compared to freshly isolated human primary alveolar type II cells (Fig. 2A–F). Up to 97% of cells were positive for surfactant protein C, 95% for NKX2.1, 89% positive for surfactant protein B, and the vast majority (94%) of the cells expressed the epithelial marker CD54 (Fig. 2E, F). In this study we evaluated the capacity of iPSC-ATII and of hATII cells to differentiate into type I-like cells using either an air–liquid interface system or through the use of small molecules.
Fig. 2.
(A) Schematic protocol for directed differentiation of iPSCs to ATII and ATI in vitro in 29 days. Cytokines were added at different steps indicated in the panel (B) Phase-contrast images of differentiated cells at day 22, which are termed ATII cells. (C–D) Immunofluorescent staining of alveolar type II markers (C) pro-surfactant protein C (ProSPC) and (D) Surfactant protein A (SPA). (E) Flow cytometry analysis for the percentage of positive cells for alveolar type II marker (NKX2.1, SPC, SPA, CD54) at day 22. (F) qRT-PCR analysis in iPSC-derived ATII cells compared to hATII cells that were derived from fresh human lung. Values from three independent experiments from the triplicate PCR reactions for a gene of interest (SPC, and SPA) were normalized against average GAPDH Ct values from the same cDNA sample. Fold change of GOI transcript levels between iPS derived-ATII and human type II cells equals 2−ΔΔCt, where ΔCt = Ct(GOI) – Ct(GAPDH), and ΔΔCt = ΔCt(ATII) - DCt(ATII). (Bar indicate ± SEM and n = 3 independent experiments for qRT-PCR and flow cytometry). Scale bar, 63 µm.
3.1. Differentiation of iPSC-ATII to AETI in an air–liquid interface system compared to small molecule administration
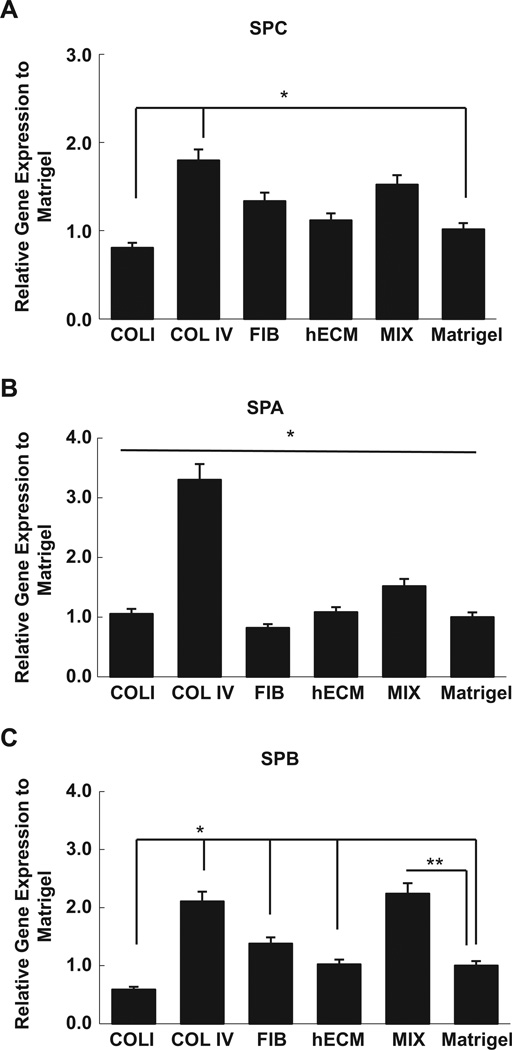
In order to test the impact of an air–liquid interface on the differentiation of iPSC-ATII cells to cells of ATI phenotype, iPSC-ATII cells were placed onto collagen IV coated Biopore membranes and inserted into a bioreactor that allows for a continuous air–liquid interface (Fig.1A–C).The choice of ECM proteins in our study was driven by the need to promote both the attachment of iPSC-ATII to the synthetic substrate. For these experiments, we focused on proteins that are found in native lung matrix and basement membranes. Collagen IV is highly expressed in the lung alveolar basement membrane, and we found that iPSC-ATII cultured on collagen IV expressed significantly higher levels of SPC, SPB, and SPA as compared to when these cells were cultured on other ECM proteins (Fig. 3A, B).
Fig. 3.
Expression of different type II marker cells on different extracellular matrix proteins after 7 days. (A) SPC, (B) SPA and (C) SPB expression in iPSC-derived ATII on collagen I (ColI), collagen IV (ColIV), fibronection (Fib), Mix of Fib, ColI, ColIV, and human ECM compared to Matrigel, quantified by qRT-PCR. The gene expression in iPS derived-ATII cells on different ECM proteins were compared to iPS derived-ATII cells on Matrigel. Ct values from three independent experiments from the triplicate PCR reactions for a gene of interest (SPB, SPC, and SPA) were normalized against average GAPDH Ct values from the same cDNA sample. Fold change of GOI transcript levels between iPS derived-ATII on each ECM protein and iPS derived-ATII cells on Matrigel equals 2–ΔΔCt, where ΔCt = Ct(GOI) –Ct(GAPDH), and ΔΔCt = ΔCt(ECM) - ΔCt(Matrigel ). Collagen IV induced significantly higher levels of SPC, SPB and SPA expression compared to each ECM proteins individually. (Bar indicate ± SEM and n= 3 independent experiments *denotes statistically significant difference p-value < 0.05).
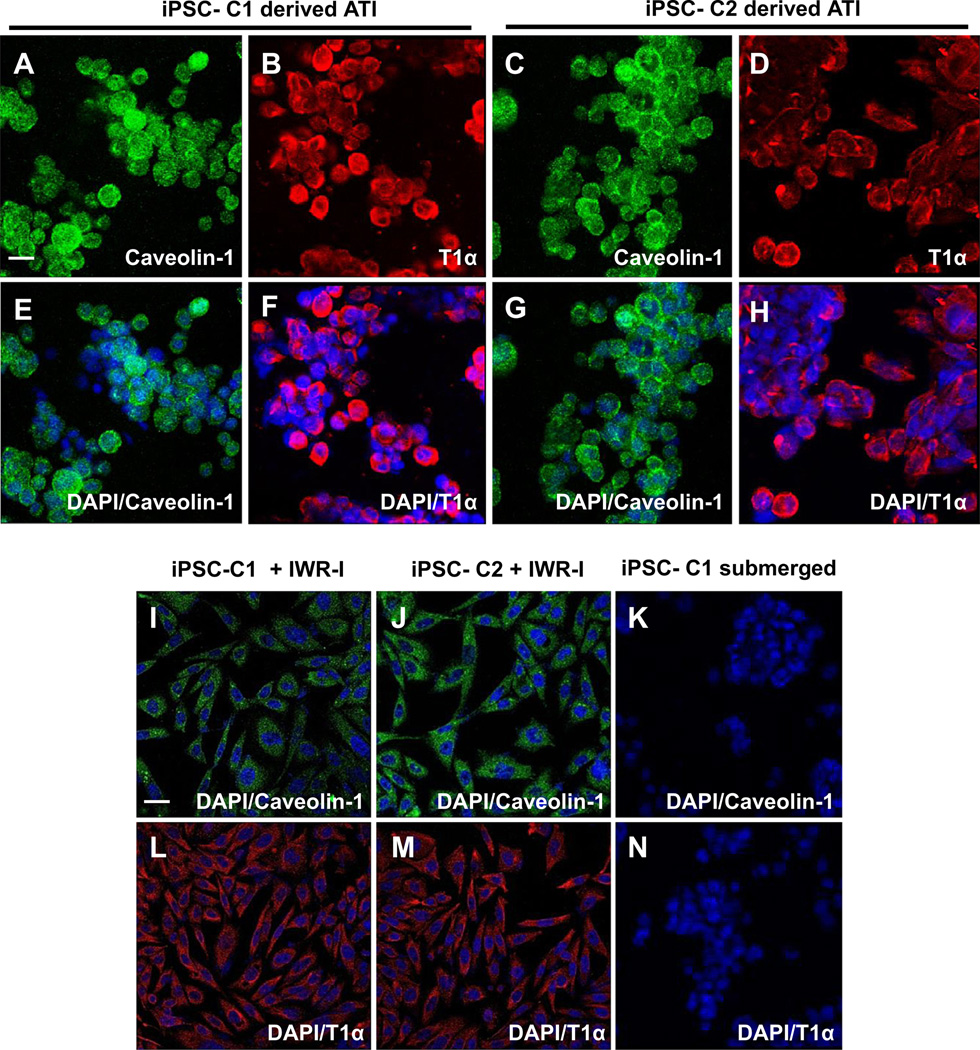
In order to assess the effects of the specialized rotating culture apparatus on type I marker expression, genes and proteins indicative of hATI, as well as markers of hATII were analyzed. In order to identify the portion of cells that express hATI markers, the presence of the following proteins and genes were assayed: aquaporin-5, caveolin-1 and T1a which are indicative of the type I phenotype and synthesized exclusively by alveolar type I cells [2,15,22]. After culturing iPSC-ATII in the rotating bioreactor for 7 days, iPSC-ATII cells derived from two clones, C1 and C2, were largely positive for the type I cell markers, T1a and caveolin-1 by immunostaining (Fig. 4A–H). These markers were not expressed in iPSC-ATII that were submerged in media used as a negative control (Fig. 4K, N).
Fig. 4.
(A–M) Immunofluorescence analysis of type I cell (ATI) markers, T1α and Caveolin-1 in cells derived from iPSCs (C1 and C2 clones), at day 7 cultured in bioreactor and grown in flasks. (A–D) shows T1α and Caveolin-1 staining in iPSC-derived ATI cells from both C1 and C2 clones cultured in the rolling bioreactor; (E–H) Merge with DAPI. (I–M) Immunofluorescent staining of alveolar type I marker, (I, L) T1α and (J, M) Caveolin-1 in differentiated ATI cells from C1 and C2 iPSC exposed to WNT inhibitors. Immunofluorescent staining of (K) Caveolin-1 and (N) T1α in iPSC-ATII cells submerged in media. Scale bar, 63 µm.
iPSC-ATII from both clones and hATII in the ALI system appeared round and formed clusters after 7 days of culture on the PTFE (Fig. 4A–H). This may be due to the culture on the PTFE uneven surface. In comparison, the flask-grown iPSC-ATII and hATII that had been treated with small molecules retained a two dimensional (2D) monolayer morphology. The organization of iPSC-ATII and hATII into clusters in the rolling bioreactor may be one reason for the enhanced type I function, and we hypothesize that cluster formation may be triggered by a being exposed to air, which is known to promote type I phenotype. Alternatively, the additional stimulus of shear stress caused by the repetitive movement into and out of the media by the attached cells may have also contributed to the formation of cells clusters.
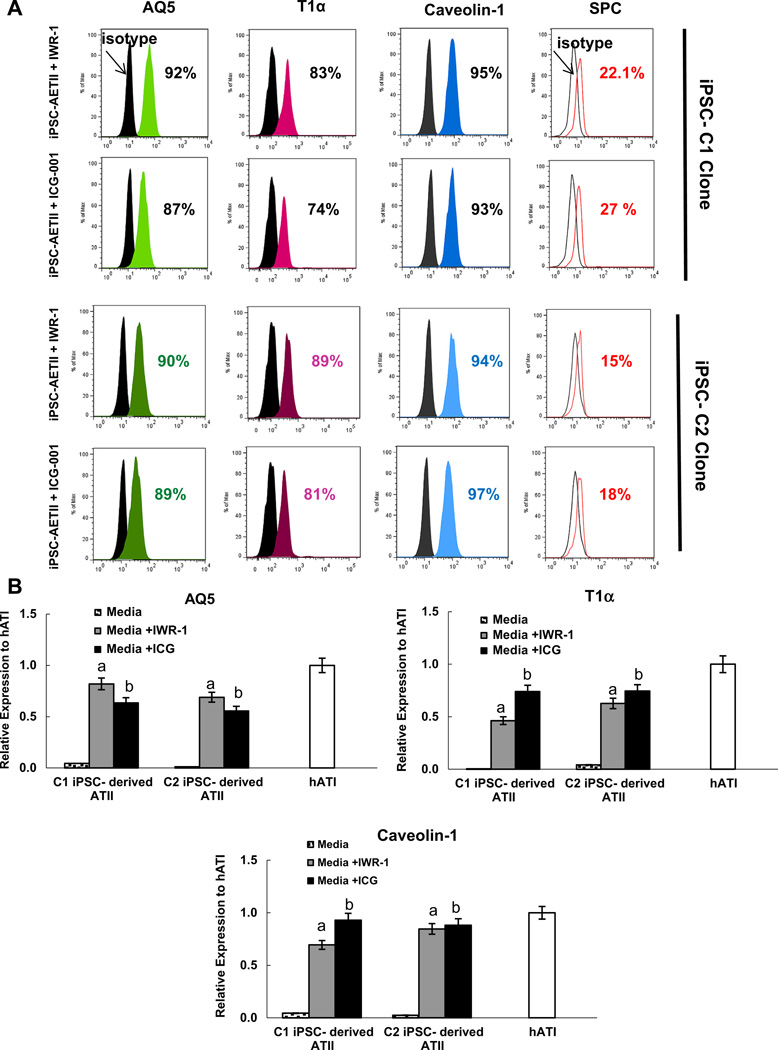
Several studies have demonstrated that alveolar epithelial type I can be generated from type II cells by manipulating the Wnt/β-catenin/Creb-binding protein (CBP) transcription pathway. Therefore, we sought to compare the impact of the rolling bioreactor system with small molecule administration. We first examined two selective Wnt/β-catenin pathway inhibitors, ICG-001 and IWR-1, on type I marker expression in iPSC-ATII cells derived from both C1 and C2 clones [21,23,25]. Data from independent experiments indicated that incubation of day 22 iPSC-ATII cells with IWR-1 (100 mM) or ICG-001 (100 mM) for 7 days induced the differentiation of iPSC-ATII cells to the ATI cell phenotype. Little or no expression of type 1 alveolar cell markers was observed in cells cultured in media without IWR-1 and ICG-001. Following treatment of iPSC-ATII with IWR-1 and ICG-001, there was a significant increase in the ATI markers AQ5, T1α and caveolin-1, in iPSC-ATII from both C1 and C2 as determined by flow cytometry and real time qRT-PCR (n =3, p < 0.05%) (Fig. 5A–C, Fig. 4I – M). There was also a concomitant decrease in the expression of the type II cell marker, SPC, following 7 days of culture in medium containing IWR-I and ICG-001. These data taken together indicate that iPSC-ATII cells exposed to IWR-1 or ICG-001 have a high percentage of expression of type I cell markers, but this increase was more pronounced in the case of IWR-1 (Fig. 5A, B).
Fig. 5.
(A) Representative flow cytometric analysis of T1α, AQ5, Caveolin-1 and SPC in C1 and C2 iPSCs derived ATI at 7 day cultured in media supplemented with WNT inhibitors; IWR-1 and ICG-001. More than 90% of the population is positive for type I cells markers. iPSC-derived ATI from both C1 and C2 clones exposed to IWR-1 express higher percentages of type I markers. (B) mRNA expression of AQ5, T1α and caveolin-1 in iPSC-derived ATI cells grown in flasks and the addition of IWR-1 and ICG001. Data expressed as quantification of mRNA normalized to GAPDH and compared to hATI cells that were derived from fresh human lung. Fold change of GOI transcript levels between iPS derived-ATI and human type I cells equals 2−ΔΔCt, where ΔCt = Ct(GOI) - Ct(GAPDH), and ΔΔCt = ΔCt(AETII)– ΔCt(ATII) Bar indicate ± SEM and n= 3 independent experiments for qRT-PCR and flow cytometry. ‘a’ marks statistically significant difference (p < 0.05) when comparing gene expression in cells cultured in media+IWR-1 vs. cells submerged in media. ‘b’ marks statistically significant difference (p < 0.05) in gene expression in cells exposed to ICG vs. cells submerged in media.
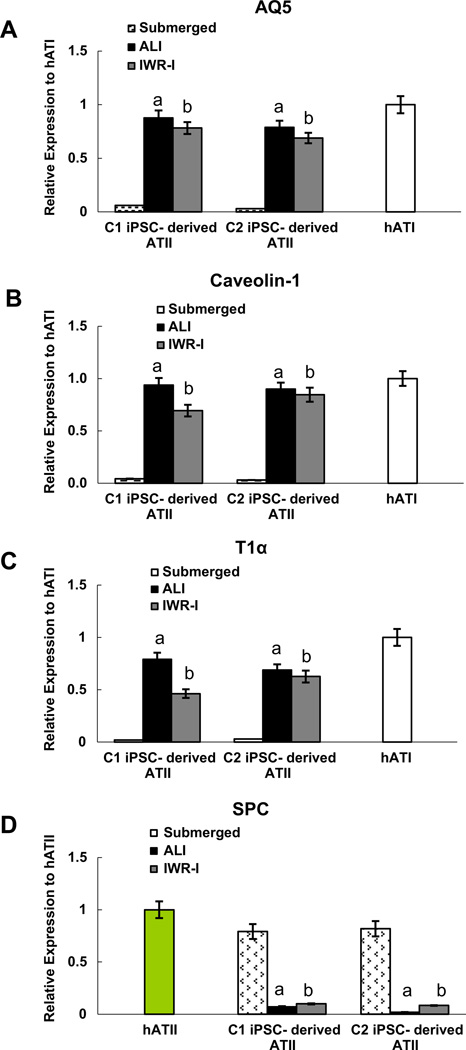
Next we compared the impact on type I marker expression of rolling bioreactor system, or air–liquid interface (ALI), to the Wnt inhibitor IWR-1. We performed real time RT-PCR on cells grown in either the rolling bioreactor or under presence of the IWR-1 for 7 days (Fig. 6A – C). A high percentage of cells express type I markers when cultured in the bioreactor system under ALI conditions, comparable to native human ATI cells, when compared with cells submerged in media only (n = 3, p < 0.05 for AQ5, T1α and a caveolin-1). iPSC-ATII treated with IWR-1 also expressed a high level of type I markers compared to iPSC-ATII submerged in media (n = 3, p < 0.05). Although there was no significant difference between the expression level of type I markers between two conditions, the expression of these markers was higher in the ALI bioreactor system as compared to IWR-1 (n = 3, p > 0.05) (Fig. 5A – C). We also monitored the expression of the hATII marker, SPC. qPCR of cells at day 7 revealed that the expression of SPC decreased progressively in both iPSC-ATII clones, when cultured in the ALI bioreactor and when treated with IWR-1. (Fig. 6D). However the decrease in the type II marker, SPC, was more pronounced in cells cultured in the bioreactor culture system indicates the majority of iPSC-ATII cells gave rise to type I cells.
Fig. 6.
mRNA levels of (A) AQ5 (B) caveolin-1, (C) T1 a and (D) SPC in iPSC-derived ATI cells cultured in the rolling bioreactor system (air–liquid interface, or ALI system) compared to cells submerged in media and cells submerged in media with addition of inhibitors. qRT-PCR data from three independent experiments expressed as quantification of mRNA normalized to GAPDH and compared to hATI cells that were derived from fresh human lung. Fold change of GOI transcript levels between iPS derived-ATI and human type I cells equals 2−ΔΔCt, where ΔCt = Ct(GOI) –Ct(GAPDH), and ΔΔCt = ΔCt(AETII) - ΔCt(ATII) (Bar indicate ± SEM and n= 3 independent experiments for qRT-PCR and flow cytometry). ‘a’ marks statistically significant difference (p < 0.05) when comparing gene expression in cells cultured in ALI only vs. cells submerged in media. ‘b’ marks statistically significant difference (p < 0.05) in gene expression in cells exposed to IWR-1 vs. cells submerged in media.
3.2. Differentiation of native human epithelium in the bioreactor and in response to small molecules
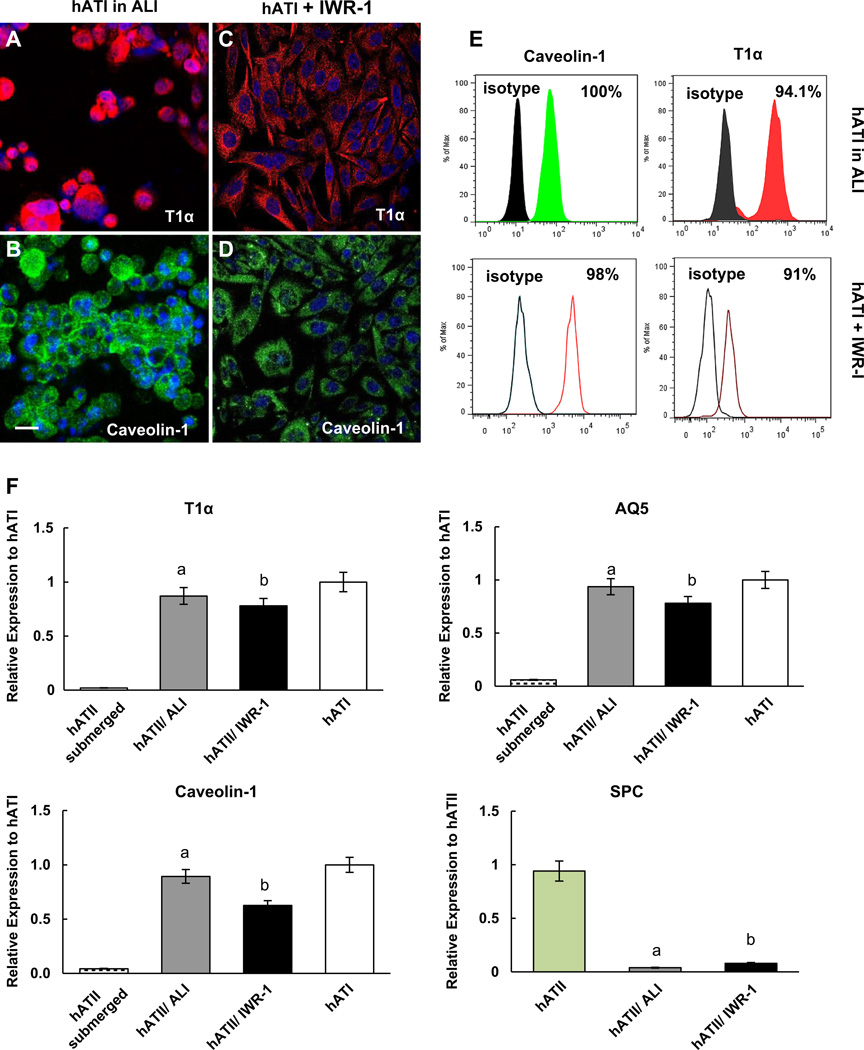
As a control for this study, hATII cells from adult human lung were seeded onto collagen IV coated membranes and placed into the rolling bioreactor culture system. As with the iPSC-ATII cells, many of the hATII cells in the bioreactor culture system gave rise to type I cells within 7 days. hATII cells cultured in the ALI system were mostly positive for type I markers, caveolin-1, and T1α at both the protein (Fig. 7A, B) and the RNA levels (Fig. 7F). The majority of cells, 94%, were positive for caveolin-1 and up to 100% of cells exposed to ALI expressed T1α as determined by flow cytometry (Fig. 7E). Importantly, the increase in type I marker expression was concomitant with the down regulation of the type II marker, SPC (Fig. 7F).
Fig. 7.
Characterization of human type II cells cultured in ALI system compare to adding WNT inhibitors at day 7 of differentiation towards type I cells (A–D) Immunofluorescence analysis of ATI markers; (A, B) show T1α and Caveolin-1 positive cells cultured in ALI system; (C, D) show T1α and Caveolin-1 positive cells cultured in media supplemented with IWR-1. Scale bar, 63 µm (E) Flow cytometric analysis of T1α and caveolin-1 during ALI–mediated and IWR-1–mediated induction of type I phenotype in human ATII cells at day 7 (compare to hATI cells stained with corresponding isotype). (F) Comparison of mRNA expression of T1α, AQ5, caveolin-1 and SPC in human ATII cells cultured in ALI system compared to cells submerged in media and submerged in media+IWR-1. Data expressed as quantification of mRNA normalized to GAPDH and compared to hATII cells that were derived from fresh human lung. Fold change of GOI transcript levels between iPS derived-ATII and human type II cells equals 2−ΔΔCt, where ΔCt = Ct(GOI) Ct(GAPDH), and ΔΔCt = ΔCt(AETII) - ΔCt(ATII). Bar indicate ± SEM and n= 3 independent experiments for qRT-PCR and flow cytometry. ‘a’ marks statistically significant difference (p < 0.05) when comparing gene expression in cells cultured in ALI vs. cells submerged in media. ‘b’ marks statistically significant difference (p < 0.05) in gene expression in cells exposed to IWR-1 vs. cells submerged in media.
The effect of IWR-1 on the differentiation of hATII cells into ATI cells was also examined. Many of the hATII cells exposed to IWR-1 gave rise to type I cells within 7 days and were positive for type I markers at both the protein and RNA levels, while the expression of SPC decreased over 7 days of exposure to IWR-1. After 7 days of differentiation, the majority of the cells stained positively for caveolin-1, and T1α (Fig. 7C, D). Flow cytometric analysis indicated that the human ATII cells exposed to IWR-1 for 7 days express a high percentage of markers associated with type I, including 98% for caveolin-1, and 91% for T1α (Fig. 7E).
We also compared the expression of type I marker expression in cells grown in the rolling bioreactor to cells grown under presence of the IWR-1 by real time RT-PCR (Fig. 7F). These analyses demonstrated the expression level of type I markers in the ALI bioreactor culture system was significantly higher compared to hATII or submerged in media only (n = 3, p < 0.05). While not significant, the expression of these genes was higher in cells in the ALI bioreactor system than in cells exposed to IWR-1 (n = 3, p > 0.05) (Fig. 7F).
3.3. Combination of the bioreactor culture system with small molecule administration
We also evaluated the impact of combining both the ALI system with the WNT inhibitor on type I marker expression. Fig. 8A – C shows the expression level of type I markers in the ALI bioreactor system, ALI+IWR-1 and ALI+ICG-001. Comparison of these conditions showed that combining ALI with Wnt inhibitors resulted in higher level of type I marker expression when compared with ALI condition in the bioreactor system only. Both ALI+ICG-001 and ALI+IWR-1 had similar effects on the differentiation of type II from iPSC towards type I, but the increase was more pronounced in the case of ALI+IWR-1. Although combining the bioreactor culture system with inhibitors resulted in a higher expression of type I markers, comparison of gene expression revealed no statistically significant difference between the ALI only, ALI+ICG-001 and ALI+IWR-1 (n = 3, p > 0.05) (Fig. 8A – C).
Fig. 8.
(A–C) Comparison of type I phenotypes induction in four different conditions (submerged in media, in ALI system, in ALI plus ICG-001 and ALI plus IWR-I) in iPC-derived ATI using quantitative RT-PCR. Applying IWR-1 and ICG-001 and ALI to iPC-derived ATII induced type I phenotypes in these cells. Comparison of the impact of combination of both ALI system and WNT inhibitor on type I markers expression by real time qRT-PCR showed higher level of type I markers expression in ALI+IWR-1 compared to when they cultured in ALI system only. Comparison of gene expression revealed no statistically significant difference between ALI only, ALI+ICG-001 and ALI+IWR-1. Bars indicate ± SEM and n= 3 independent experiments for qRT-PCR, ELISA and flow cytometry.
4. Discussion
The differentiation and maintenance of ATI cells derived from pluripotent stem cells are critically dependent upon providing physiological conditions to maintain proper phenotypic characteristics in vitro [2,32]. In this paper,wetriedto mimic physiological conditions with an air–liquid interface rolling culture system, to provide a differentiation signal to iPSC-derived ATII and to native type II epithelial cells. Current respiratory epithelium differentiation protocols have largely focused on seeding respiratory epithelium in transwell culture systems [2,33,34] or on designing microsystems to simulate breathing using lung-on-a-chip technology [35]. These methods suffer from several flaws, including difficulty of use with regard to attention to fluid levels, and inconsistency of set-up.
Previously, Macchiarini and colleagues reported a rotating bioreactor for culturing a recellularized airway construct, essentially moving the construct alternately between liquid and air [36]. We designed a different rotating culture bioreactor in which cells are cultured on a membrane and rolled in and out of fluid and exposed to air and liquid as the cell substrate is rolled. This design of the ALI bioreactor took into account the physiological requirements for the maintenance of ATI cells. By rolling a porous, absorbent substrate containing iPSC-ATII cells, alternately through media and air, we were able to generate a functional ALI system that promotes the differentiation of iPSC-ATII cells toward ATI-like cells. iPSC-ATII cells cultured in this manner had expression of type I cells markers including AQ5, T1a, and caveolin-1.
This rolling bioreactor culture system provides a promising alternative to current methods for air–liquid interface modeling, and can be used to expand and differentiate tens of millions of cells in vitro. Transwell culture systems are not well-suited for scale-up production of alveolar cell epithelium for use in both cell therapy and, ultimately, for producing human tissues. The ability to “scale up” a functional alveolar population will be particularly valuable when translating these technologies for use in producing human tissues, and allows for the possibility of using autologous iPSC derived cells in future lung bioengineering work.
Low-maintenance bioreactor systems that are capable of producing cells with little manual attention are becoming increasingly important in tissue engineering. Physiological, biomimetic systems allowed for perhaps better differentiation ATI phenotype when compared to Wnt/β-catenin inhibitors (ICG-001, IWR-1) that were added to static cultures. Moreover, using small molecules and changes in cell signaling pathways may have an impact on other cellular functions which may make them less ideal strategies. Therefore, the technique presented here provides one example of a system that could fulfill this need to grow large numbers of differentiated epithelial type I cells and have significant implications for the field of stem cell, alveolar epithelium research and tissue engineering. Future work will concentrate on the direct production of ATI from iPSC in a parallel effort to widen the pool of cells that are available for lung cell therapy and tissue engineering.
5. Conclusions
Our study demonstrates that the differentiation of IPS derived ATII cells towards an ATI phenotype can be achieved with the use of a rotating bioreactor as well as through the use of small molecule inducers. Our data show that use of the rotating bioreactor, in which cells are placed on a membrane, and undergo air/liquid interface is more efficient in differentiating the ATII cells towards the ATI phenotype. A key advantage to using this bioreactor is that the use of small molecules is not necessary thus eliminating the possibility of off-target, negative effects that may be had by the small molecules on various signaling pathways. The use of the bioreactor in the differentiation process has significant implications for the field of stem cell research and regenerative medicine, especially when the need is present to grow large amounts of epithelial cell types. This bioreactor ultimately allows for reduced costs associated with stem cell differentiation as well as the possibility for large scale production of AT1 cells.
Acknowledgments
We gratefully acknowledge Prof. James Thomson for providing human iPS cells clones. This work was supported by United Therapeutics, Inc. United Therapeutics did not affect the content or conclusions contained in this manuscript. Work also supported by NIH U01 HL111016 and R01 HL098220 (both to LEN). JJM is supported by T32 GM086287.
Abbreviations
- ALI
air–liquid interface
- hATII
human alveolar type II
- iPSC-ATII
iPSC-derived alveolar type II
- hATI
human alveolar type I
Footnotes
Disclosure statement
L.E.N. has a financial interest in Humacyte, Inc., a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Author contributions
Laura L. Niklason designed the research and wrote the manuscript; Mahboobe Ghaedi differentiated the iPSCs and performed most the analyses and wrote the manuscript. Julio Mendez performed a part of staining and flow cytometry, Peter F. Bove provided isolated human ATII. Amogh Sivarapatna performed cell culture; Micha Sam Brickman Raredon designed and built the bioreactor.
References
- 1.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133(13):2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 2.Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respir Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8(4):541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- 4.Dobbs LG. Isolation and culture of alveolar type II cells. Am J Phys. 1990;258(4 Pt 1):L134–L147. doi: 10.1152/ajplung.1990.258.4.L134. [DOI] [PubMed] [Google Scholar]
- 5.Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- 6.Petersen TH, Calle EA, Colehour MB, Niklason LE. Bioreactor for the long-term culture of lung tissue. Cell Transplant. 2011;20(7):1117–11126. doi: 10.3727/096368910X544933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotton DN. Next-generation regeneration: the hope and hype of lung stem cell research. Am J Respir Crit Care Med. 2012;185(12):1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 8.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 10.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 11.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green MD, Chen A, Nostro MC, d’Souza SL, Schaniel C, Lemischka IR, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nat Biotechnol. 2011;29(3):267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943–952. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 14.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104(11):4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12(4):867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 17.Ghaedi M, Calle ES, Mendez JJ, Gard AL, Balesterini J, Booth AJ, et al. Human iPS cell–derived alveolar epithelium repopulates lung extracellular matrix. JCI. 2013;123(11) doi: 10.1172/JCI68793. http://dx.doi.org/10.1172/JCI68793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto WR. Lung stem cells. Int J Exp Pathol. 1997;78(5):291–310. doi: 10.1046/j.1365-2613.1997.370366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asselin-Labat ML, Filby CE. Adult lung stem cells and their contribution to lung tumourigenesis. Open Biol. 2012;2:120094. doi: 10.1098/rsob.120094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barkauskas CE, Cronce MJ, Rackley CR, Bowie EJ, Keene DR, Stripp BR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujino N, Kubo H, Suzuki T, Ota C, Hegab AE, He M, et al. Isolation of alveolar epithelial type II progenitor cells from adult human lungs. Lab Invest. 2011;91(3):363–378. doi: 10.1038/labinvest.2010.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fehrenbach H. Alveolar epithelial type II cell: defender of the alveolus revisited. Respir Res. 2001;2(1):33–46. doi: 10.1186/rr36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee ER, Laflamme MA, Papayannopoulou T, Kahn MMC, Henderson WR., Jr Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One. 2012;7(3):e33165. doi: 10.1371/journal.pone.0033165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcorn D, Adamson TM, Lambert TF, Maloney JE, Ritchie BC, Robinson PM. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123(Pt 3):649–660. [PMC free article] [PubMed] [Google Scholar]
- 25.Ostrowski LE, Nettesheim P. Inhibition of ciliated cell differentiation by fluid submersion. Exp Lung Res. 1995;21(6):957–970. doi: 10.3109/01902149509031773. [DOI] [PubMed] [Google Scholar]
- 26.Gutierrez JA, Gonzalez RF, Dobbs LG. Mechanical distension modulates pulmonary alveolar epithelial phenotypic expression in vitro. Am J Phys. 1998;274(2 Pt 1):L196–l202. doi: 10.1152/ajplung.1998.274.2.L196. [DOI] [PubMed] [Google Scholar]
- 27.Kubo A, Shinozaki K, Shannon JM, Kouskoff V, Kennedy M, Woo S, et al. Development of definitive endoderm from embryonic stem cells in culture. Development. 2004;131(7):1651–1662. doi: 10.1242/dev.01044. [DOI] [PubMed] [Google Scholar]
- 28.Duan Y, Ma X, Zou W, Wang C, Bahbahan IS, Ahuja TP, et al. Differentiation and characterization of metabolically functioning hepatocytes from human embryonic stem cells. Stem Cells. 2010;28(4):674–686. doi: 10.1002/stem.315. [DOI] [PubMed] [Google Scholar]
- 29.Ghaedi M, Duan Y, Zern MA, Revzin A. Hepatic differentiation of human embryonic stem cells on growth factor-containing surfaces. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1595. http://dx.doi.org/10.1002/term.1595. [DOI] [PubMed] [Google Scholar]
- 30.Bove PF, Grubb BR, Okada SF, Ribeiro CM, Rogers TD, Randell SH, et al. Human alveolar type II cells secrete and absorb liquid in response to local nucleotide signaling. J Biol Chem. 2010;285(45):34939–34949. doi: 10.1074/jbc.M110.162933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raredon MS, Calle EA, Niklason LE. A rotating bioreactor for large-scale culture and differentiation of respiratory epithelium. Cell Transplant. 2013 doi: 10.3727/215517914X681794. ounder revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTRTR protein. Nat Biotechnol. 2012;30(9):876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin H, Li H, Cho HJ, Bian S, Roh HJ, Lee MK, et al. Air-liquid interface (ALI) culture of human bronchial epithelial cell monolayers as an in vitro model for airway drug transport studies. J Pharm Sci. 2007;96(2):341–350. doi: 10.1002/jps.20803. [DOI] [PubMed] [Google Scholar]
- 34.Hermanns MI, Unger RE, Kehe K, Peters K, Kirkpatrick CJ. Lung epithelial cell lines in coculture with human pulmonary microvascular endothelial cells: development of an alveolo-capillary barrier in vitro. Lab Invest. 2004;84(6):736–752. doi: 10.1038/labinvest.3700081. [DOI] [PubMed] [Google Scholar]
- 35.Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–1668. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372(9655):2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]