Abstract
Background:
Withania somnifera is an herbal medicine that has been known to possess memory-enhancing properties. The current study involved an assessment of cognitive and psychomotor effects of Withania somnifera extract in healthy human participants.
Materials and Methods:
In this prospective, double-blind, multi-dose, placebo-controlled, crossover study, 20 healthy male participants were randomized to receive 250 mg two capsules twice daily of an encapsulated dried aqueous extract of roots and leaves of Withania somnifera or a matching placebo for a period of 14 days. Cognitive and psychomotor performance was assessed pre-dose (day 1) and at 3 hrs post-dose on day 15 using a battery of computerized psychometric tests. After a washout period of 14 days, the subjects crossed-over to receive the other treatment for a further period of 14 days as per prior randomization schedule. Same battery of test procedures were performed to assess cognitive and psychomotor performance.
Results:
Significant improvements were observed in reaction times with simple reaction, choice discrimination, digit symbol substitution, digit vigilance, and card sorting tests with Withania somnifera extract compared to placebo. However, no effect can be seen with the finger tapping test.
Conclusion:
These results suggest that Withania somnifera extract can improve cognitive and psychomotor performance and may, therefore, be a valuable adjunct in the treatment of diseases associated with cognitive impairment.
Keywords: Central nervous system, psychometric tests, Withania somnifera extract
INTRODUCTION
Evaluation of effects of drugs on psychomotor performance during the new drug development process is an important aspect, because of their potential to contribute to accidents, whether on the road, at work place, or at home. Effects of drugs on the central nervous system (CNS) can be assessed in terms of effects on behavior, vigilance, attention, cognition, neurophysiological activity of the brain and on the neuroendocrine functions. Psychometric tests are used in clinical pharmacological studies for quantitative documentation of CNS effects of drugs during the early phases of drug development.[1]
Withania somnifera, also known as ashwagandha, is an important herb in Ayurvedic and indigenous medical systems. The plant is known as “Medhya Rasayan”[2,3,4] or mind rejuvenate, used in enhancing memory and overall brain functioning. The active principles of Withania somnifera sitoindosides VII-X and Withaferin A (glycowithanolides) have shown an antioxidant effect in the brain, which may be responsible for its diverse pharmacological properties. Withania somnifera has been used to promote physical and mental health, to provide defense against disease and adverse environmental factors, and to arrest the aging process. Several studies[5,6,7,8,9] indicated that Withania somnifera possesses antioxidant, anti-tumor, anti-stress, anti-inflammatory, immunomodulatory, hematopoietic, anxiolytic, anti-depressive, rejuvenating properties and was found to play a significant role in the prevention of different CNS disorders, especially in the conditions of stress and neurodegenerative diseases, which includes Parkinson's and Alzheimer's disorders, tardive dyskinesia, cerebral ischemia, and also in the management of the drug addiction. It has a cognition promoting effect and was found to be useful in children with memory deficit and old age people with memory loss. In a study, Schliebs et al.[10] reported that Withania somnifera improves the cognitive capabilities of the brain by increasing the capacity of muscarinic receptors. The present study was thus planned to evaluate the effect of Withania somnifera extract on cognitive and psychomotor performance in healthy human participants.
MATERIALS AND METHODS
Study medication
Each capsule contains either an inert placebo or 250 mg of highly standardized dried aqueous extract of the roots and leaves of Withania somnifera[11] (SENSORIL®, Natreon Inc, USA). The Withania somnifera extract was derived from withaferin A and corresponding withanolide glycoside-predominant, genetically uniform chemotype, cultivated in the central and northern provinces of India. Withania somnifera root and leaf material was processed using a water-based extraction protocol and assessed using high performance thin layer chromatography analysis of fractions against standard references (CAMAG Linomat V applicator, CAMAG TLC Scanner, and WinCats software version 1.3.4; CAMAG, Sonnenmattstr. Muttenz, Switzerland). Each Withania somnifera extract capsule contains not less than 10% withanolide glycosides, not more than 0.5% of withaferin-A and not less than 32% of oligosaccharides. The placebo capsules were identical in appearance and contain 49.7% (w/w) of microcrystalline cellulose, 49.5% (w/w) of lactose, and 0.69% (w/w) of magnesium stearate.
Study methodology
Twenty-six healthy male participants, aged 20-35 yrs, were enrolled into the study. The study was approved by the Institutional Ethics Committee and registered in the clinical trial registry of India (CTRI/2013/04/003537) and was carried out in accordance with the Declaration of Helsinki. Experimental procedures were explained in detail to the study participants, and written informed consent was obtained prior to testing. A detailed medical history (including smoking, alcohol habits), physical examination, hematological, hepatic and renal parameters, electrocardiogram (ECG), and chest X-ray were done in all the participants. Subjects were excluded if there was any evidence of physical illness, drug abuse or aberrant laboratory findings during screening. All the subjects abstained from nicotine, caffeine, and alcohol intake for at least 24 hrs, prior to and during the test day. The participants were trained in study procedures on at least two occasions prior to the study to make them familiar with the testing device.
This was a prospective, double-blind, multi-dose, placebo-controlled, crossover study, with participants randomized to receive either Withania somnifera extract or placebo capsules in Run I. On the study day, after an overnight fast, participants were asked to relax and sit comfortably for half an hour before initiation of test procedures in the clinical research unit. All the test measurements were recorded at baseline between 8:00 and 9:00 AM in an adequately lit, quiet experimental room with an ambient temperature of 22° ± 2° C to avoid distraction and increase the comfort level of the subject. Subjects then received the study medication as per prior randomization schedule, with instructions for them to take two capsules twice-a-day (i.e., in the morning and in the evening) with 240 ml of water daily for 14 days. All the test measurements were recorded 3 hrs after the morning dose administration on Day-15. A washout period of 14 days was given between the treatments. Subjects were then crossed over to receive the second treatment i.e., 2 capsules twice daily for 14 days for Run II. All the test procedures were repeated pre- and post-treatment as described for Run I. Participants were asked to report any side-effects with either treatment. If there was any adverse event, same was noted down in the case record form. Compliance was evaluated by questioning the subject and counting the leftover capsules. The drugs assigned for each subject was prepared by a staff member otherwise not involved in the present study using a randomization program (Statistica: Stat Soft, Inc, USA). After the study, the assigned drugs were unblinded for statistical analysis. Figure 1, provides an overview of the requirement, allocation, and follow-up of participants.
Figure 1.
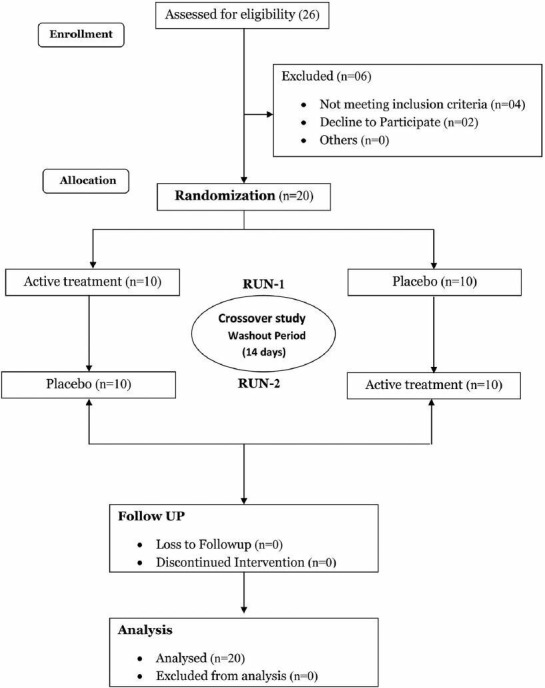
Requirement, allocation, and follow-up of participants
Assessments
The following tests were administered
Tests of psychomotor performance
Cognitive and Psychomotor performance was assessed using an indigenously developed automated psychometric test battery, whose sensitivity and validity has been previously established in the department.[12] The tests used in the study are sensitive and can detect drug-induced changes in psychomotor performance in healthy subjects. The entire session of tests presented took approximately 20 minutes, and in each session, tests were administered in the following order. Each test was done thrice, and an average of three readings was taken.
Finger tapping test
The duration of the test is 10 sec, during which the subject has to continuously tap on the “Enter Button” on the response box in quick succession. The results were presented in terms of average reaction time in milliseconds as well as total number of clicks attempted. The test provides information on motor system performance.
Simple reaction test
The duration of the test is 60 sec. In this test, on the click of a start button, the test time will begin and a picture of a boy will appear on the center of the screen for 20 times. The subject has to press the ‘BOY’ symbol button on response box as quickly as possible every time the ‘BOY’ picture appears on the monitor. Each time, the BOY picture will remain on the screen for 1 sec, and there will be a time gap of 1.5-2.5 sec between the appearances of subsequent BOY pictures. Results are presented in terms of average reaction time, correct attempts, and wrong attempts. This test assesses attention and sensory-motor performance of brain.
Choice discrimination test
The duration of the test is 60 sec. In this test, on the click of a start button, the test time will begin and a picture of a boy or girl will appear randomly for 10 times each on the center of the screen. The subject has to press the ‘BOY’ symbol button with right index finger and ‘GIRL’ symbol button with left index finger on the response box as quickly as possible corresponding to the picture. Each time, the picture will remain on the screen for 1 sec, and there will be a time gap of 1.5-2.5 sec between the appearances of the subsequent pictures. Results are presented in terms of average reaction time, correct attempts, and wrong attempts. This test assesses the attention and sensory-motor performance of the brain and estimates the psychomotor response speed.
Digit symbol substitution test
In this test, the upper panel of the screen will display 1-9 digits with their corresponding target symbol placed over each digit. The subject has to carefully concentrate and remember the corresponding digit for these symbols. On click of start button, the symbols that are shown in the panel will appear randomly one after other on the center of the screen. The subject has to press the corresponding digit as quickly as possible on the response box when the target symbol appears. The total duration of the test is 90 sec. Results are presented in terms of average reaction time, total attempts, correct attempts, and wrong attempts. This test assesses attention, response speed, central integration, and visuo-motor coordination.
Digit vigilance task
A target digit was randomly selected and constantly displayed to the right of the monitor screen. On click of start button, a series of digits will appear one at a time on the center of the screen at the rate of 105 digits/min. The subject has to press the “Enter Button” on the response box as quickly as possible every time the digit in the series matched the target digit. The task lasts for 60 sec, and there will be 45 stimulus-target matches. The results are presented in terms of average reaction time (millisec), total attempts, correct attempts, and wrong attempts. The test assesses alertness and vigilance while placing minimal demands on two other components of attention: Selectivity and capacity.
Card sorting test
In this test, the participant was asked to sort a set of 52 cards based on the different colors and shape using a digital pen. Results are presented in terms of the average time taken to complete the sorting and the number of correct and wrong cards. This test assesses sensory, motor, central integrative and executive functions.
Safety assessments
Physical examination, systemic examination, vital signs, hematological and laboratory tests like hemoglobin, complete blood picture, renal function tests, liver function tests, lipid profile, and complete urine examination including ECG were performed at screening visit, baseline, and end of treatment during each cross-over period.
Statistical analysis
The statistical analysis was carried using Graphpad prism software, Version 4 (Graphpad software Inc. Sandiego, California, USA). All the data were presented as Mean ± SD. Within subject and pair-wise comparisons between the two treatments (Withania somnifera extract Vs Placebo) were tested for statistical significance using one way ANOVA and paired t-test. Statistical significance was at P < 0.05.
Sample size calculation
Assuming a 15% difference between two treatments, sample size of 20 subjects was calculated based on two-sided differences with the type I error α being 0.05 and type II error β at 80%. Taking into account study design and total number of times subject visiting the study center and expected dropout of 30%, a total of 26 subjects were enrolled into the study.
RESULTS
Total 26 subjects were screened, out of which four subjects were excluded because of abnormal laboratory investigations and two subjects did not report to randomization visit.
The mean age of the 20 subjects was 24.90 ± 4.18 yrs, with a mean BMI of 22.38 ± 1.15 kg/m2 . There were no significant differences in baseline characteristics between the two groups.
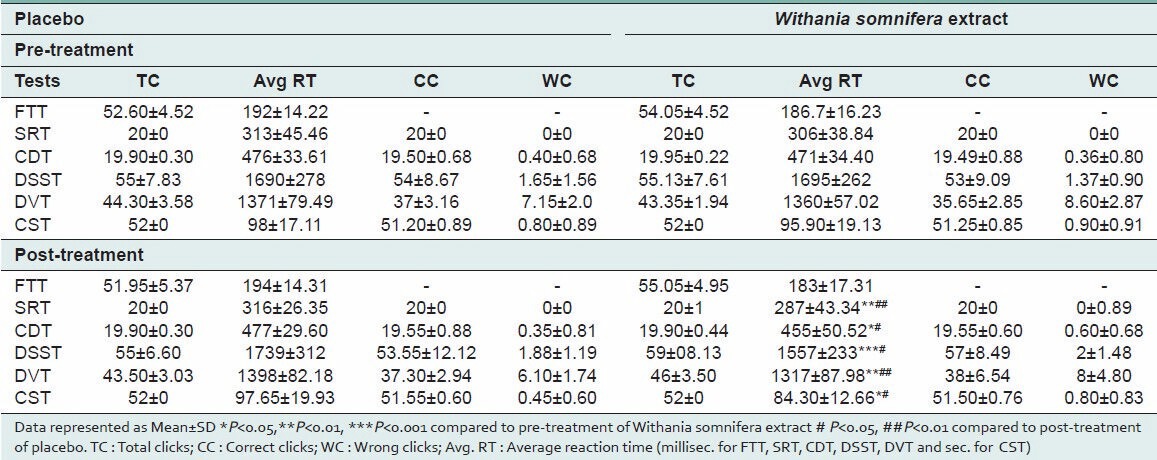
The results of the outcome measures are depicted in Table 1. As seen from the table, Withania somnifera extract significantly decreased reaction time (RT) in SRT, CDT, DSST, DVT, and CST compared to that of baseline and placebo. However, no significant effect can be seen with FTT.
Table 1.
Effects of placebo and Withania somnifera extract on psychomotor performance tests
Compared to placebo, the mean percentage change in reaction time for the CDT, DSST, and DVT decreased significantly. However, no significant change can be seen with FTT, SRT, and CST [Figures 2 and 3].
Figure 2.
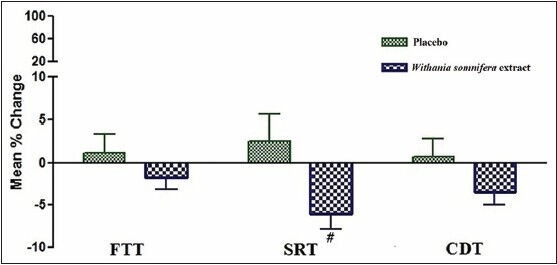
Mean % change in RT in FTT, SRT, and CDT before and after intake of Placebo and Withania somnifera extract #P < 0.05 compared to placebo
Figure 3.
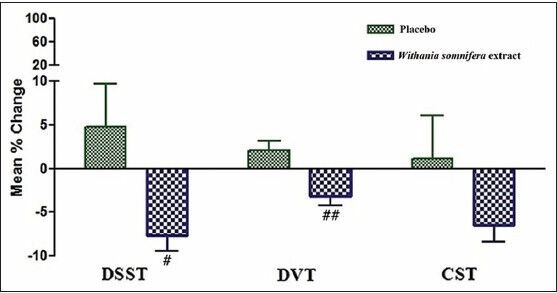
Mean % change in RT in DSST, DVT, and CST before and after intake of Placebo and Withania somnifera extract #P < 0.05, ##P < 0.01 compared to placebo
All subjects completed both treatments, and no participant discontinued the study. Compliance to study medication was good, and all the subjects in the Withania somnifera extract group and in the placebo group took more than 90% of the study drugs. Safety laboratory parameters were within normal range, and none of the treatments affected the safety lab parameters.
DISCUSSION
The present study demonstrated the favorable effects of Withania somnifera extract on cognitive and psychomotor performance in healthy participants. Psychometric tests like FTT, CDT, DSST, DVT, and CST were performed using computerized psychometric test system, which have gained popularity in recent times in clinical studies of psychiatric disorders and to evaluate the effects of psychotropic drugs.[13] Psychometric tests are used as biological markers in early phase 1 trials to establish preliminary objective pharmacodynamic data.[1] In the present study, the psychomotor performance tests used are well-established with sensitivity to the depressant and stimulant effects for a wide range of psychotropic drugs.
Withania somnifera is a known brain tonic in Ayurveda postulated to act in the human body by modulating the neuro-endocrino-immune systems and have been found to be a rich source of antioxidants.
In the present study, we have observed that Withania somnifera extract decreased the reaction time significantly in SRT, CDT, DSST, DVT, and CST following 14 days treatment (1000 mg/day) compared to placebo, indicating its positive effect on psychomotor function. Similarly, there was significant reduction in mean percent reaction time by 6% in SRT, 7% in DSST, and 3% in DVT compared to placebo.
The decrease in the reaction time could be interpreted as a central effect, since Withania somnifera helps to maintain homeostasis and psychomotor abilities in times of stress. Clinical trials have shown that Withania somnifera can alleviate a reactive type of depression without causing sedation. Instead, it optimizes mental and psychomotor performance by easing the mental stress bundle.[8] Our results are in par with the above data where we have not witnessed any sedative effect in the subjects during treatment with Withania somnifera extract. The mechanism by which Withania somnifera exerted beneficial effects is presently not clear; however, animal studies have shown that Withania somnifera is capable of improving memory and enhancing cognitive function by modulation of cholinergic neurotransmission. As per the literature, leaf and fruit extracts of Withania somnifera consisting of mixtures of sitoindosides VII-X and withaferin-A produces an increase in the cortical muscarinic acetylcholine capacity, which may partly explain cognition-enhancing effect demonstrated in animal and human models. Schliebs et al.[10] studied the effects of sitoindosides VII-X and withaferin isolated from aqueous methanol extracts from the roots of Withania somnifera on brain cholinergic, glutamatergic, and GABAergic receptors in male Wistar rats. The activity of AchE levels is enhanced in lateral septum and globus pallidus, whereas it is decreased in vertical diagonal band. Similarly, the activity of M1 cholinergic receptor binding is enhanced in lateral septum and frontal cortex, whereas M2 receptor binding sites increased in frontal and parietal cortex regions. The data suggests that the compounds - sitoindosides VII-X and withaferin preferentially affect events in the cortical cholinergic-signal transduction cascade. The drug-induced increase in cortical muscarinic acetylcholine receptor capacity might partly explain the cognition-enhancing and memory-improving effects of Withania somnifera extracts in animals and in humans.
Similarly, Dhuley et al.[14] tested Withania somnifera, comprising sitoindosides VII-X and Withaferin-A, to prove their effect on improving short-term memory in mice. The test was done using electric shock therapy, treatment with scopolamine (an anti-cholinergic agent that produces deficits in learning and memory retention) as well as testing mice that had never been on the electric grid before. The mice treated with Withania somnifera (200 mg/kg of body weight) produced a significant decrease in the amount of errors while trying to reach the shock-free zone after 1 hr of training. The effect of Withania somnifera on the scopolamine-treated mice reversed the deficits in retrieval and acquisition. This study showed an effectiveness of Withania somnifera in improving short-term memory in both naive and amnesiac mice.
Though there is not much data on the effects of Withania somnifera on psychomotor performance in healthy participants, research by Karnick CR[15] in a randomized, double-blind study reported that Withania somnifera significantly improved integrated sensorimotor function, auditory reaction time, and mental arithmetic ability compared to Panax ginseng and placebo in 30 healthy subjects. The results of the present study demonstrate that the natural products like Withania somnifera is a promising realm that awaits neuropharmacologist to exploit.
CONCLUSION
The measurement of cognitive and psychomotor performance is highly important in evaluating and predicting treatment outcomes in healthy subjects as well as in patients. In the present study, Withania somnifera extract has shown to improve the cognitive and psychomotor performance in healthy human participants. However, multicentric long-term clinical studies in patients are required to confirm its therapeutic efficacy in disease states associated with impaired cognition and psychomotor function.
ACKNOWLEDGMENTS
The authors are grateful to the Natreon INC. USA for providing study medications (SENSORIL® and placebo capsules) and Dr. I. Sravanthi, Ayurvedic Physician, for her expert advice. We extend our thanks to Indian Council of Medical Research, New Delhi, India for providing the extramural grant for development of instrument for evaluating cognitive and psychomotor performance under Advanced ICMR Clinical Pharmacodynamic Center for Evaluation of Pharmacodynamic Effects of Drugs.
Footnotes
Source of Support: Natreon INC. USA for providing study medications (SENSORIL® and placebo capsules), Indian Council of Medical Research, New Delhi, India for providing the extramural grant for development of instrument
Conflict of Interest: None declared.
REFERENCES
- 1.Wiedemann K. Biomarkers in development of psychotropic drugs. Dialogues Clin Neurosci. 2011;13:225–34. doi: 10.31887/DCNS.2011.13.2/kwiedemann. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya SK, Satyan KS, Ghosal S. Antioxidant activity of glycowithanolides from Withania somnifera. Indian J Exp Biol. 1997;35:236–9. [PubMed] [Google Scholar]
- 3.Singh N, Bhalla M, de Jager P, Gilca M. An overview on ashwagandha: A Rasayana (rejuvenator) of ayurveda. Afr J Tradit Complement Altern Med. 2011;8(Suppl 5):S208–13. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Radheyshyam T, Tripathi JS, Sanjay G, Reddy KR. Pharmaceutical and clinical studies on compounds ayurvedic formulations, Saraswatha churna. International Research Journal of Pharmacy. 2011;2:77–84. [Google Scholar]
- 5.Alam N, Hossain M, Khalil MI, Moniruzzaman M, Sulaiman SA, Gan SH. Recent advances in elucidating the biological properties of Withania somnifera and its potential role in health benefits. Phytochem Rev. 2012;11:97–112. [Google Scholar]
- 6.Singh G, Sharma PK, Dudhe R, Singh S. Biological activities of Withania somnifera. Ann Biol Res. 2010;1:56–63. [Google Scholar]
- 7.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): A Review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 8.Gupta GL, Rana AC. Withania somnifera (Ashwagandha): A review. Pharmacogn Rev. 2007;1:129–36. [Google Scholar]
- 9.Malhotra CL, Mehta VL, Das PK, Dhalla NS. Studies on Withania-ashwagandha, Kaul. V. The effect of total alkaloids (ashwagandholine) on the central nervous system. Indian J Physiol Pharmacol. 1965;9:127–36. [PubMed] [Google Scholar]
- 10.Schliebs R, Liebmann A, Bhattacharya SK, Kumar A, Ghosal S, Bigl V. Systemic administration of defined extracts from Withania somnifera (Indian Ginseng) and Shilajit differentially affects cholinergic but not glutamatergic and GABAergic markers in rat brain. Neurochem Int. 1997;30:181–90. doi: 10.1016/s0197-0186(96)00025-3. [DOI] [PubMed] [Google Scholar]
- 11.Auddy B, Hazra J, Mitra A, Abedon B, Ghosal S. A standardized Withania somnifera extract significantly reduces stress related parameters in chronically stressed humans: A double blind, randomized, placebo controlled study. [Last Accessed: 13 April 2013];Journal of American Nutraceutical Association. 2008 11:50–6. Available from: http://www.lifeforce.net/pdfs/withania_review.pdf . [Google Scholar]
- 12.Raveendranadh P, Naidu MU, Usharani P, Shobha JC. Evaluation of a new computerized psychometric test battery: Effects of zolpidem and caffeine. J Pharmacol Pharmacother. 2013;4:247–55. doi: 10.4103/0976-500X.119710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gualtieri CT. Computerized neurocognitive testing and its potential for modern psychiatry. Psychiatry (Edgmont) 2004;1:29–36. [PMC free article] [PubMed] [Google Scholar]
- 14.Dhuley J. Nootropic-like effect of ashwaganda (Withania somnifera L.) in mice. Phytother Res. 2002;15:524–8. doi: 10.1002/ptr.874. [DOI] [PubMed] [Google Scholar]
- 15.Karnick CR. A double-blind, placebo-controlled clinical studies on the effects of Withania somnifera and Panax Ginseng Extracts on psychomotor performance in healthy Indian volunteers. Indian Med. 1991;3:1–5. [Google Scholar]


