Abstract
Background:
Acute and chronic stress is a risk factor for the development and progression of coronary artery disease. Increased arterial stiffness is an independent marker for cardiovascular disease. Cold pressor test (CPT) is known to be associated with substantial activation of the autonomic nervous system.
Objective:
The aim of this study was to evaluate the effect of Phyllanthus emblica extract on cold pressor stress test induced changes on cardiovascular parameters and aortic wave reflections in healthy human subjects.
Materials and Methods:
This was a double-blind, placebo-controlled, crossover study. Participants were randomized to receive either two capsules of P. emblica extract 250 mg (containing aqueous extract of P. emblica, highly standardized by high-performance liquid chromatography to contain low molecular weight hydrolysable tannins emblicanin-A, emblicanin-B, pedunculagin and punigluconin) or two capsules of placebo twice daily for 14 days. Pharmacodynamic parameters such as heart rate, augmentation pressure, augmentation index (AIx), subendocardial viability ratio (SEVR), radial and aortic blood pressure (BP) were recorded before and after CPT at baseline and end of treatment. After washout period of 14 days, subjects crossed over to the other treatment and the same test procedure was repeated again. Safety assessments were done at baseline and at the end of treatment.
Results:
A total of 12volunteers completed the study. Compared with baseline and placebo, P. emblica extract produced a significant decrease of mean percent change in the indices of arterial stiffness (AIx, radial and aortic BP) and increase in SEVR, an index of myocardial perfusion with CPT. Both treatments were well-tolerated and no serious adverse events were reported.
Conclusion:
Proprietary P. emblica extract, showed a significant decrease in cold pressor stress test induced changes on aortic wave reflections.
Keywords: Augmentation index, cardiovascular disease, cold pressor test, Phyllanthus emblica
INTRODUCTION
The human body is in a state of dynamic equilibrium, also known as allostasis.[1] Stress is the state of threatened homeostasis provoked by psychological, physiological or environmental stressors.[2] The stress response is initiated when external and internal forces, the stressors challenge this allostasis. The sympathetic nervous system and the hypothalamic-pituitary-adrenal (HPA) axis are the main mediators of stress response, which might negatively affect the cardiovascular system by alterations in blood pressure (BP) and lipids.[3] Most allostatic mediators have a biphasic role with protective effects in the short run and damaging effects under chronic stress. Experimental studies have found that acute stress leads to pathophysiological changes in cardiac risk profile and thereby more directly explain a link to cardiovascular disease.[3] Stress releases free radicals which results in oxidative stress damage, resulting in imbalance between oxidant/antioxidant systems. Generation of free radicals is an integral feature of normal cellular functions. In contrast to excessive generation, inadequate removal results in destructive and irreversible cell damage.[4]
Antioxidants play an important protective role against reactive oxygen species.[5] Research indicates that there is an inverse relationship between the dietary intake of antioxidant rich foods and the cardiovascular morbidity.[6] Phyllanthus emblica, also known as Emblica officinalis (Family: Euphorbiaceae) is used in Ayurveda as potent rasayanas, a class of plant derived drugs reputed to promote health and longevity by improving defense against diseases.[7] P. emblica is a rich source of vitamin C, which plays an important role in scavenging free radicals, thus attenuates stress induced cardiovascular changes through its antioxidant action.[8] E. officinalis has been reported to reduce arterial BP and heart rate (HR) in rats with deoxycorticosterone acetate/1% sodium chloride high salt-induced hypertension.[9]
The present study was undertaken to evaluate the effect of P. emblica on cold pressor stress test induced changes on cardiovascular parameters and aortic wave reflections in healthy human subjects. We hypothesized that cold pressor stress test would result in increased central BP, wave reflection and aortic stiffness.
MATERIALS AND METHODS
This was a prospective, randomized, double blind study conducted after approval of the Institutional Ethics Committee of Nizam's Institute of Medical Sciences (NIMS), Hyderabad, India. The study was registered in the Clinical Trial Registry of India (2013/05/003656). All subjects gave written informed consent prior to participation in the study. The cold pressor stress test model equipment used in the present study was designed and validated by the department of clinical pharmacology and Therapeutics, NIMS, Hyderabad.[10]
Study participants
The 15 healthy male participants aged between 20 and 30 years, were screened following a full medical history, physical examination, hematological, hepatic, renal, biochemical, electrocardiogram and chest X-ray. Urine drug screening was done, for drug of abuse. The volunteers were selected from departmental database, which includes some of them working with the institute and some with private firms. Volunteers were excluded if there was any evidence of physical illness, drug abuse or abnormal laboratory parameters. Subjects were trained on the test procedure on two prior occasions so as to introduce them to the test procedure and to make them familiar with the testing device. All the recordings were carried out in the morning between 7.30 am and 10:00 am after a light breakfast.
Study medication
Each CAPROS® 250mg capsule,is an aqueous extract of the edible fruits of Phyllanthus emblica (Amla), containing not less than 60% of low molecular weight hydrolysable tannins comprising Emblicanin-A, Emblicanin-B, Punigluconin and Pedunculagin as the bioactives. Each placebo capsule contains microcrystalline cellulose (49.7% w/w), lactose (49.5%) and magnesium stearate (0.69% w/w). Both study medications were supplied by Natreon, Inc. USA.
Study procedure
The study was a double-blind placebo controlled crossover design. All eligible subjects were randomized to receive either two capsules of proprietary P. emblica extract 250 mg twice-a-day or two capsules of placebo twice-a-day for 14 days. Subjects were housed in the department and after an overnight fast and abstinence of caffeine containing beverages, alcohol and smoking for at least 12 h, the study procedures were initiated. Before any testing, each subject rested in a supine position for 20 min in a quiet, temperature-controlled (26°C ± 1°C) room. Subjects were asked to breathe normally and to remain at rest during the cardiovascular measurement. Subjects were permitted to listen to music and to read, except during periods of cardiovascular measurement. After stabilizing in an ambient environment the baseline arterial stiffness was recorded with sphygmocor, after which cold pressor test (CPT) was performed as described below. Then the arterial stiffness was again recorded within 2 min of performing CPT. Then the subject was given study medication and asked to take two capsules twice-a-day of the study medication allocated to them as per prior randomization schedule with 240 ml of water. The same procedure was repeated after 2 weeks of treatment. A washout period of 14 days was given between the treatments. Subjects then crossed over to receive the second formulation two capsules twice daily for 2 weeks. All the same test procedures were repeated before and after treatment.
Recording of vital parameters
Brachial BP and HR were measured with an automated digital BP monitor (OMRAN, SEM-1) and a mean of three readings was taken. All readings were taken with cuff placed on the subject's non-dominant arm positioned at heart level with the forearm resting on a table.
Cold stimulation technique
The recording was performed in a temperature controlled room at 24°C. After 10 min of rest, subject's BP was recorded. Then the subject placed his non-dominant hand up to the wrist with fingers wide apart in a temperature controlled hot water bath at 35°C for 2 min. 15 s before transferring the arm into cold water bath, the BP cuff was inflated to 20 mm Hg below subject's diastolic BP. Then the subject was asked to keep his hand in cold water bath up to the wrist with fingers wide open maintained at 1°C ± 0.5°C, until he was able to sense unbearable pain (indicated by raising the finger of dominant hand). Then the subject was then asked to immerse his hand again in hot water bath maintained at 35°C for 1 min for normalization of temperature.[10]
Measurement of wave reflection indices
Arterial stiffness was measured by using a validated, commercially available system (SphygmoCor; AtCor Medical, Australia) that employs the principle of applanation tonometry and appropriate acquisition and analysis software for non-invasive recording and analysis of the arterial pulse.[11,12,13] Augmentation index (AIx) and augmented pressure of the central (aortic) pressure waveforms were measured as indices of wave reflections. The AIx (defined as augmented pressure divided by pulse pressure and expressed as a percentage) is a composite measure of the magnitude of wave reflections and arterial stiffness, which affects timing of wave reflections. The subendocardial viability index, an indicator of myocardial workload and perfusion (O2 supply vs. demand) was calculated as the ratio of the integral of diastolic pressure and time to the integral of systolic pressure and time.[14]
Statistical analysis
As this is a pilot study, the test has been carried out in 15 healthy volunteers. ANOVA and paired t-test was used for evaluation. Two-way ANOVA was used for the analysis. P < 0.05 was considered to be statistically significant. All statistical analysis was performed using GraphPad Prism 4, Sandiego, CA, USA.
RESULTS
A total of 15 male volunteers were screened and three were excluded due to abnormal laboratory results (two volunteers had abnormal elevated Serum glutamic pyruvic transaminase (SGPT) and one had mildly elevated BP). The remaining 12 volunteers were randomized to receive either P. emblica or placebo capsule in crossover manner. All the randomized subjects completed the study and were evaluated for cardiovascular measurements. The mean age of the study participants was 25.62 ± 2.32 years and mean body mass index 22.42 ± 2.32 kg/m2 .
In healthy human subjects, CPT is known increase BP. In the present study, the CPT produced marked increase in cardiovascular parameters when compared to baseline in both groups. It has been suggested that increased sympathetic activation and BP in response to cold exposure may contribute to cardiovascular changes.[15,16] Sympathetic activation could lead to an increase in peripheral arterial stiffness via vasoconstriction, resulting in increased BP.[17] The present study also showed significant increase in BP after CPT compared with baseline. The BP response to CPT was attenuated after 2 weeks of treatment with P. emblica extract, contributing to decrease in mean systolic and diastolic BP compared with baseline and placebo [Table 1]. CPT also increases arterial stiffness, which results in increase in aortic augmentation pressure and AIx as measured by sphygmocor. The increase in AIx produced by CPT was also significantly reduced by P. emblica extract [Table 1], resulting in 7% decrease in AIx compared with baseline and placebo [Figure 1].
Table 1.
Effect of Phyllanthus emblica and Placebo on cold pressor induced changes in wave reflections
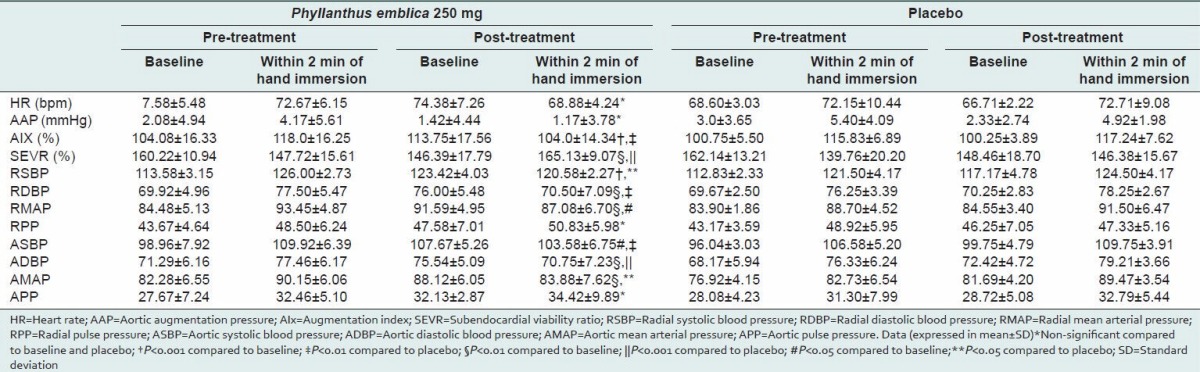
Figure 1.
Mean percent change in augmentation index induced by cold pressor test after treatment with Phyllanthus emblica and placebo
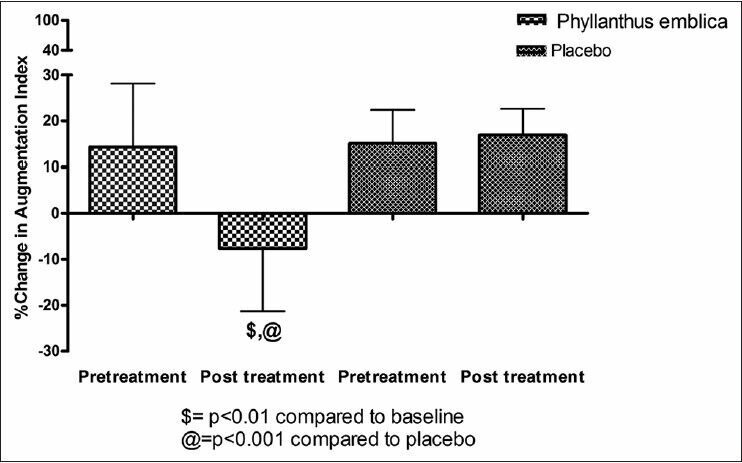
Sub-endocardial viability ratio (SEVR) is an indicator of myocardial perfusion, which is reduced by CPT as compared to baseline [Table 1]. The present study also showed 14% increase in SEVR on treatment with P. emblica extract, which was significant compared to baseline and placebo. Further no significant changes were observed in the evaluated parameters in placebo group [Figure 2].
Figure 2.
Mean percent change in subendocardial viability ratio induced by cold pressor test after treatment with Phyllanthus emblica and placebo
Both study medications were well-tolerated with two volunteers reported mild adverse effects, abdominal discomfort in P. emblica extract and one with headache in placebo group and none of the subjects discontinued the drugs because of these adverse effects. There was no significant change in safety lab parameters at the end of treatment when compared to baseline.
DISCUSSION
In the present study, CPT was used as a model to produce the stress induced cardiovascular changes in healthy volunteers. Increased aortic stiffness, enhanced wave reflection, increased systolic pressure and pulse pressure (and especially central pulse pressure) have been identified as independent components of cardiovascular risk.[18] E. officinalis has been reported to produce cardiovascular adaptation by augmenting endogenous antioxidants and protects heart from oxidative stress in animal models.[19] The antioxidant and cardioprotective effect of E. officinalis was also elaborated by Bhattacharya et al., in animal models.[20]
Cold pressor stress is an experimental stress paradigm based on a short-term painful stimulation by immersing the hand into ice-cold water. This paradigm has been frequently used in stress research and is known to be associated with substantial activation of the autonomic nervous system as well as mild to moderate activation of the HPA axis.[21,22] Studies have shown that CPT model can be used to examine the cold induced sympathetic activation on vascular function. The cold exposure results in sympathetic activation, vasoconstriction and increased systolic and diastolic BP and also increases aortic augmentation index (AIx), a measure of wave reflection, leading to augmented central systolic pressure.[23,24,25,26] The increase in AIx induced by CPT in the present study was significantly reduced on treatment with P. emblica extract.
Tapas et al., in their study demonstrated that immersion of the right hand up to the wrist in 4°C cold water for 60 s stressed the healthy subjects. Stress exerted through the CPT for a minute stimulated the sympathetic nervous system and produced an acceleration of the HR and rise in BP, both systolic and diastolic, in comparison to those recorded before the foregoing test in all the normotensive volunteers.[27] Our study observations are in agreement with these published reports. In the present study, there was increase in, radial and aortic systolic and diastolic BP after CPT. These stress induced changes on cardiovascular parameters were attenuated on treatment with P. emblica extract, whereas the placebo group did not show any significant change in response to CPT induced stress. The effect of CPT on HR response and BP is less well-defined, more variable on an individual basis and not homogeneous for the entire CPT period as reported by Mourot et al., in normal subjects and the similar variations in HR and BP in response to CPT was also observed in the present study.[28] This explains the variability in vascular reactivity in response to cold pressor stimulation in healthy volunteers.
Chart 1.
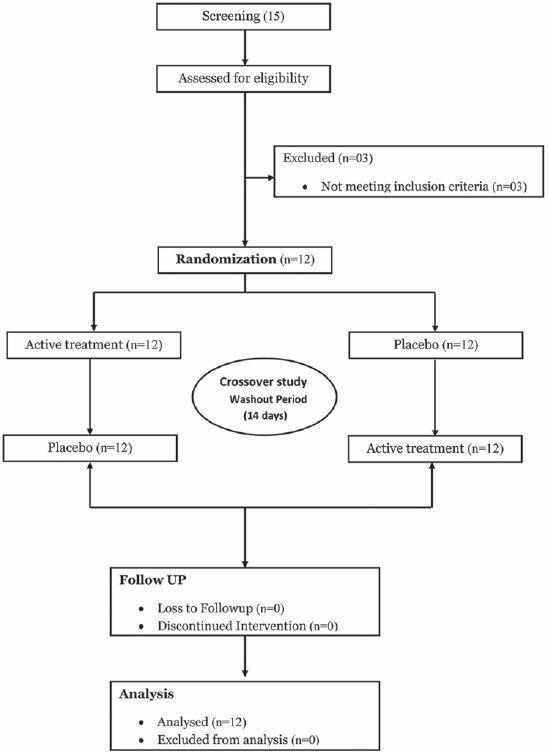
Consort flow chart
SEVR is an index of myocardial oxygen supply and demand. Low SEVR has been shown to be associated with coronary artery disease, severity of Type I and Type II diabetes, decreased renal function and a history of smoking.[14] Estimation of SEVR by using applanation tonometry may provide a reliable tool for the assessment of coronary microcirculation in essential hypertensives with indications of myocardial ischemia and normal coronary arteries. The decrease in SEVR observed in the present study after cold pressor induced stress, is due to vasoconstriction and decreased myocardial perfusion.
Oxidative stress has been implicated in the pathophysiology of stress and associated cardiovascular changes. Thangaraj et al., investigated the antioxidant property of E. officinalis during restrain stress in albino rat. Administration of E. officinalis (500 mg/kg body weight for 30 days) significantly prevented the restrain-stress-induced oxidative stress and elevation in lipid peroxidation levels.[29] In another recent study, the antihypertensive effect of administration of hydro-alcoholic lyophilized extract of E. officinalis given in different doses (75, 150 and 300 mg/kg/day) for 5 weeks caused reduction in arterial BP in rats. Increased thiobarbituric acid substances and decreased endogenous antioxidants including glutathione s- transferase and superoxide dismutase activity in serum, heart and kidney tissues of hypertensive rats were also normalized.[13] Gopa et al., demonstrated that Amla therapy given in the dose of 500 mg capsule once daily at night for 42 days produced reduction in BP in hypertensive patients.[30] The well-established antioxidant and cardio protective actions of E. officinalis explains the possible mechanism of P. emblica extract in reducing cardiovascular changes produced by CPT.
CONCLUSION
Proprietary P. emblica extract used in the present study showed significant decrease in cold pressor stress test induced changes on aortic wave reflections, suggesting the beneficial effects of this formulation in reducing stress induced cardiovascular changes. Further clinical studies are warranted to evaluate the beneficial effects of P. emblica in patients with other associated diseases and in those residing in cold climatic conditions.
ACKNOWLEDGMENTS
Authors would like to thank Natreon Inc., USA for providing study medications (Phyllanthus emblica extract and Placebo) and literature. Authors thank ICMR (New Delhi) for extramural grant to develop cold pressor test equipment and Dr. I. V. Sravanthi (Ayurvedic Physician) for her expert advice. Authors thank Mr. N. Muralidhar for helping in carrying out study related procedures.
Footnotes
Source of Support: This study was supported by Natreon INC. USA
Conflict of Interest: None declared.
REFERENCES
- 1.McEwen BS, Wingfield JC. The concept of allostasis in biology and biomedicine. Horm Behav. 2003;43:2–15. doi: 10.1016/s0018-506x(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 2.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 3.Rozanski A, Blumenthal JA, Kaplan J. Impact of psychological factors on the pathogenesis of cardiovascular disease and implications for therapy. Circulation. 1999;99:2192–217. doi: 10.1161/01.cir.99.16.2192. [DOI] [PubMed] [Google Scholar]
- 4.Lopacszyski W, Zeisel SH. Antioxidants, programmed cell death and cancer. Nutr Res. 2001;21:295–307. [Google Scholar]
- 5.Eqbal MD, Abdullah A, Sani AH. Natural antioxidants, lipid profile, lipid peroxidation, antioxidant enzymes of different vegetable oils. Adv J Food Sci Technol. 2011;3:308–16. [Google Scholar]
- 6.Hallibell V. Advances in Pharmacology. Vol. 38. Academic Press; 1997. Antioxidants in Disease Mechanisms and Therapy; pp. 3–17. [Google Scholar]
- 7.Udupa KN, Singh RH. Clinical and experimental studies on rasayana drugs and panchakarma therapy. New Delhi: Central Council for Research in Ayurveda and Siddha; 1995. [Google Scholar]
- 8.Kenjale RD, Shah RK, Sathaye SS. Anti-stress and anti-oxidant effects of roots of Chlorophytum borivilianum (Santa Pau and Fernandes) Indian J Exp Biol. 2007;45:974–9. [PubMed] [Google Scholar]
- 9.Bhatia J, Tabassum F, Sharma AK, Bharti S, Golechha M, Joshi S, et al. Emblica officinalis exerts antihypertensive effect in a rat model of DOCA-salt-induced hypertension: Role of (p) eNOS, NO and oxidative stress. Cardiovasc Toxicol. 2011;11:272–9. doi: 10.1007/s12012-011-9122-2. [DOI] [PubMed] [Google Scholar]
- 10.Reddy KS, Rani PU, Naidu MU, Rao TR. A simple cold pressure technique for the evaluation of analgesic drugs in healthy subjects. Indian J Pharmacol. 2012;44:571–5. doi: 10.4103/0253-7613.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamanoglu M, O’Rourke MF, Avolio AP, Kelly RP. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–7. doi: 10.1093/eurheartj/14.2.160. [DOI] [PubMed] [Google Scholar]
- 12.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, et al. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–36. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 13.Vlachopoulos C, Hirata K, O’Rourke MF. Effect of caffeine on aortic elastic properties and wave reflection. J Hypertens. 2003;21:563–70. doi: 10.1097/00004872-200303000-00022. [DOI] [PubMed] [Google Scholar]
- 14.Doonan RJ, Scheffler P, Yu A, Egiziano G, Mutter A, Bacon S, et al. Altered arterial stiffness and subendocardial viability ratio in young healthy light smokers after acute exercise. PLoS One. 2011;6:e26151. doi: 10.1371/journal.pone.0026151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culić V. Acute risk factors for myocardial infarction. Int J Cardiol. 2007;117:260–9. doi: 10.1016/j.ijcard.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Marchant B, Ranjadayalan K, Stevenson R, Wilkinson P, Timmis AD. Circadian and seasonal factors in the pathogenesis of acute myocardial infarction: The influence of environmental temperature. Br Heart J. 1993;69:385–7. doi: 10.1136/hrt.69.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boutouyrie P, Lacolley P, Girerd X, Beck L, Safar M, Laurent S. Sympathetic activation decreases medium-sized arterial compliance in humans. Am J Physiol. 1994;267:H1368–76. doi: 10.1152/ajpheart.1994.267.4.H1368. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell GF. Arterial stiffness and wave reflection: Biomarkers of cardiovascular risk. Artery Res. 2009;3:56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajak S, Banerjee SK, Sood S, Dinda AK, Gupta YK, Gupta SK, et al. Emblica officinalis causes myocardial adaptation and protects against oxidative stress in ischemic-reperfusion injury in rats. Phytother Res. 2004;18:54–60. doi: 10.1002/ptr.1367. [DOI] [PubMed] [Google Scholar]
- 20.Bhattacharya A, Chatterjee A, Ghosal S, Bhattacharya SK. Antioxidant activity of active tannoid principles of Emblica officinalis (amla) Indian J Exp Biol. 1999;37:676–80. [PubMed] [Google Scholar]
- 21.McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: Biological and subjective responses to the cold pressor and trier social stressors. Hum Psychopharmacol. 2006;21:377–85. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- 22.Schwabe L, Haddad L, Schachinger H. HPA axis activation by a socially evaluated cold-pressor test. Psychoneuroendocrinology. 2008;33:890–5. doi: 10.1016/j.psyneuen.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 23.Edwards DG, Gauthier AL, Hayman MA, Lang JT, Kenefick RW. Acute effects of cold exposure on central aortic wave reflection. J Appl Physiol. 2006;100:1210–4. doi: 10.1152/japplphysiol.01154.2005. [DOI] [PubMed] [Google Scholar]
- 24.Brown CM, Sanya EO, Hilz MJ. Effect of cold face stimulation on cerebral blood flow in humans. Brain Res Bull. 2003;61:81–6. doi: 10.1016/s0361-9230(03)00065-0. [DOI] [PubMed] [Google Scholar]
- 25.Patel HM, Mast JL, Sinoway LI, Muller MD. Effect of healthy aging on renal vascular responses to local cooling and apnea. J Appl Physiol. 2013;115:90–6. doi: 10.1152/japplphysiol.00089.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwards DG, Roy MS, Prasad RY. Wave reflection augments central systolic and pulse pressures during facial cooling. Am J Physiol Heart Circ Physiol. 2008;294:H2535–9. doi: 10.1152/ajpheart.01369.2007. [DOI] [PubMed] [Google Scholar]
- 27.Tapas P, Regmi P, Adhikari P, Roychowdhury P. Cold pressor test as a predictor of hypertension. J Tehran Univ Heart Cent. 2009;3:177–80. [Google Scholar]
- 28.Mourot L, Bouhaddi M, Regnard J. Effects of the cold pressor test on cardiac autonomic control in normal subjects. Physiol Res. 2009;58:83–91. doi: 10.33549/physiolres.931360. [DOI] [PubMed] [Google Scholar]
- 29.Thangaraj R, Rathakrishnan SA, Pannerselvam M, Jayaraman B. Antioxidant property of Emblica officinalis during experimentally induced restrain stress in rats. J Health Sci. 2007;53:496–9. [Google Scholar]
- 30.Gopa B, Bhatt J, Hemavathi KG. A comparative clinical study of hypolipidemic efficacy of Amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor simvastatin. Indian J Pharmacol. 2012;44:238–42. doi: 10.4103/0253-7613.93857. [DOI] [PMC free article] [PubMed] [Google Scholar]


