Abstract
Background:
Obesity is a metabolic disorder that can lead to adverse metabolic effects on blood pressure, cholesterol, triglycerides and insulin resistance and also increases the risk of coronary heart disease, ischemic stroke and type 2 diabetes mellitus. This study was designed to determine the effect of Coleus forskohlii on obesity and associated metabolic changes in rats fed with cafeteria diet.
Objective:
The aim of this study was to evaluate antiobesogenic and metabolic benefits of C. forskohlii in cafeteria diet induced obesity rat model.
Materials and Methods:
Rats were randomly divided into five groups of six animals in each group and as follows: Normal pellet diet group; cafeteria diet group; cafeteria diet followed by 50 mg/kg/d Coleus forskohlii extract (CFE), 100 mg/kg/d CFE and 45 mg/kg/d orlistat groups, respectively. Indicators of obesity such as food intake, body weight and alteration in serum lipid profiles were studied.
Results:
Feeding of cafeteria diet induced obesity in rats. Administration of CFE significantly halted increase in food intake and weight gain associated with cafeteria diet. Development of dyslipidemia was also significantly inhibited.
Conclusion:
The observed effects validate that supplementation of CFE with cafeteria diet could curb the appetite and mitigate the development of dyslipidemia.
Keywords: Appetite, cholesterol, Cafeteria diet, Coleus forskohlii, lipid profile
INTRODUCTION
Obesity is a metabolic disorder that can lead to adverse metabolic effects on blood pressure, cholesterol, triglycerides (TGs) and insulin resistance and also increases the risk of coronary heart disease, ischemic stroke and type 2 diabetes mellitus. Overweight and obesity are the fifth leading risk for global deaths. At least 2.8 million adults die each year as a result of being overweight or obese. In addition, 44% of the diabetes burden, 23% of the ischemic heart disease burden and between 7% and 41% of certain cancer burdens are attributable to overweight and obesity.[1] The most associated reasons for increase in the prevalence rates are increasing rates of obesity in children and adolescents due to sedentary life-style and unhealthy food habits.
In spite of better understanding of obesity and number of pharmacological approaches to treatment obesity, only few antiobesity drugs are safe and most of these have adverse effects.[2] Thus, the best way to control obesity is to prevention its development. Traditional system of medicine provides a rich source of herbs, which are used over thousands of years safely for prevention and treatment of obesity.
Coleus forskohlii Briq. (Lamiaceae), native Indian plant is one of the oldest medicinal plants mentioned in the traditional system of medicine to treat various ailments including obesity.[3] It has also got long food use in India. Numerous preclinical and clinical studies, with C. forskohlii have reported significant weight reduction activity, without any noticeable adverse effects. The most known mechanism behind its antiobesity activity is the direct activity of forskolin on adenylate cyclase, an enzyme that activates the synthesis of cyclic adenosine monophosphate or Cyclic adenosine monophosphate (cAMP) in the cell. cAMP promotes the breakdown of stored fats in animal and human fat cells.[4]
In the present study, the effect of Coleus forskohlii extract (CFE) on cafeteria diet induced obesity and indicators of obesity such as appetite, Weight gain, dyslipidemia and adipocyte area was determined.
MATERIALS AND METHODS
Test substance
C. forskohlii root extract M/s. Olive Lifesciences Pvt. Ltd., India.
Drugs/chemicals used
Cholesterol AR (HiMedia Laboratories Pvt. Ltd., India), Cholic acid (HiMedia Laboratories Pvt. Ltd., India), Oleic acid (Qualigens fine chemicals), orlistat (Meyer Pharmaceuticals Bangalore.; Trade name: Xenical 20). Cholesterol estimation kit (ERBA Diagnostics Mannheim GmbH, Germany), TGs estimation kit (ERBA Diagnostics Mannheim GmbH, Germany) and high density lipoprotein (HDL) cholesterol kit (ERBA Diagnostics Mannheim GmbH, Germany).
Animals
Thirty female albino wistar rats (Central Animal Facility, Sree Siddaganga College of Pharmacy, Tumkur) (45 days old), were acclimatized for a week and maintained under standard laboratory conditions with free access to standard pellet feed (M/s Pranava Agro Industries Sangli, Maharashtra) and ultraviolet purified and filtered water, ad libitum.
Diets
The cafeteria diet consisted of three diets (a) 48 g of condensed milk, 48 g of bread, (b) 18 g of chocolate, 36 g of dried coconut, 36 g of biscuit and (c) 48 g of cheese, 60 g of potatoes. The three diets were presented to the individual rats on day 1, 2 and 3 respectively and then repeated for 42 days in the same succession in addition to normal pellet chow diet.[5]
Treatment protocol
The animals were divided into five groups of six animals each and individually housed in cages. The normal control group continued to be fed a laboratory pellet chow ad libitum. The cafeteria diet-control group received the cafeteria diet in addition to the normal pellet diet (NPD). The remaining three groups were fed with the cafeteria diet and NPD along with CFE (50 mg/kg, p.o.), CFE (100 mg/kg, p.o.) and orlistat (45 mg/kg, p.o.), respectively. Treatment was continued for 6 weeks. The animals were weighed at the start of the experiment and then every week thereafter.
Measurement of food intake
The food intake of each animal was determined initially and then every week thereafter by measuring the difference between the pre-weighed chows and the weight of the food that remained after 24 h and the results were expressed as a gram per week for a group of six rats each.[6]
Blood biochemical analysis
On the day 42, blood was collected by retro-orbital puncture from the ether-anesthetized rats and subjected to centrifugation to obtain the serum. The serum levels of cholesterol and lipoproteins were estimated using ERBA estimation kits.
Estimation of organ and tissue weights, parametrial adipose tissue weight, and liver TG content
Animals were sacrificed with an overdose of diethyl ether. The liver, heart, kidney and Parametrial adipose tissue (PAT) were quickly removed and weighed. The liver tissue was stored at −80°C until analysis was performed. The liver TG content was estimated as follows; a portion (0.5 g) of liver tissue was homogenized in the Krebs Ringer Phosphate buffer (pH 7.4, 4.5 mL) and the homogenate (0.2 mL) was extracted with a chloroform-methanol mixture (2:1, v/v, 4 mL). The extract was concentrated and the residue was analyzed using ERBA TG E-test kit.[7]
Fat excretion in feces
Feces wet weight and TG content in feces obtained during the last 24 h was measured using ERBA TG E-test kit.[7]
Statistical analysis
Results were expressed as mean ± Standard error of the mean. The data were analyzed by one-way analysis of variance followed by Dunnett's multiple comparisons test and P < 0.05 was considered as significant.
RESULTS
Effect on body weight
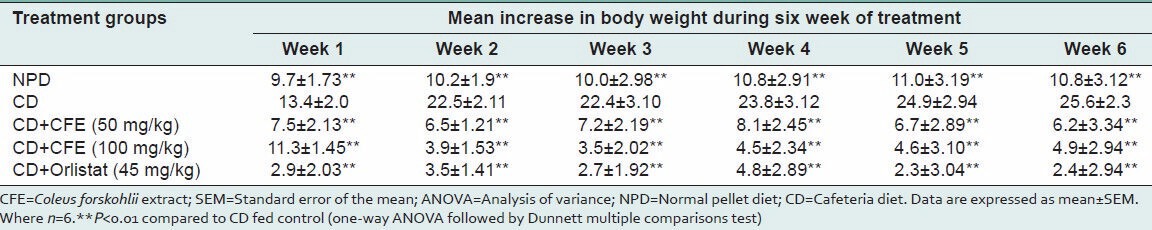
Cafeteria diet significantly increased the body weight of rats compared to NPD fed group over 6 weeks. Both 50 and 100 mg/kg CFE significantly (P < 0.01) inhibited weight gain over 6 weeks compared with cafeteria diet alone fed group. There was no significant difference between both the doses of CFE on body weight gain. Orlistat (45 mg/kg) also showed a significant reduction in weight gain compared with cafeteria diet alone fed group [Table 1].
Table 1.
Mean body weight gain of rats fed experimental diets for 6 weeks
Effect on food intake
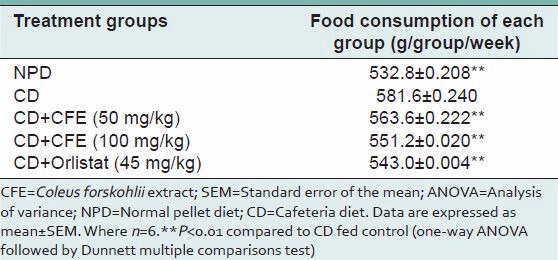
Feeding behavior was monitored in the test animals for 6 weeks. As expected, food intake was significantly greater in cafeteria diet fed group than in the group given NPD (P < 0.01). Concurrent administration of CFE (50 mg/kg or 100 mg/kg) with cafeteria diet, reduced food intake considerably compared with cafeteria diet alone group (P < 0.01). Orlistat (45 mg/kg) also showed a significant (P < 0.01) decrease in feed intake per week [Table 2].
Table 2.
Food intake of rats fed experimental diets for 6 weeks
Effect on serum biochemical parameters
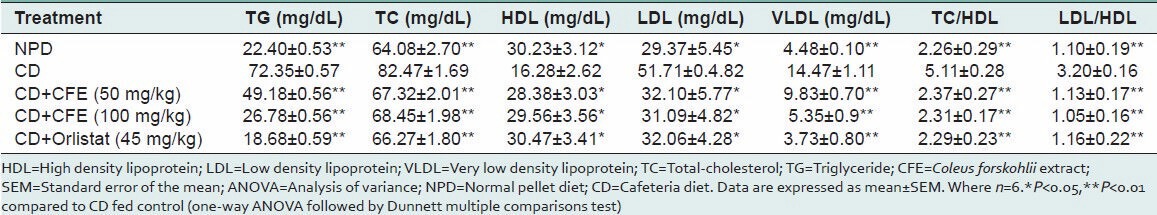
Cafeteria diet significantly increased levels of total-cholesterol (TC), low density lipoprotein (LDL), very low density lipoprotein (VLDL), TGs and significantly reduced HDL as compared to NPD-control [Table 3]. Both the doses of CFE administered for 6 weeks to rats significantly decreased serum TC, TG, VLDL, LDL and significantly increased serum HDL-cholesterol level compared to cafeteria diet-control. Orlistat showed significant changes in all the serum biochemical parameters compared to cafeteria diet alone fed group [Table 3]. Dyslipidemia indices such as TG/HDL and LDL/HDL were also significantly improved by CFE administration.
Table 3.
Plasma biochemistry of rats fed experimental diets for 6 weeks
Effect on organs weight, PAT weight, and liver TG content
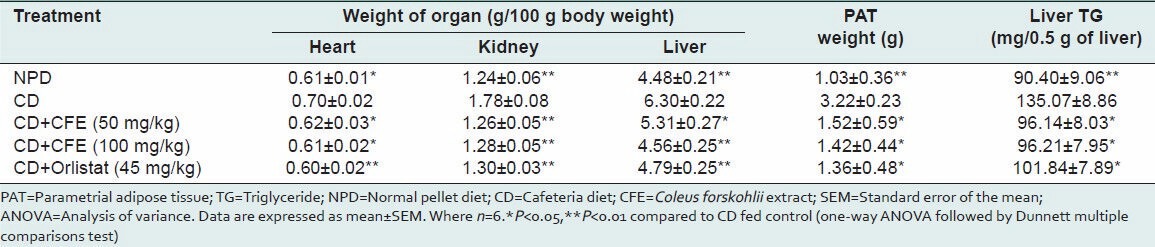
Cafeteria diet produced a significant increase in heart, liver, kidney and PAT weights compared to NPD fed rats [Table 4]. Furthermore, the cafeteria diet also induced fatty liver, with the accumulation of TGs in the liver when compared with the normal control group. Treatment with CFE (both low and high dose) and orlistat significantly inhibited the weight gain of the heart, liver, kidney, and PAT. Both doses of CFE prevented the accumulation of TGs in liver [Table 4].
Table 4.
Organs weight, PAT weight and liver TG level of rats fed experimental diet for 6 weeks
Fat excretion in feces
Fecal wet weight was significantly decreased in cafeteria diet fed rats than in NPD fed rats. Treatment with CFE (50 and 100 mg/kg) and orlistat to cafeteria diet fed rats significantly (P < 0.01) increased the feces weight at the end of 6 weeks, compared to cafeteria diet fed control [Table 5].
Table 5.
Wet weight of feces and TG in feces of rats fed experimental diet for 6 weeks
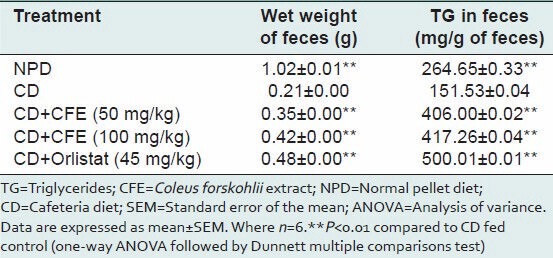
The amount of TG in the feces was dramatically reduced in rats fed with cafeteria diet for 6 weeks compared to NPD fed rats. Treatment with different doses of CFE (50 and 100 mg/kg) and orlistat for 6 weeks showed significantly higher TG in feces compared with cafeteria diet fed control. The results are summarized in a Table 5.
DISCUSSION
Feeding rats with cafeteria diet inevitably causes weight gain compared to normal pellet chow diet.[8] A cafeteria diet induced obesity model is the simplest obesity-induced model and possibly the one that most closely resembles the reality of obesity in humans.[9] Our results demonstrated that feeding of CFE with cafeteria diet to rats for 6 weeks produced significantly reduced food intake (appetite suppressant effect) in this model. These effects were reflected in the reduced body weight, liver weight, PAT and serum lipid profiles of rats. The possible mechanism of appetite suppression may be a reduction of digestion of carbohydrates and lipids in the digestive track of rats. The most common complication of obesity includes significant alteration of lipid profiles. Apart from weight reduction, treatment with CFE caused significant normalization in the lipid profiles, which include significant decrease in levels of TC, LDL, VLDL and TG and increase in HDL compared with cafeteria diet alone fed rats. The reduction in the ratios of TC/HDL and LDL/HDL observed in CFE treated rats might be due to the higher proportion of HDL. Elevated serum TG is considered as an independent risk factor for cardiovascular diseases.[10] A significant decline in the serum TG was observed in CFE treated rats. The mechanism by which CFE lower the serum TG concentration could be either by decreasing VLDL synthesis, by channeling VLDL through pathways other than to LDL or an increase in lipoprotein lipase activity. CFE supplementation suppressed fat absorption, consequently resulting in increased excretion of TG in feces and this could partly contribute to its lipid lowering effect.
Based on the present study CFE might elicit beneficial effects by halting the development of obesity and obesity associated metabolic change (dyslipidemia) in cafeteria diet fed rats by its hypophagic and antihyperlipidemic properties. CFE, 50 mg/kg/day appear to be the optimal dose for preventing cafeteria diet induced changes in body weight, PAT and dyslipidemia. Present study supports the use of CFE as an antiobesogenic agent. CFE supplementation mitigates the development of high fat diet induced obesity and associated metabolic imbalance like dyslipidemia. Possible mechanisms may be due to its hypophagic and antihyperlipidemic activities. Further studies are required to gain more insight into the mechanism of antiobesogenic activity of C. forskohlii.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Who.int [homepage on the internet] Obesity and overweight. [Updated on 2013 Mar]. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/
- 2.Ryan DH, Bray GA, Helmcke F, Sander G, Volaufova J, Greenway F, et al. Serial echocardiographic and clinical evaluation of valvular regurgitation before, during, and after treatment with fenfluramine or dexfenfluramine and mazindol or phentermine. Obes Res. 1999;7:313–22. doi: 10.1002/j.1550-8528.1999.tb00414.x. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal KC, Parks RE., Jr A potential antimetastatic agent. Int J Cancer. 1983;32:801–4. doi: 10.1002/ijc.2910320622. [DOI] [PubMed] [Google Scholar]
- 4.Litosch I, Hudson TH, Mills I, Li SY, Fain JN. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol Pharmacol. 1982;22:109–15. [PubMed] [Google Scholar]
- 5.Kumar S, Alagawadi KR, Rao MR. Effect of Argyreia speciosa root extract on cafeteria diet-induced obesity in rats. Indian J Pharmacol. 2011;43:163–7. doi: 10.4103/0253-7613.77353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ukwuani AN, Abukakar MG, Shehu RA, Hassan LG. Anti obesity effects of pulp extract Tamarindus indica in albino rat. Asian J Biochem. 2008;3:221–7. [Google Scholar]
- 7.Han LK, Zheng YN, Yoshikawa M, Okuda H, Kimura Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Complement Altern Med. 2005;5:9. doi: 10.1186/1472-6882-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sampey BP, Vanhoose AM, Winfield HM, Freemerman AJ, Muehlbauer MJ, Fueger PT, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity (Silver Spring) 2011;19:1109–17. doi: 10.1038/oby.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sclafani A, Springer D. Dietary obesity in adult rats: Similarities to hypothalamic and human obesity syndromes. Physiol Behav. 1976;17:461–71. doi: 10.1016/0031-9384(76)90109-8. [DOI] [PubMed] [Google Scholar]
- 10.Fungwe TV, Cagen LM, Cook GA, Wilcox HG, Heimberg M. Dietary cholesterol stimulates hepatic biosynthesis of triglyceride and reduces oxidation of fatty acids in the rat. J Lipid Res. 1993;34:933–41. [PubMed] [Google Scholar]


