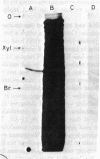
Abstract
The binding of a few molecules [1-6] of RNA bacteriophage coat protein to 1 molecule of RNA represses in vitro translation of the RNA synthetase cistron. Digestion of the complex, R17 coat protein-R17 RNA, by T1 RNase yields an RNA fragment bound to the coat protein. The nucleotide sequence of this fragment (59 residues) reveals that it contains the punctuation signal between the coat protein and RNA synthetase cistrons, suggesting that this is the site on the RNA where the coat protein acts as a translational repressor.
Keywords: RNA bacteriophages, translational repression, RNA synthetase
Full text
PDF
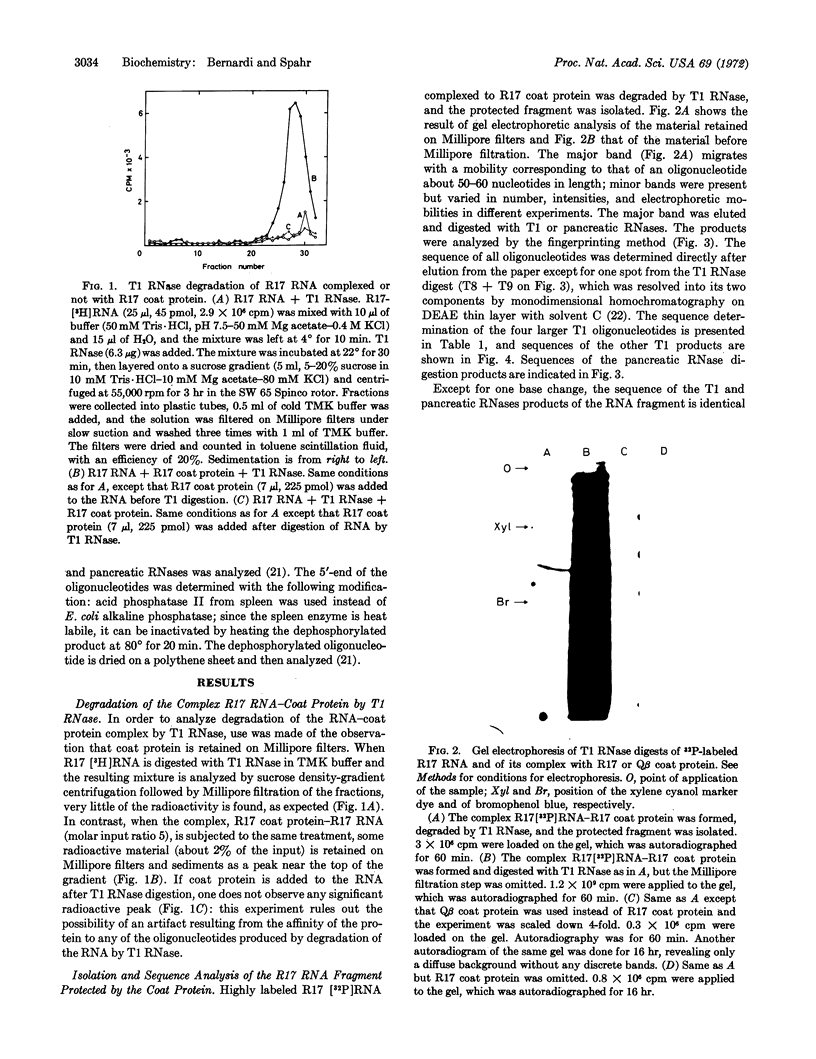
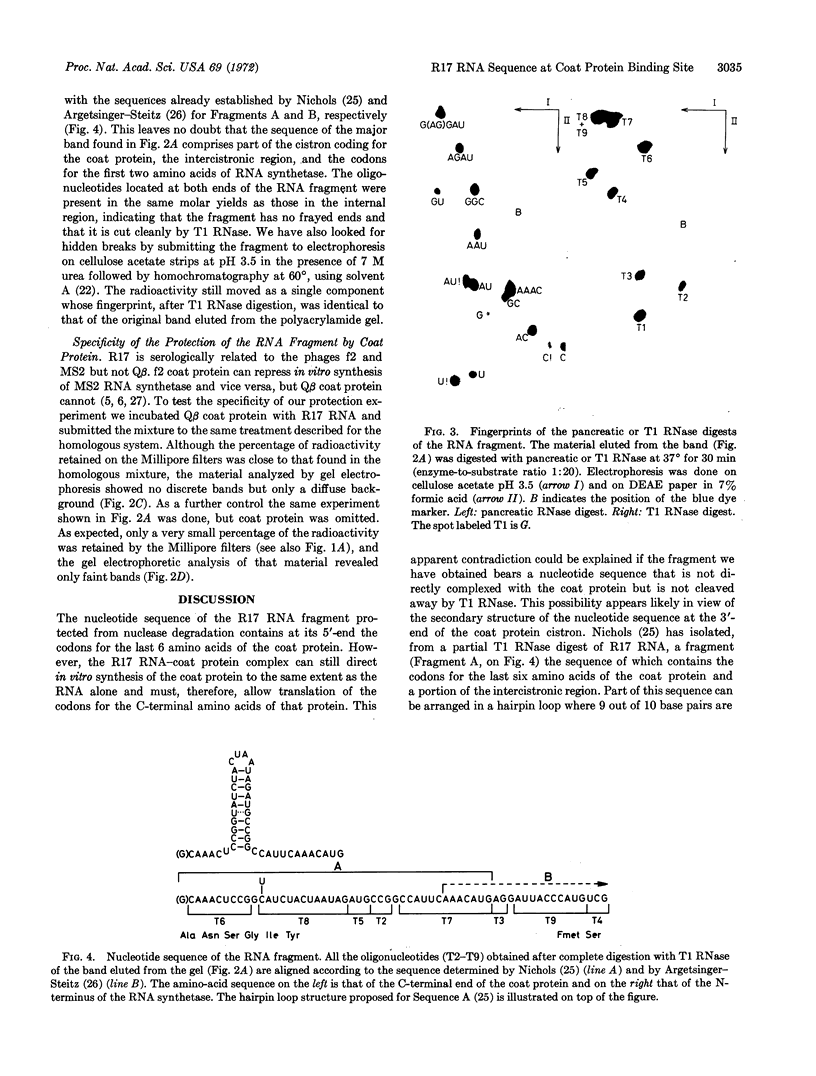


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Jeppesen P. G., Sanger F., Barrell B. G. Nucleotide sequence from the coat protein cistron of R17 bacteriophage RNA. Nature. 1969 Sep 6;223(5210):1009–1014. doi: 10.1038/2231009a0. [DOI] [PubMed] [Google Scholar]
- Adams J. M., Spahr P. F., Cory S. Nucleotide sequence from the 5' end to the first cistron of R17 bacteriophage ribonucleic acid. Biochemistry. 1972 Mar 14;11(6):976–988. doi: 10.1021/bi00756a006. [DOI] [PubMed] [Google Scholar]
- Bernardi A., Bernardi G. Studies on acid hydrolases. 3. Isolation and properties of spleen acid ribonuclease. Biochim Biophys Acta. 1966 Oct 24;129(1):23–31. [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Eggen K., Nathans D. Regulation of protein synthesis directed by coliphage MS2 RNA. II. In vitro repression by phage coat protein. J Mol Biol. 1969 Jan;39(2):293–305. doi: 10.1016/0022-2836(69)90318-0. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Spahr P. F. A new procedure for the preparation of highly labeled intact 32P RNA from bacteriophage R-17. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1267–1272. doi: 10.1016/0006-291x(70)90224-x. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Spahr P. F. Translation of R-17 RNA fragments. Cold Spring Harb Symp Quant Biol. 1969;34:707–716. doi: 10.1101/sqb.1969.034.01.080. [DOI] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Denaturation and renaturation of viral ribonucleic acid. I. Annealing R17 ribonucleic acid with denatured replicative form or with denatured replicative intermediate. J Virol. 1967 Aug;1(4):793–803. doi: 10.1128/jvi.1.4.793-803.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen P. G. The nucleotide sequences of some large ribonuclease T 1 products from bacteriophage R17 ribonucleic acid. Biochem J. 1971 Sep;124(2):357–366. doi: 10.1042/bj1240357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent initiation of translation of two bacteriophage f2 proteins. Biochem Biophys Res Commun. 1969 Sep 24;37(1):127–136. doi: 10.1016/0006-291x(69)90890-0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F., Zinder N. D. Mutants of the bacteriophage f2. 8. Control mechanisms for phage-specific syntheses. J Mol Biol. 1966 Aug;19(2):333–348. doi: 10.1016/s0022-2836(66)80008-6. [DOI] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Nathans D., Oeschger M. P., Polmar S. K., Eggen K. Regulation of protein synthesis directed by coliphage MS2 RNA. I. Phage protein and RNA synthesis in cells infected with suppressible mutants. J Mol Biol. 1969 Jan;39(2):279–292. doi: 10.1016/0022-2836(69)90317-9. [DOI] [PubMed] [Google Scholar]
- Nichols J. L. Nucleotide sequence from the polypeptide chain termination region of the coat protein cistron in bacteriophage R17 RNA. Nature. 1970 Jan 10;225(5228):147–151. doi: 10.1038/225147a0. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Robertson H. D. Sequences of RNA fragments from the bacteriophage f2 coat protein cistron which differ from their R17 counterparts. Biochim Biophys Acta. 1971 Feb 11;228(3):676–681. doi: 10.1016/0005-2787(71)90731-3. [DOI] [PubMed] [Google Scholar]
- Robertson H., Webster R. E., Zinder N. D. Bacteriophage coat protein as repressor. Nature. 1968 May 11;218(5141):533–536. doi: 10.1038/218533a0. [DOI] [PubMed] [Google Scholar]
- Roufa D. J., Leder P. Biosynthesis of phage-specific initiation dipeptides. A purified biosynthetic system derived from Escherichia coli. J Biol Chem. 1971 May 25;246(10):3160–3167. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Skogerson L., Roufa D., Leder P. Characterization of the initial peptide of Q-beta RNA polymerase and control of its synthesis. Proc Natl Acad Sci U S A. 1971 Feb;68(2):276–279. doi: 10.1073/pnas.68.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr P. F., Farber M., Gesteland R. F. Binding site on R17 RNA for coat protein. Nature. 1969 May 3;222(5192):455–458. doi: 10.1038/222455a0. [DOI] [PubMed] [Google Scholar]
- Staples D. H., Hindley J., Billeter M. A., Weissmann C. Localization of Q-beta maturation cistron ribosome binding site. Nat New Biol. 1971 Sep 15;234(50):202–204. doi: 10.1038/newbio234202a0. [DOI] [PubMed] [Google Scholar]
- Steitz J. A. Polypeptide chain initiation: nucleotide sequences of the three ribosomal binding sites in bacteriophage R17 RNA. Nature. 1969 Dec 6;224(5223):957–964. doi: 10.1038/224957a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Hebert R. R., Hartman K. A. Ribonucleoprotein complexes formed between bacteriophage MS2 RNA and MS2 protein in vitro. J Mol Biol. 1967 May 14;25(3):455–463. doi: 10.1016/0022-2836(67)90198-2. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Nakada D. Translational control of bacteriophage MS2 RNA cistrons by MS2 coat protein: polyacrylamide gel electrophoretic analysis of proteins synthesized in vitro. J Mol Biol. 1968 Feb 14;31(3):431–440. doi: 10.1016/0022-2836(68)90419-1. [DOI] [PubMed] [Google Scholar]
- Viñuela E., Algranati I. D., Feix G., Garwes D., Weissmann C., Ochoa S. Virus-specific proteins in Escherichia coli infected with some amber mutants of phage MS2. Biochim Biophys Acta. 1968 Feb 26;155(2):558–565. doi: 10.1016/0005-2787(68)90199-8. [DOI] [PubMed] [Google Scholar]
- Ward R., Strans M., Valentine R. C. Translational repression of f2 protein synthesis. Biochem Biophys Res Commun. 1968 Feb 15;30(3):310–317. doi: 10.1016/0006-291x(68)90452-x. [DOI] [PubMed] [Google Scholar]
- Weber K. Amino acid sequence studies on the tryptic peptides of the coat protein of the bacteriophage R17. Biochemistry. 1967 Oct;6(10):3144–3154. doi: 10.1021/bi00862a023. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Wittmann H. G. Coat proteins of strains of two RNA viruses: comparison of their amino acid sequences. Mol Gen Genet. 1967;100(4):358–363. doi: 10.1007/BF00334062. [DOI] [PubMed] [Google Scholar]