Abstract
Background:
The Newcastle disease represents as one of the most infectious viral disease, which afflicts almost every species of the birds. The causative agent of the disease is a single-stranded RNA virus with rapid replication capability.
Objective:
This study was performed to evaluate the comparative anti-viral efficacy and toxicity of Glycyrrhiza glabra aqueous extract and ribavirin against the Newcastle disease virus.
Materials and Methods:
The embryonated eggs were divided into six groups (A, B, C, D, E and F). Groups A, B, C, and D were further subdivided into three subgroups. The virus was identified by hemagglutination inhibition test. Spot hemagglutination test and viability of embryos were also evaluated. Three different concentrations i-e., 30 mg/100 ml, 60 mg/100 ml, and 120 mg/100 ml of the Glycyrrhiza aqueous extract and 10 μg/ml, 20 μg/ml, and 40 μg/ml ribavirin in deionized water were evaluated for their toxicity and anti-viral activity in the embryonated eggs.
Results:
60 mg/100 ml concentration of Glycyrrhiza extract did not produce any toxicity in the embryonated eggs and showed anti-viral activity against the virus. Similarly, 20 μg/ml ribavirin was non-toxic in the embryonated eggs and contained anti-viral activity.
Conclusion:
It may conclude from the presented study that 60 mg/100 ml Glycyrrhiza extract inhibits replication of Newcastle disease virus and is non-toxic in the embryonated eggs. So, Glycyrrhiza glabra extract may be further evaluated in future to determine the potentially active compounds for their anti-viral activity against Newcastle disease virus. Furthermore, the mechanism of action of these active phytochemicals as an antiviral agent would be helpful to elucidate the pathogenesis of the disease.
Keywords: Anti-viral agents, embryonated eggs, Glycyrrhiza glabra, Newcastle disease virus, Ribavirin
INTRODUCTION
Newcastle disease (ND) is a highly contagious disease, which affects almost all species of the birds. It was first recognized in Indonesia and England in 1926,[1] and the disease virus has a worldwide prevalence.[2] The disease is caused by a single-stranded, enveloped, non-segmented RNA virus, which resembles in genome configuration to Avian Paramyxoviridae serotype-1 (APMV-1) of genus Avulavirus and the family Paramyxoviridae.[3] The genome of the Newcastle disease virus (NDV) is about 15.0 kb (kilo base) long[4] and encodes for six structural proteins in the order 3’- NP-P-M-F-HN-L-’5 respectively.[5] It is believed that fusion (F) protein is a major determinant of the virulence.[6]
Medicinal plants have been used all over the world for their therapeutic benefits, although their use remained restricted to China, India, Japan, Pakistan, Sri Lanka, Thailand and a number of African countries.[7] Similarly, the developed nations are also encouraging the use of natural medicinal products in their health care systems. Natural medicinal products in the forms of herbs have been commercially added in the dietary supplement industry as well as in holistic medicine in the United States. It has been estimated that one-third person in the United States has tried some form of natural medicine at least once.[8]
The traditional sources for the use of Glycyrrhiza species as an herbal medicine are reported in ancient manuscripts from China, India, and Greece. Its use for symptoms of viral respiratory tract infections and hepatitis has been documented by a number of researchers. Randomized controlled trials of the Glycyrrhiza glabra derived compound “glycyrrhizin,” and its derivatives showed reduced hepatocellular damage in chronic hepatitis B- and C-infected patients. In hepatic cirrhosis induced by hepatitis C virus, the risk to develop hepatocellular carcinoma was reduced in those infected patients who administered with glycyrrhizin.[9] Glycyrrhizin (licorice root extract) has anti-inflammatory and antioxidant activities. Glycyrrhizin inhibits CD4+ T-cell and tumor necrosis factor (TNF) - mediated cytotoxicity.[10] Glycyrrhizin has a membrane stabilizing effect[11] and also stimulates endogenous production of interferon.[12] 18-β glycyrrhetinic acid, an active constituent of glycyrrhizic acid, shows anti-viral activity against a number of DNA and RNA viruses, possibly due to activation of nuclear factor (NF-κB and induction of IL-8 secretion).[13]
Ribavirin is a nucleoside analog (also known as a nucleoside reverse transcriptase inhibitor), broad-spectrum anti-viral drug, which demonstrates anti-viral activity against a wide range of RNA and DNA viruses, including the hepatitis B, C, and retroviruses.[14] The drug's exact mechanism of action is still unclear; however, it is proposed that after phosphorylation into the cell, ribavirin inhibits inosine 5’-monophosphate dehydrogenase (IMPDH).[15] IMPDH inhibitors like ribavirin decrease the intracellular synthesis and storage of “guanine,” a nucleotide base essential for DNA and RNA replication, consequently inhibiting viral replication.[16] The ribavirin pharmacokinetic profile, preclinical toxicity, safety, and clinical efficacy studies are well documented. The studies also show the use of ribavirin to treat respiratory syncytial virus infection in infants and young children and to treat influenza A and B virus infections in young adults.[17] Ribavirin aerosol has been used successfully to treat respiratory syncytial virus and para-influenza virus infection of immunodeficient children.[18] The aim of the current study was to investigate the efficacy of plant extract Glycyrrhiza glabra as an anti-viral agent against the Newcastle disease virus and to compare the efficacy of Ribavirin and Glycyrrhiza for the chemotherapy and prophylaxis of Newcastle disease virus.
MATERIALS AND METHODS
Embryonated eggs of 9th to 10th days were obtained from Hi-Tech Laboratories Pvt. Ltd. and were placed in WTO Laboratory. Live and dead eggs were separated by candling. A total of 90 eggs was collected and incubated at 37°C temperature in an egg incubator (70% humidity). The eggs were divided into six groups (A, B, C, D, E, and F). Groups A, B, C, and D were further divided into three subgroups.
Experimental procedure
Group-A1, A2, and A3 were designed to evaluate the anti-viral activity of Glycyrrhiza. Group-A1 contains 4HA virus with antibiotics (i-e., Penicillin 500000 IU/ml, Gentamycin 100 mg/ml, Streptomycin 500 mg/ml, and Amphotericin B 250 μg/ml) +2X (30 mg/100 ml) aqueous Glycyrrhiza extract. Group-A2 contains 4HA virus with antibiotics + 2X (60 mg/100 ml) aqueous Glycyrrhiza extract. Group-A3 contains 4HA virus with antibiotics + 2X (120 mg/100 ml) aqueous Glycyrrhiza extract. While groups-B-1, B-2, and B-3 were designed to evaluate the toxicity of Glycyrrhiza. Group-B-1 contains normal saline + 2X (30 mg/100 ml) aqueous Glycyrrhiza extract. Group-B2 contains normal saline + 2X (60 mg/100 ml) aqueous Glycyrrhiza extract. Group-B3 contains normal saline + 2X (120 mg/100 ml) aqueous Glycyrrhiza extract.
Groups-C1, C2, and C3 were designed to evaluate the anti-viral activity of Ribavirin.
Group-C-1 contains 4HA virus with antibiotics (i-e., Penicillin 500000 IU/ml, Gentamycin 100 mg/ml, Streptomycin 500 mg/ml, and Amphotericin B 250 μg/ml) +2X (10 μg/ml) aqueous solution of Ribavarin. Group-C2 contains 4HA virus with antibiotics + 2X (20 μg/100 ml) aqueous solution of Ribavarin. Group-C3 contains 4HA virus with antibiotics + 2X (40 μg/100 ml) aqueous solution of Ribavirin. Groups-D1, D2, and D3 were designed to evaluate the toxicity of Ribavarin. Group-D1 contains normal saline + 2X (10 μg/ml) aqueous solution of Ribavarin. Group-D2 contains normal saline + 2X (20 μg/ml) aqueous solution of Ribavirin. Group-D3 contains normal saline + 2X (40 μg/100 ml) aqueous solution of Ribavirin.
Group-E was kept as negative control, in which only normal saline was inoculated.
Group-F was kept as a positive control, in which only virus with normal saline was inoculated. 0.1 ml from each of the groups was inoculated into respective groups of embryonated eggs. The experiment was performed to evaluate the anti-viral effect as well as the toxicity of Glycyrrhiza extract (Root) and Ribavirin.
Glycyrrhiza extraction
Collection of plant material
The plant was obtained from the botanical gardens of Govt. College University, Lahore, Pakistan and ground to form a paste and was dried for overnight in Desiccators (MILLIPORE Desiccators).
Maceration
One reagent bottle of 500 ml capacity was used for maceration. Five-hundred ml of solvent i.e. deionized distilled water was taken with a bottle for Glycyrrhiza.
Five-hundred grams of Glycyrrhiza powder was weighed accurately using a digital balance and added in the bottle. The bottle was placed in a vibrator for 24 hours, and the plant material was allowed to macerate. The extract was filtered using Whatmann's filter paper. The water extract was subjected to Syringe Filtration, using filters of 0.2 μm size in safety cabinets.
Bacterial contamination test
One ml of extract was poured on a nutrient agar plate. The plate was left undisturbed for 2 minutes and after that, the excess of the extract was discarded from the plate. The plate was incubated overnight, and the growth of bacteria was observed on the plate. The aqueous extract of the plant did not show any growth of the bacteria.
Drying of the herbal extract
The filtrate was kept at 40˚C for 90 hours in the incubator for drying. The extract (powder) was concentrated and weighed for Glycyrrhiza. After weighing, different aqueous experimental concentrations of Glycyrrhiza (15 mg/100 ml, 30 mg/100 ml and 60 mg/100 ml) were prepared accordingly to evaluate its toxicity and anti-viral activity.
Ribavirin
Four-hundred mg of ribavirin ("Variba®" of Bosch pharmaceutical Pvt. Limited) was mixed into 200 ml of deionized distilled water. From this aqueous solution, different experimental concentrations of ribavirin were prepared (5 μg/ml, 10 μg/ml, and 20 μg/ml) accordingly to evaluate its toxicity and anti-viral activity.
Source of virus
The Newcastle disease virus was obtained from Department of Microbiology, University of Veterinary and Animal Sciences, Lahore, Pakistan.
The virus was identified by Hemagglutination inhibition test (HI). The test was performed to estimate the 4HA activity of the test virus according to the method described by Allan and Gough, 1974.[19] The viability of the embryo in embryonated eggs i.e. live and dead embryo was evaluated by candling.
The air sacs and the heads of the embryos were demarcated by candling by using led pencils. Inoculum virus and virus plus a drug mixture were prepared by admixing 4HA suspension of ND virus along with the tested antibiotic and anti-fungal drug concentration (Penicillin 500000 IU/ml, Gentamycin 100 mg/ml, Streptomycin 500 mg/ml, and Amphotericin B 250 μg/ml).
The experimental groups, which were designed for the evaluation of toxicity and anti-viral activity of Glycyrrhiza extract and Ribavirin as well as the control groups, were inoculated with respective inoculums by making a pinpoint hole. The holes were sealed by using wax. The eggs were positioned with broadened ends upside and incubated for 72 hours with frequent candling after every 24 hours. The dead embryonated eggs were separated and were kept in the refrigerator at 4˚C to 8˚C till the final evaluation. After 72 hours, the groups designed for the anti-viral activity were checked for the replication of ND virus by means of spot Hemagglutination tests, whereas the groups designed for toxicity were checked for the viability of the embryo by candling and by opening the embryonated eggs.
The spot hemagglutination test was performed by taking one drop of allanto amniotic fluid from each egg and one drop of the chick RBCs suspension on slides. The slide which showed agglutination was considered as positive, while the slide without any agglutination was considered as negative.
RESULTS
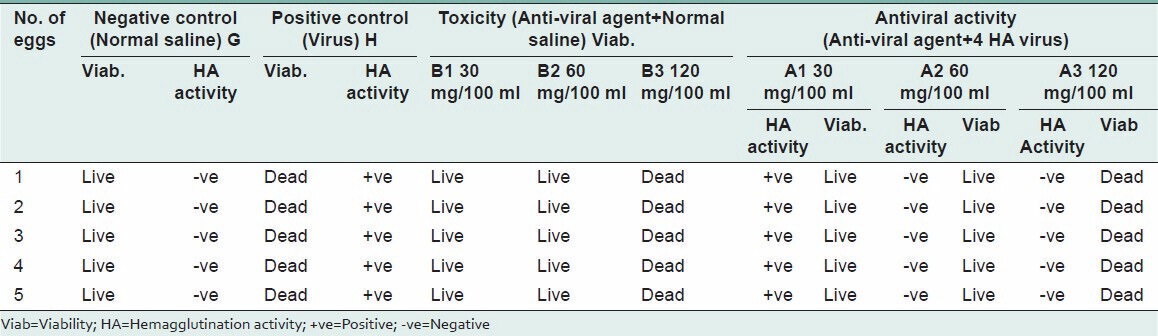
The presence or absence of the virus was confirmed by using the hemagglutination property of the Newcastle disease virus. The Glycyrrhiza aqueous extract concentration 30 mg/100 ml failed to stop the replication of virus, while 60 mg/100 ml and 120 mg/100 ml inoculated embryonated egg's fluid showed absence of HA (Hemagglutination) activity. On the other hand, 30 mg/100 ml and 60 mg/100 ml concentrations did not cause the death of the embryo, whereas embryos in eggs inoculated with 120 mg/100 ml died, which indicate that 30 mg/100 ml and 60 mg/100 ml were non-toxic, whereas 120 mg/100 ml was a toxic drug concentration. Finally, it may conclude that the 60 mg/100 ml aqueous Glycyrrhiza extract is non-toxic and has anti-viral activity as shown in the Table 1.
Table 1.
Toxicity and antiviral effect of Glycyrrhiza glabra extract against Newcastle disease virus
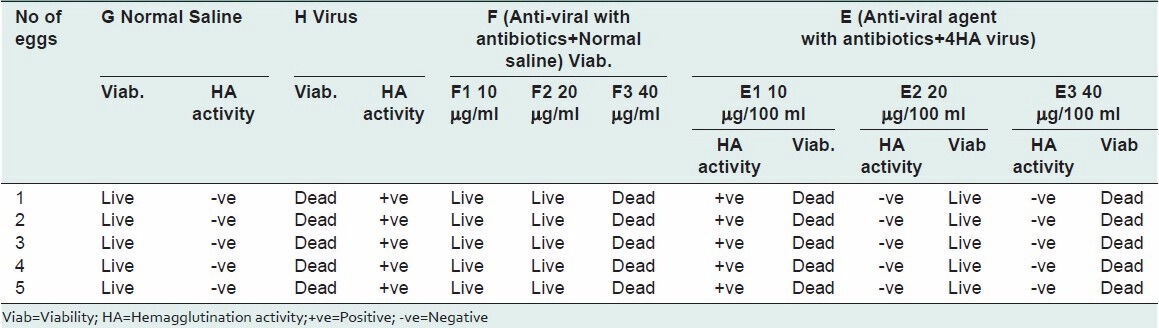
For ribavirin 400 mg, the presence and absence of virus were verified by using hemagglutination property of Newcastle disease virus. The first drug concentration i.e., 10 μg/ml did not stop the replication of the virus, while 20 μg/100 ml and 40 μg/100 ml concentration showed no hemagglutination activity in inoculated embryonated eggs. 10 μg/ml and 20 μg/ml concentrations did not cause the death of the embryo. On the other hand, embryos in eggs inoculated with 40 μg/ml died, which indicate that 20 μg/ml was non-toxic drug concentration while 40 μg/ml concentration was toxic. Finally, it may conclude that the drug concentration 20 μg/ml is non-toxic and has anti-viral activity, as shown in Table 2.
Table 2.
Toxicity and anti-viral effect of Ribavirin against Newcastle disease virus in embryonated eggs
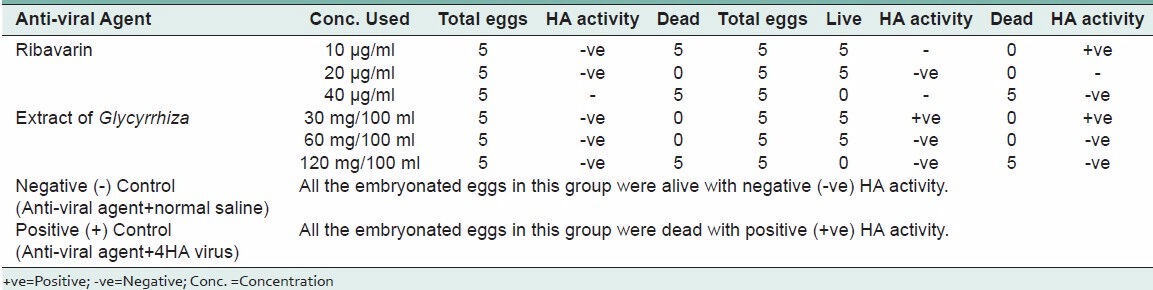
The Table 3 shows the comparison of anti-viral activity of Glycyrrhiza glabra extract and ribavirin at different drug concentrations.
Table 3.
Comparative efficacy of Glycyrrhiza glabra extract and Ribavirin against Newcastle disease virus in embryonated eggs
DISCUSSION
ND virus was propagated in 9 to 10 days old embryonated eggs. The ND virus was identified by serological test known as a hemaagglutination inhibition test. In this test, the HA receptors on the ND virus were neutralized by specific antibodies, due to which ND virus failed to cause the hemagglutination of chicken RBCS . This was in an agreement with the findings of Brugh et al.[20] and Rizwana et al.[21] who described that ND virus can be confirmed by hemagglutination inhibition activity along with the means death time (MDT), interacerebral pathogenicity index (ICPI), and an intravenous pathogenicity index. The virus caused some lesions on the embryo, when propagated in the chicken embryonated eggs.
The anti-viral activity of Glycyrrhiza glabra extract is due to the presence of anti-viral agent known as glycyrrhizic acid, which is water-soluble. The glycyrrhizic acid acts on different levels of the ND viral infection including adsorption, penetration, transcription, translation, assembly and releasing from the host cell. These findings are in line to the Pompei et al.,[22] Naksahima,[23] Baba and Shageta,[24] Badam,[25] Utsunomiya,[26] Harada,[27] Cristina et al.,[9] and Shiuan et al.[28] In these studies, the researchers indicated that glycyrrhizic acid inhibits the virus infection by the reduction of membrane fluidity, by the production of Gama interferon, inhibition of phosphorylating enzymes and reduction in viral latency. Furthermore, Crance et al.[29] described in 2003 that glycyrrhizic acid is comparable to the ribavirin because this is not only less toxic than ribavirin but also as a potent anti-viral as ribavirin when he studied it in tissue cultures. The results of the presented study are in line with these arguments as it was found out that only higher doses can be proved toxic when used as an anti-viral drug [Table 1].
The toxicity and anti-viral screening of Glycyrrhiza extract were comparable with ribavirin, which is a standard, recognized, and FDA-approved anti-viral agent [Table 3]. Ribavirin has been recently used for the treatment of hepatitis B, C, and Herpes Megalo viral infections. Ribavirin stops particularly the viral replication by inhibiting the mechanism of duplication of viral nucleic acid as after the penetration in a host cell, the virus gets the control of cellular metabolism and uses the cellular machinery for its own replication and virus assembly. These facts are supported by the findings of Fenton and Potter,[30] John,[31] Connor et al.,[32] Hultgren et al.,[33] Lucia et al.,[34] Robert et al.,[35] Fernandez et al.,[18] Marina et al.,[36] Ramos et al.,[37] and Wang et al.[38] Herbal extracts contain a large number of therapeutically active compounds, and these phytochemicals are a potential source of molecular drug design and development against a large number of infectious viral diseases. It may conclude here that the aqueous extract of Glycyrrhiza contains the anti-viral activity due to glycyrrhizic acid; however, further studies are needed to determine the exact role of the potentially active ingredient in the viral inhibition in Newcastle disease. Similarly, complementary studies about the role of the active ingredients of Glycyrrhiza extract and its mechanism of action will also be helpful to postulate the exact role of each active component in viral inhibition and pathogenesis of the disease in the near future. Equivocally, glycyrrhizic acid and ribavirin use in combination to inhibit viral replication may be studied in the future for synergistic therapeutic effects. Further studies are required to know the histological changes in the viral infected host cells with or without aqueous Glycyrrhiza extract.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Doyle TM. A hitherto unrecorded disease of fowls due to a filter passing Virus. J Comp Pathol Ther. 1927;40:14–169. [Google Scholar]
- 2.Mayo MA. A summary of the changes recently approved by ICTV. Arch Virol. 2002;147:1655–56. doi: 10.1007/s007050200039. [DOI] [PubMed] [Google Scholar]
- 3.Aldous EW, Alexander DJ. Detection and differentiation of New castle disease virus (Avian Paramyxovirus type 1) Avian Pathol. 2001;30:117–28. doi: 10.1080/03079450120044515. [DOI] [PubMed] [Google Scholar]
- 4.De Leeuw OS, Peeters BP. Complete nucleotide sequence of Newcastle disease virus: Evidence for the existence of a new genus within the subfamily Paramyxovirinae. J Gen Virol. 1999;80:131–6. doi: 10.1099/0022-1317-80-1-131. [DOI] [PubMed] [Google Scholar]
- 5.Wilde A, McQuain C, Morrison TG. Identification of the sequence content of four polycistronic transcripts synthesized in Newcastle disease virus infected cells. Virus Res. 1986;5:77–95. doi: 10.1016/0168-1702(86)90067-5. [DOI] [PubMed] [Google Scholar]
- 6.Peeters BP, De Leeuw OS, Koch G, Gielkens AL. Rescue of Newcastle disease virus from cloned cDNA: Evidence that cleavability of the fusion protein is a major determinant for virulence. J Virol. 1999;73:5001–9. doi: 10.1128/jvi.73.6.5001-5009.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoareau L, DaSilva EJ. Medicinal plants: A re-emerging health aid. Electr J Biotechnol. 1999. [Last cited on 1999 Jan 2]. Available from: http://www.ejb.org/content/vol2/issue2/full2 .
- 8.Eisenberg DM, Kessler RC, Foster C, Norlock FE, Calkins DR, Delbanco TL. Unconventional medicine in the United States. Prevalence, costs, and patterns of use. N Engl J Med. 1993;328:246–52. doi: 10.1056/NEJM199301283280406. [DOI] [PubMed] [Google Scholar]
- 9.Cristina F, Eisenhut M, Krausse R, Ragazzi E, Pellati D, Armanini D, et al. Antiviral effects of Glycyrrhiza species. Phytother Res. 2007;22:141–8. doi: 10.1002/ptr.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshikawa M, Matsui Y, Kawamoto H, Umemoto N, Oku K, Koizumi M, et al. Effects of glycyrrhizin on immune-mediated cytotoxicity. J Gastroenterol Hepatol. 1997;12:243–8. doi: 10.1111/j.1440-1746.1997.tb00416.x. [DOI] [PubMed] [Google Scholar]
- 11.Shiki Y, Shirai K, Saito Y, Yoshida S, Mori Y, Wakashin M. The effect of glycyrrhizin on lysis of hepatocyte membranes induced by anti-liver cell membrane antibody. J Gastroenterol Hepatol. 1992;7:12–6. doi: 10.1111/j.1440-1746.1992.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 12.Abe Y, Ueda T, Kato T, Kohli Y. Effectiveness of interferon, glycyrrhizin combination therapy in patients with chronic hepatitis C. Nippon Rinsho. 1994;52:1817–22. [PubMed] [Google Scholar]
- 13.Shaneyfelt ME, Burke AD, Graff JW, Jutila MA, Hardy ME. Natural products that reduce rotavirus infectivity identified by a cell-based moderate-throughput screening assay. Virol J. 2006;3:68–75. doi: 10.1186/1743-422X-3-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy KR, Shiffman ML, Morgan TR, Zeuzem S, Hadziyannis S, Hamzeh FM, et al. Impact of ribavirin dose reductions in hepatitis C virus genotype 1 patients completing peginterferon Alfa-2a/ribavirin treatment. Clin Gastroenterol Hepatol. 2007;5:124–9. doi: 10.1016/j.cgh.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Afdhal NH, Dieterich DT, Pockros PJ, Schiff ER, Shiffman ML, Sulkowski MS, et al. Epoetin Alfa maintains ribavirin dose in HCV infected patients: A prospective, double-blind, randomized controlled study. Gastroenterology. 2004;126:1302–11. doi: 10.1053/j.gastro.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 16.Shiffman ML, Salvatore J, Hubbard S, Price A, Sterling RK, Stravitz RT, et al. Treatment of chronic hepatitis C virus genotype 1 with peginterferon, ribavirin, and epoetin alpha. Hepatology. 2007;46:371–9. doi: 10.1002/hep.21712. [DOI] [PubMed] [Google Scholar]
- 17.Marcellin P, Gish RG, Gitlin N, Heise J, Halliman DG, Chun E, et al. Safety and efficacy of viramidine versus ribavirin in viser2: Randomized, double-blind study in therapy-naive hepatitis C patients. J Hepatol. 2010;52:32–8. doi: 10.1016/j.jhep.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Fernandez H, Banks G, Smith R. Ribavirin: A clinical overview. Eur J Epidemiol. 2004;2:1–14. doi: 10.1007/BF00152711. [DOI] [PubMed] [Google Scholar]
- 19.Allan WH, Gough RE. A standard Haemagglutination inhibition test for New castle disease. A comparison of macro and micro methods. Vet Rec. 1974;95:120–3. doi: 10.1136/vr.95.6.120. [DOI] [PubMed] [Google Scholar]
- 20.Brugh M, Erickson GA, Beard CW. Embryonated eggs compared with fragments of chorioallantois attached to egg shell for isolation of Newcastle disease virus. Avian Dis. 1980;24:486–92. [PubMed] [Google Scholar]
- 21.Rizwana A, Farzana R, Anjum AD, Abeera M. Pathogenicity of field isolates of the New castle disease virus. Pakistan J Biol Sci. 2000;3:1083–5. [Google Scholar]
- 22.Pompei R, Paghi L, Ingianni, Uccheddu P. Glycyrrhizic acid inhibits influenza virus growth in embryonated eggs. Microbiologica. 1983;6:247–50. [PubMed] [Google Scholar]
- 23.Nakashima H, Baba M, Pauwels R, De Clercq E, Shigeta S, Yamamoto N. Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus. Antiviral Res. 1987;7:127–37. doi: 10.1016/0166-3542(87)90001-5. [DOI] [PubMed] [Google Scholar]
- 24.Baba M, Shigeta S. Antiviral activity of Glycyrrhizin against Varicella Zoster virus in vitro. Antiviral Res. 1987;7:99–107. doi: 10.1016/0166-3542(87)90025-8. [DOI] [PubMed] [Google Scholar]
- 25.Badam L. In vitro antiviral activity of indigenous glycyrrhizin, licorice and glycyrrhizic acid (Sigma) on Japanese encephalitis virus. J Commun Dis. 1997;29:91–9. [PubMed] [Google Scholar]
- 26.Utsunomiya T, Kobayashi M, Pollard RB, Suzuki F. Glycyrrhizin, an Active component of licorice roots, reduces morbidity and mortality of mice infected with lethal doses of influenza virus. Antimicrob Agents Chemother. 1997;41:551–6. doi: 10.1128/aac.41.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harada S. The broad anti-viral agent glycyrrhizin directly modulates the fluidity of plasma membrane and HIV-1 envelope. Biochem J. 2005;15(392 (PT 1)):191–9. doi: 10.1042/BJ20051069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiuan PL, Shang YT, Yu CH, Pei DL. Glycyrrhizin and Licorice Significantly Affect the Pharmacokinetics of Methotrexate in Rats. J Agric Food Chem. 2009;57:1854–59. doi: 10.1021/jf8029918. [DOI] [PubMed] [Google Scholar]
- 29.Crance JM, Scaramozzino N, Jouan A, Garin D. Interferon, ribavirin, 6-azauridine and glycyrrhizin: Antiviral compounds active against pathogenic flaviviruses. Antiviral Res. 2003;58:73–9. doi: 10.1016/s0166-3542(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 30.Fenton RJ, Potter CW. Dose-response activity of ribavirin against influenza virus infection in ferrets. J Antimicrob Chemother. 1977;3:263–71. doi: 10.1093/jac/3.3.263. [DOI] [PubMed] [Google Scholar]
- 31.John WH. Prospects for Treatment of Viral Hemorrhagic Fevers with Ribavirin, a Broad-Spectrum Antiviral Drug. Rev Infect Dis. 1989;11:750–61. doi: 10.1093/clinids/11.supplement_4.s750. [DOI] [PubMed] [Google Scholar]
- 32.Connor E, Morrison S, Lane J, Oleske J, Sonke RL, Connor J. Safety, tolerance, and pharmacokinetics of systemic ribavirin in children with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1993;37:532–9. doi: 10.1128/aac.37.3.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hultgren C, Milich DR, Weiland O, Sallberg M. The antiviral compound ribavirin modulates the T helper (TH) 1/TH2 subset balance in hepatitis B and C virus-specific immune responses. J Gen Virol. 1998;79:2381–91. doi: 10.1099/0022-1317-79-10-2381. [DOI] [PubMed] [Google Scholar]
- 34.Lucia DF, Giovanna F, Franco T, Kodjo A, Carlo B, Franco M, et al. Hemolytic anemia induced by ribavirin therapy in patients with chronic hepatitis C virus infection: Role of membrane oxidative damage. Hepatology. 2003;31:997–10004. doi: 10.1053/he.2000.5789. [DOI] [PubMed] [Google Scholar]
- 35.Robert E, Bernadette G, Helen L, Devron R, Brad P, Deborah C, et al. Antiviral effect and virus-host interactions in response to alpha interferon, gamma interferon, Poly (I) -Poly (C), tumor necrosis factor alpha, and ribavirin in Hepatitis C Virus sub genomic replicons. J Virol. 2003;77:1092–104. doi: 10.1128/JVI.77.2.1092-1104.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marina N, Celia M, Miguel AB, Elena L, Koldo A, Antonio O, et al. Role of weight-based ribavirin dosing and extended duration of therapy in chronic Hepatitis C in HIV-Infected patients: The PRESCO Trial. AIDS Res Hum Retroviruses. 2007;23:972–82. doi: 10.1089/aid.2007.0011. [DOI] [PubMed] [Google Scholar]
- 37.Ramos B, Nunez M, Rendon A, Berdun MA, Losada E, Santos I, et al. Critical role of ribavirin for the achievement of early virological response to HCV therapy in HCV/HIV-coinfected patients. J Viral Hepat. 2007;14:387–91. doi: 10.1111/j.1365-2893.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Zhou J, Yang QW, Chen Y, Li X, Pioa YA, et al. An improved embryonated chicken egg model for the evaluation of antiviral drugs against influenza A virus. J Virol Methods. 2008;153:218–22. doi: 10.1016/j.jviromet.2008.06.022. [DOI] [PubMed] [Google Scholar]


