Abstract
Background:
Fruits, leaves, and tuberous roots of Momordica dioica are used as a folk remedy for diabetes mellitus (DM) in India. The aqueous extract of Momordica dioica fruit possesses very good anti-diabetic activity and is having high margin of safety.
Objectives:
The aim of the present study was to investigate the antioxidative effect of Momordica dioica fruits in alloxan-induced diabetic Wistar rats.
Materials and Methods:
Effect of aqueous extract of Momordica dioica (AEMD) on thiobarbituric acid reactive substances (TBARS), hydroperoxide (HP), non-enzymatic and enzymatic antioxidants in liver, kidney, pancreas, and serum was evaluated in diabetic rats after 21 days treatment.
Results:
Increase in the levels of TBARS, HP and decrease in the levels of non-enzymatic antioxidants and activity of enzymatic antioxidants was observed in liver, kidney, pancreas, and serum of diabetic rats when compared with normal healthy rats. TBARS and HP levels were reduced while non-enzymatic and enzymatic antioxidant enzymes activity was increased in AEMD and glibenclamide-treated rats. Furthermore, histological examination of liver, kidney, and pancreas of diabetic rats showed degenerative changes. AEMD treatment for 21 days rejuvenated liver, kidney, and pancreas histoarchitecture.
Conclusion:
In conclusion, the present results showed the protective role of AEMD on liver, kidney, and pancreas in severe diabetic rats justifying support for its anti-diabetic use in folk medicine.
Keywords: Alloxan, momordica dioica, oxidative stress, Wistar rat
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disorder characterized mainly by chronic hyperglycemia resulting from defects in insulin secretion and/or its action. Chronic hyperglycemia leads to improper regulation of carbohydrate, protein, and lipid metabolism and contributes to the progression of micro and macrovascular complications.[1] Oxidative stress has been suggested as mechanism underlying DM and diabetic complications.[2]
Free radicals are continuously produced in the body as a result of normal metabolic processes and interaction with environmental stimuli. Oxidative stress results from an imbalance between radical-generating and radical-scavenging systems that has increased free radical production or reduced activity of antioxidant defenses or both. Role of oxidative stress in the pathogenesis of DM is suggested not only by oxygen free-radical generation but also due to non-enzymatic protein glycosylation, auto-oxidation of glucose, impaired glutathione metabolism, alteration in antioxidant enzymes, and formation of lipid peroxides.[3]
It is well-known that superoxide anion is the primary radical formed by the reduction of molecular oxygen that may lead to secondary radicals or reactive oxygen species (ROS) such as hydrogen peroxide and hydroxyl radical. ROS are part of the defense mechanism against infection, but excessive generation of free oxygen radicals may damage tissues (Ahmed, 2005).[3] Co-operative defense systems that protect the body from free radical damage include enzymatic and non-enzymatic antioxidants.[4] Enzymatic antioxidants namely glutathione-s-transferase (GST), superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) and non-enzymatic antioxidants such as vitamins C and E, reduced glutathione (GSH), and ceruloplasmin (CP) play an important role in preventing tissue damage due to the formation of free radicals.[2] High levels of glucose or glycated proteins enhances lipid peroxidation.[5] The efficiency of antioxidant defense system altered in DM and, therefore, the ineffective scavenging of free radicals may play a crucial role in pathophysiology of DM.
A number of synthetic chemical agents have been developed for management of DM. The challenge is to optimize the suitable anti-diabetic drug for effective glycemic control, cost of the therapy, adverse effect profiles, ease of administration, and the urgency for blood sugar normalization. Long term uses of present day anti-diabetic agents is often costly and have adverse health effects, hence always there is need for safer and cost-effective drugs. Several medicinal plants have been used in management of DM. Safety and low cost of treatment are the merits of the plant-based anti-diabetic agents. Plants often contain substantial amounts of antioxidants, and are likely to slow down the progress of pancreatic damage and progression of DM.[6]
Momordica dioica Roxb. EX. Wild is a wild plant of the genus momordica. Fruits, leaves, and tuberous roots of M. dioica are used as a folk remedy for DM in India.[7] M. dioica exhibited anti-hyperglycemic[8] and renoprotective[9] activity. Recently, our group has reported anti-diabetic activity of aqueous extract of M. dioica (AEMD) fruits.[10] We observed increase in serum insulin level in diabetic rats treated with AEMD. The findings indicated that anti-diabetic effect of AEMD may be partly due to increase in insulin level. Increase in insulin level may be either due to insulin secretagogues activity of AEMD or due to pancreatic β-cell protecting activity or both. The protection of pancreatic β-cell by AEMD may be due to its antioxidant activity. Hence, the present study was planned to evaluate antioxidative effect of AEMD in alloxan-induced diabetic Wistar rats. The selected model is widely used in animal research for studying the anti-diabetic or antioxidant effect of purified drugs or plant extracts.
MATERIALS AND METHODS
Materials
Fresh fruits of the plant were collected in the rainy season from the hills of Bundelkhand University, Jhansi. The plant was identified with the help of experts in Department of Botany, Bundelkhand University, and the voucher specimen no. BU/BMS/VS/2010/03 was preserved in Department of Biomedical Sciences. The plant material was shade-dried and ground to coarse powder. The powder was soaked in distilled water for 12 hours at 25° C, with continuous stirring. The extract was filtered, centrifuged (10,000 rpm, 10 min, at room temperature) to remove any residual or fibrous material, lyophilized, and named as AEMD (aqueous extract of Momordica dioica).
Chemicals and reagents
Alloxan and kits for the estimation of SOD, GSH, GST, CAT, GPx, and GR were purchased from Sigma Aldrich, USA. Kits for lipid peroxidation and hydroperoxides were purchased from Cayman, USA, and one-touch glucometer (Optium) from Roche Diagnostics. Other chemicals and reagents used in the study were of high purity.
Animals
Male Wistar rats weighing about 150-200 g, obtained from Central Drug Research Institute (CSIR), Lucknow, were reared in animal house of Bundelkhand University. Animals were kept for acclimatization at ambient temperature of 25° C and 45-55% relative humidity, with 12 h each of dark and light cycles and were fed pelleted diet and water ad-libitum. Principles of Laboratory Animal Care (NIH publication no, 85-23, revised 1985) were followed for animal care during the course of experimentation.
Induction of diabetes
Diabetes was induced in rats using alloxan. Alloxan solution was freshly prepared in neutral saline and kept in ice bath and used within half an hour of its preparation. Diabetes was induced by single intraperitoneal injection of alloxan at the dose of 160 mg/kg bw in overnight-fasted rats. Rats with stabilized diabetes having FBG more than equal to 250 mg/dl were used in the experiment.
Experimental design
The animals were divided into four groups of six rats each. Group I was taken as normal control, group II diabetic control, group III AEMD-treated, and group IV glibenclamide-treated. Dosage of AEMD (200 mg/kg bw) and glibenclamide (5 mg/kg bw) were according to Singh et al.[11] AEMD and glibenclamide were dissolved in water and given to rats once daily for 21 days by gavaging. The animal experiments were according to Institutional Animal Ethical Committee guidelines (BU/Pharm/IAEC/08/40).
Tissues and serum biochemical estimation
Rats were sacrificed under ether anesthesia, blood was taken from the ventricle of the heart, and serum was separated. Liver, kidney, and pancreas were removed, cut into small pieces, and homogenized with a teflon homogenizer in 0.025 M Tris-HCl buffer (pH 7.4). The homogenate was centrifuged at 2000 rpm for 30 min, and the supernatant was used to estimate the levels of TBARS, HP, non-enzymatic and enzymatic antioxidants in tissues and serum.
Estimation of lipid peroxidation
Lipid peroxidation levels in plasma and tissues were measured by Cayman's TBARS assay kit.
Estimation of lipid peroxidation
Reduced glutathione, SOD, CAT, GST, GPx, and GR levels in the plasma and tissues were measured by using Kit from Sigma.
Histological study
For histological examinations, small pieces of liver, kidney, and pancreas were fixed in Bouin's solution for 24 h, dehydrated through graded concentration of ethanol, embedded in paraffin wax, sectioned at 5 μm thicknesses and stained with Mayer's hematoxylin and eosin (HandE) and observed under light microscope.
Statistical analysis
Results were expressed as Mean ± SEM. Data was subjected to one-way analysis of variance (ANOVA). The treatment groups were compared with control group using Dunnett's test. All statistics were carried out in graph pad instat software inc., v. 3.06, Sandigeo, USA.
RESULTS
Effect of AEMD on TBARS and HP in diabetic rats
The diabetic rats showed significant increase in the concentration of TBARS (liver 140, kidney 100, pancreas 894, serum 134%, Figure 1) and HP (liver 30.6, kidney 41, pancreas 91.8, serum 71.3%, Figure 2) as compared to normal rats. Administration of AEMD to diabetic rats significantly (P < 0.0001) decreased the levels of TBARS (liver 57, kidney 50, pancreas 83.5, serum 45.5%, Figure 1) and HP (liver 24.5, kidney 23.1, pancreas 41.8, serum 45.2%, Figure 2). Glibenclamide-treated animals also showed significant (P < 0.0001) decrease in TBARS (liver 48, kidney 42.2, pancreas 76.1, serum 41.6%, Figure 1) and HP (liver 18.5, kidney 20.6, pancreas 32.9, and serum 31.8%, Figure 2) as compared to diabetic rats.
Figure 1.
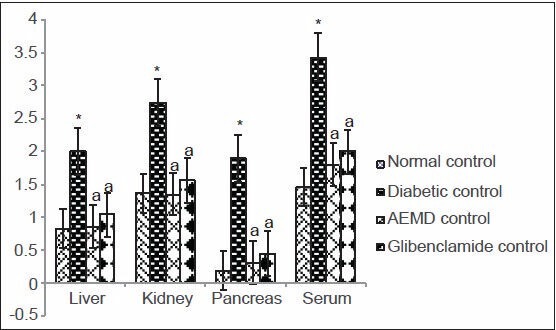
Effect of AEMD and glibenclamide on the levels of TBARS. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Figure 2.
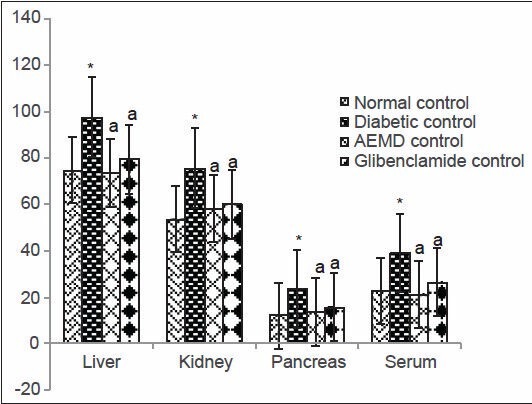
Effect of AEMD and glibenclamide on levels of HP. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Effect of AEMD on non-enzymatic antioxidant in diabetic rats
The diabetic rats showed significant decrease in GSH in tissues (liver 42.3, kidney 25%, and pancreas 47.5%) and serum (45.7%). Administration of AEMD and glibenclamide significantly increased (P < 0.0001) GSH level in liver (90.1% and 75.6%), kidney (40.3% and 23.5%), pancreas (90% and 76.6% and serum (87.3% and 69.4%), respectively, as compared to diabetic control [Figure 3].
Figure 3.
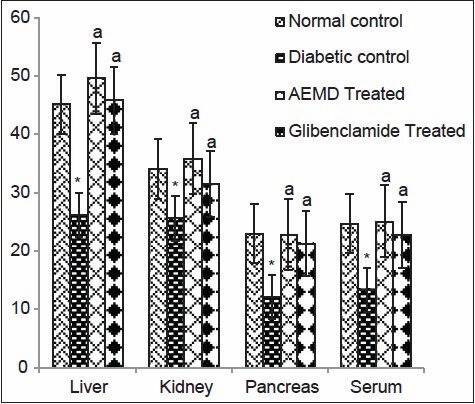
Effect of AEMD and glibenclamide on levels of GSH. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Effect of AEMD on enzymatic antioxidants in diabetic rats
The diabetic rats showed significant P < 0.0001 decrease in SOD (liver 53.1, kidney 46.8, pancreas 59.5, serum 60.6%, Figure 4), CAT (liver 40.7, kidney 40.5, pancreas 59.2, serum 38.3%, Figure 5), GST (liver 44.5, kidney 44.6, pancreas 24.7, serum 54.2%, Figure 6), GPx (liver 52.9, kidney 43.4, pancreas 39.1, serum 41.3%, Figure 7), GR (liver 43.7, kidney 27.4, pancreas 22.1, serum 33.7%, Figure 8) as compared to control. Administration of AEMD to diabetic rats significantly (P < 0.0001) increased the activity of SOD (liver 113.4, kidney 92.9, pancreas 141.1, serum 192.3%, Figure 4) CAT (liver 70.8, kidney 60.5, pancreas 135.6, serum 61.9%, Figure 5), GST (liver 68.2, kidney 75, pancreas 24.2, serum 117.5%, Figure 6), GPx (liver 105.1, kidney 70, pancreas 68.8, serum 83%, Figure 7) and GR (liver 82.7, kidney 35.8, pancreas 16.5, serum 47.7%, Figure 8). Glibenclamide-treated rats showed a significant (P < 0.0001) increase in SOD (80.7, 47.8, 117.6, 123%, Figure 4), CAT (62.8, 50.7, 110.6,46.9%, Figure 5), GST (29.2, 66.6, 18, 85%, Figure 6), GPx (87.1, 62.5, 55.5, 23.7%, Figure 7), GR (55.4, 21.1, 16.5, 22.5%, Figure 8) activity in liver, kidney, pancreas, and serum, respectively.
Figure 4.
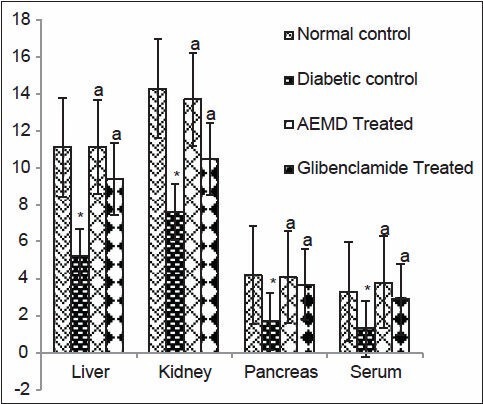
Effect of AEMD and glibenclamide on levels of SOD. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Figure 5.
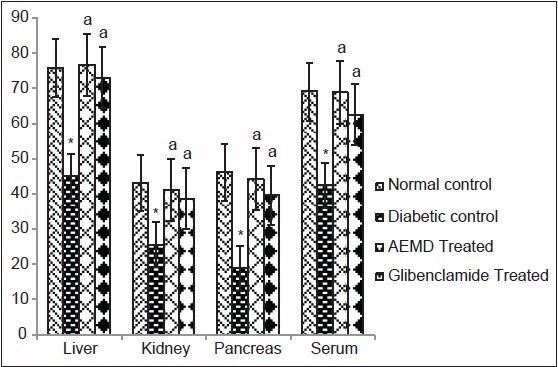
Effect of AEMD and glibenclamide on levels of CAT. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Figure 6.
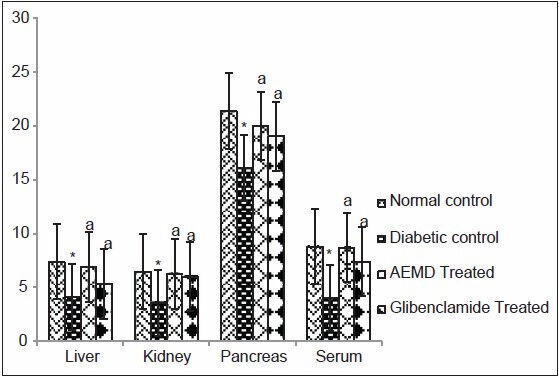
Effect of AEMD and glibenclamide on levels of GST. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P P < 0.0001) compared with diabetic control
Figure 7.
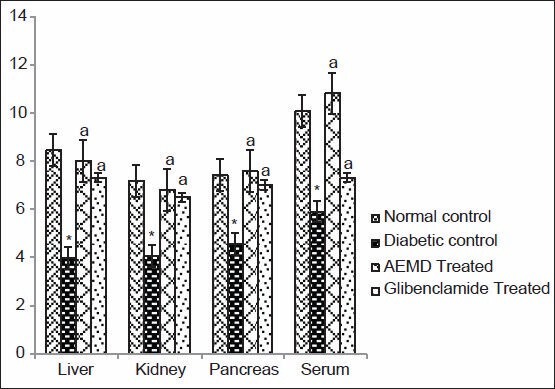
Effect of AEMD and glibenclamide on levels of GPx. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Figure 8.
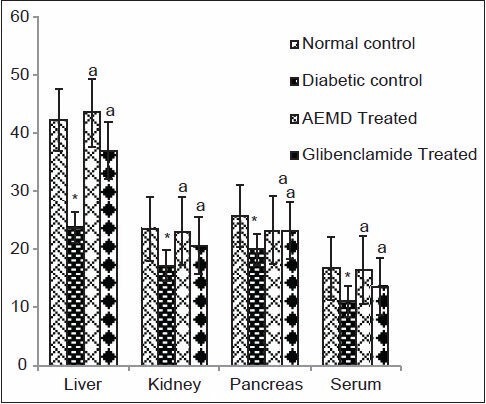
Effect of AEMD and glibenclamide on levels of GR. Each bar expressed as Mean ± SEM for 6 rats. *Indicates significant difference (P < 0.0001) compared with normal control. A indicates significant difference (P < 0.0001) compared with diabetic control
Histological examinations
Liver
Microscopic examination of liver of control group rats [Figure 9a] showed normal histology with sinusoidal cards of hepatocytes with central vein and portal tracts. The portal tracts showed portal triad with portal vein, hepatic artery, and bile duct. The liver of diabetic rats [Figure 9b] showed distortion in the arrangement of cells around the central vein, enlargement and thickening of the walls of veins and capillaries, development of fibrosis with necrosis of hepatocytes, slight congestion of the liver, and periportal fatty infiltration. The liver of AEMD-treated diabetic rats [Figure 9c] showed well-rejuvenated normal cellular arrangement around the central vein and reduced necrosis and normal blood vessels. The liver of glibenclamide- [Figure 9d] treated diabetic rats showed normal cellular arrangement around the central vein and reduced necrosis with mild to moderate intrahepatic hemorrhage and few erythrocytes in central vein.
Figure 9.
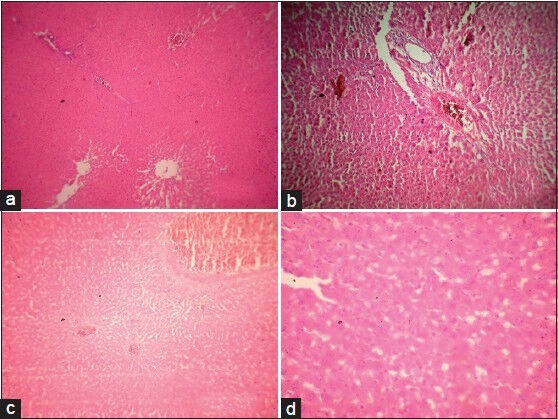
(a) Microphotograph of liver from normal rat (group 1), (b) Microphotograph of liver from diabetic rat (alloxan) (group 2), (c) Microphotograph of liver from diabetic rats treated with AEMD (group 3), (d) Microphotograph of liver from diabetic rats treated with glibenclamide (group 4)
Kidney
The kidney of control rats [Figure 10a] showed normal histology with well-developed proximal and distal convoluted tubules and normal glomeruli and Bowman's capsule. The kidney of diabetic rats showed [Figure 10b] shrunken glomerulus, thickening of vesicles, glomerulus show some cellular proliferation with fibrosis and some congestion, marked tubular damage, hemorrhage in the Bowman's space. The kidney of AEMD- [Figure 10c] treated diabetic rats showed well-rejuvenated proximal and distal convoluted tubules with glomerulus, Bowman's capsule, and renal tubules. Also, tubules with no congestion and hemorrhage, no tubular damage, and no fibrosis were observed. The kidney of glibenclamide-treated diabetic rats [Figure 10d] showed glomeruli, which appeared normal with mild dilated tubules and degenerative changes of tubular epithelium with moderate hemorrhages in glomeruli.
Figure 10.
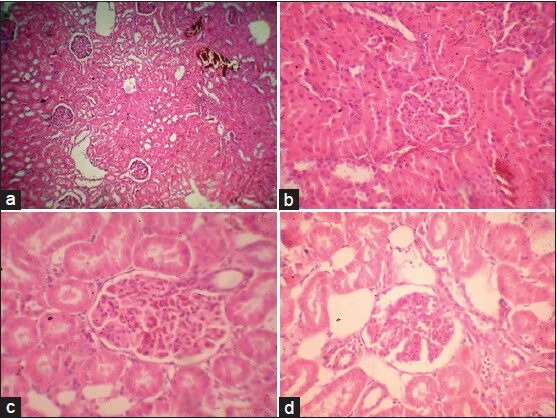
(a) Microphotograph of kidney from normal rat (group 1), (b) Microphotograph of kidney from diabetic rat (alloxan) (group 2), (c) Microphotograph of kidney from diabetic rats treated with AEMD (group 3), (d) Microphotograph of kidney from diabetic rats treated with glibenclamide (group 4)
Pancreas
The pancreas of control rats showed normal histoarchitecture with well-developed globules of acini with normal islet cells, no fibrosis or inflammation [Figure 11a]. The pancreas of diabetic rats showed atrophy of islet cells with inflammatory edema, necrosis, fibrotic changes, and shrinkage of islet cells [Figure 11b] The pancreas of AEMD-treated diabetic rats showed well-regenerated pancreatic cells with normal islet cells and prominent nucleus and nucleolus, no atrophy or necrosis, and fibrotic changes [Figure 11c]. The pancreas of glibenclamide- [Figure 11d] treated diabetic rats showed minimal necrosis and mild to moderate atrophy with mild fibrotic changes.
Figure 11.
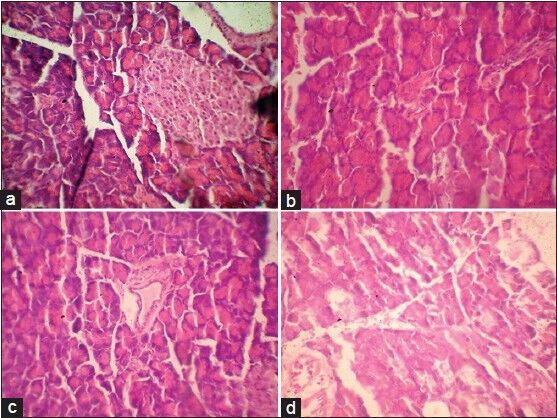
(a) Microphotograph of pancreas from normal rat (group 1), (b) Microphotograph of pancreas from diabetic rat (alloxan) (group 2), (c) Microphotograph from diabetic rats treated with AEMD (group 3), (d) Microphotograph from diabetic rats treated with glibenclamide (group 4)
DISCUSSION
The present study assessed the antioxidant effect of M. dioica in liver, kidney, pancreas, and serum of alloxan-induced diabetic Wistar rats. Alloxan, a β-cytotoxin, induces DM in a wide variety of animal species through damage of insulin-secreting cells.[11] Alloxan not only destroys the pancreatic β-cells but also damages the kidney[12] and generates superoxides, hydrogen peroxides hydroxyl radicals. Alloxan administration elevated the renal function markers like creatinine and urea in the previous study.[10] Oral administration of AEMD for 21 days resulted in improving the antioxidant levels in diabetic rats.
Increase in concentrations of lipid peroxides and hydroperoxides in tissues and serum of diabetic rats indicates an increase in free radicals generation.[13] Increased lipid peroxidation impairs membrane functions by decreasing membrane fluidity and changing the activity of membrane-bound enzymes and receptors. Its products (lipid radical and lipid peroxide) are harmful to the cells in the body and are associated with atherosclerosis and brain damage.[14] The results of this study showed decrease in lipid peroxidation and hydroperoxides on treatment of diabetic rats with AEMD. This suggests the antioxidative effect of AEMD, which act as strong superoxide radicals and singlet oxygen quenchers. Antioxidant activity of anti-diabetic extracts of Phyllantus niruri[15] and Coriandrum sativum[16] has been reported earlier.
Decline in GSH content in the tissues and serum of diabetic rats and its normalization in AEMD- and glibenclamide-treated animals also revealed the anti-lipid peroxidative effect of M. dioica. The decrease in GSH in diabetic rats may probably be due to its increased utilization by the hepatocytes in an attempt to counteract the increased formation of lipid peroxides on alloxan administration. GSH has a multifaceted role in antioxidant defense as a direct scavenger of free radicals and co-substrate for peroxide detoxification by glutathione peroxidase.[17] The findings of this study indicated that AEMD can either increase the biosynthesis of GSH or reduce the oxidative stress and slow GSH degradation, or have both effects.
The diabetic animals showed decrease in activity of SOD, CAT, GST, GPx, and GR. Decreased antioxidant enzyme activity resulted in over accumulation of O2- and H2 O2, which further generated OH- . SOD, CAT, GPx, and GR are enzymes that destroy the peroxides and play a significant role in providing antioxidant defense. SOD, CAT, and GPx are involved in the elimination of H2 O2 . SOD converts superoxide radical to H2 O2, which is then acted upon by CAT and GPx. The functions of all three enzymes are interconnected, and a lowering of their activities resulted in the accumulation of lipid peroxides and increased oxidative stress in diabetic rats. Administration of AEMD increased the activity of these enzymes and thus facilitated fast metabolism and inactivation of free radicals generated during DM.
GST play a major cellular antioxidant role. GST also have peroxidase and isomerase activities. It binds covalently with activated metabolites of xenobiotics and non-covalently with lipophilic compounds, thereby offering protection against oxidative stress.[18] GST plays an essential role in liver by eliminating toxic compounds by conjugating them with GSH. We found significant elevation in the activity of this enzyme in tissues and serum of AEMD-treated rats suggesting the hepatoprotective and antioxidant role of M. dioica.
Protective effect of AEMD was also evident from the histological evaluation of liver, kidney, and pancreas of the treated rats, whereas diabetic rats showed extensive degeneration and multiple necrotic changes.
Liver, kidneys, and pancreas histology of diabetic rats showed marked structural alterations, possibly due to hyperglycemia-induced oxidative stress. All structural changes in kidney resulting from alloxan administration in rats can thus be attributed to altered metabolism in DM. In a previous study, normalization of liver, kidney, and pancreas histopathology has been observed with the treatment of alloxan-induced diabetic rats with Trigonella foenum graecum seed powder by Thakran et al.[19]
CONCLUSION
The present study demonstrated that aqueous extract of Momordica dioica possess potent antioxidant activity. Protection and regeneration of pancreatic β-cell function and enhancement in insulin secretion may be one of the reasons for its anti-diabetic activity of M. dioica fruits. The study validates the claim of use of this plant in management of DM as folklore medicine.
ACKNOWLEDGEMENT
The authors are thankful to Council of Scientific and Industrial Research (CSIR) Govt. of India for financial assistance 38(1221)/09/EMR-II for this research work.
Footnotes
Source of Support: Council of Scientific and Industrial Research (CSIR) Govt. of India vide Ref No (38(1221)/09/EMR-II)
Conflict of Interest: None declared.
REFERENCES
- 1.Adisakwattana S, Roengsamran S, Hsu WH, Yibchoknnun S. Mechanisms of antihyperglycemic effect of p-methoxycinnamic acid in normal and Streptozotozin-induced diabetic rats. Life Sci. 2005;78:406–12. doi: 10.1016/j.lfs.2005.04.073. [DOI] [PubMed] [Google Scholar]
- 2.Kanchana G, Shyni WJ, Malini P, Rajadurai M. Effect of sinapic acid on antiperoxidative and antioxidant potential in normal and streptozotocin-induced diabetes in wistar rats. Int J Pharm Clin Res. 2011;3:5–9. [Google Scholar]
- 3.Ahmed RG. The physiological and biochemical effects of diabetes on the balance between oxidative stress and antioxidant defense system. Med J Islam World Acad Sci. 2005;15:31–42. [Google Scholar]
- 4.Kim KS, Lee S. Antioxidant activities of the extracts from the herbs of Artemisis apiacea. J Ethnopharmacol. 2003;85:69–72. doi: 10.1016/s0378-8741(02)00338-0. [DOI] [PubMed] [Google Scholar]
- 5.Kawamura M, Heinecke JW, Chait A. Pathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide-dependent pathway. J Clin Invet. 1994;94:771–8. doi: 10.1172/JCI117396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayakumar M, Balasubramaniam A, Manivannan R, Kumar NS. Antioxidant potential of ethanolic extract of Bauhinia tomentosa (Linn) flower. Res J Pharm Biol Chem Sci. 2010;1:143–7. [Google Scholar]
- 7.Sadyojatha AM, Vaidya VP. Chemical constituents of the roots of Momordica dioica Roxb. Indian Drugs. 1996;330:473–8. [Google Scholar]
- 8.Reddy TG, Kumar BR, Mohan KG, Ramesh M. Anti hyperglycemic activity of Momordica dioica fruits in alloxan-induced diabetic rats. Asian J Pharmacol. 2006;6:327–9. [Google Scholar]
- 9.Gupta R, Katariya P, Mathur M, Bajaj VK, Yadav S, Kamal R, et al. Antidiabetic and renoprotective activity of Momordica dioica in diabetic rats. Diabetol Croat. 2011;40:81–8. [Google Scholar]
- 10.Singh R, Seherawat A, Sharma P. Hypoglycemic, antidiabetic and toxicological evaluation of momordica dioica fruit extracts in alloxan induced diabetic rats. J Pharmacol Toxicol. 2011;6:454–67. [Google Scholar]
- 11.Raghavan B, Krishna KS. Effect of terminalia arjuna stem bark on antioxidant status in liver and kidney of alloxan diabetic rats. Indian J Physiol Pharmacol. 2006;50:133–42. [PubMed] [Google Scholar]
- 12.Goldner M, Gomori N. Alloxan induced diabetes. Endocrinology. 1943;33:297–9. [Google Scholar]
- 13.Matsunami T, Sato Y, Sato T, Yukawa M. Antioxidant status and lipid peroxidation in diabetic rats under hyperbaric oxygen exposure. Physiol Res. 2010;59:97–104. doi: 10.33549/physiolres.931711. [DOI] [PubMed] [Google Scholar]
- 14.Soon YY, Tan BK. Evaluation of the hypoglycemic and anti- oxidant activities of morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J. 2002;43:77–85. [PubMed] [Google Scholar]
- 15.Nwanjo HU, Oze G, Okafor MC, Nwosu D, Nwankpa P. Protective role of Phyllantus niruri extract on serum lipid profiles and oxidative stress in hepatocytes of diabetic rats. Afr J Biotechnol. 2007;6:1744–9. [Google Scholar]
- 16.Balasubramaniam D, Carani VA. Antioxidant potential of Coriandrum sativum L seed extract. Indian J Exp Biol. 2011;49:30–8. [PubMed] [Google Scholar]
- 17.Kaleem M, Asif M, Ahmed QU, Bano B. Antidiabetic and antioxidant activity of Annona squamosa extract in streptozotocin-induced diabetic rats. Singapore Med J. 2006;47:670–5. [PubMed] [Google Scholar]
- 18.Semiz A, Sen A. Antioxidant and chemoprotective properties of Momordica charantia L (bitter melon) fruit extract. Afr J Biotechnol. 2007;6:273–7. [Google Scholar]
- 19.Thakran S, Siddiqui MR, Baquer NZ. Trigonella foenum graecum seed powder protects against histopathological abnormalities in tissues of diabetic rats. Mol Cell Biochem. 2004;266:151–9. doi: 10.1023/b:mcbi.0000049153.14295.0d. [DOI] [PubMed] [Google Scholar]


