Abstract
Background:
In this randomized, double-blinded study, we investigated the preemptive effects of propofol, remifentanil or ketamine on post-operative pain scores and analgesic requirements in elective lower abdominal surgeries under general anesthesia during the first 24 h of post-operative period.
Materials and Methods:
Seventy five patients, American Society of Anesthesiologists physical status I or II candidate for elective lower abdominal surgery under general anesthesia were randomized to three groups (25 each). According to their allocated group, patients received either propofol 0.25 mg/kg, remifentanil 0.25 mic/kg or ketamine 0.3 mg/kg as preemptive analgesia immediately after the induction of general anesthesia. Post-operative pain scores with a numerical rating scale (visual analogue scale 0-10) were assessed and analgesic requirements and side-effects were compared through analysis using the SPSS version 18 in the post-operative period; post-anesthesia care unit 2, 6, 12 and 24 h.
Results:
Patients’ demographics were similar in all groups. The pain scores were significantly lower in remifentanil group immediately after recovery and also at 2 and 6 h post-operatively, but it reversed at 12 and 24 h after recovery comparing with propofol and ketamine. However, the mean of administered morphine in the first 24 h was significantly lower in propofol group (18.97 ± 6.6) comparing with remifentanil group (21.96 ± 6.55) and ketamine group (24.26 ± 5.84) (P value, 0.01).
Conclusion:
Prophylactic preemptive single dose of intravenous (IV) 0.25 mg/kg propofol significantly decreased post-operative analgesia requirements comparing with IV 0.3 mg/kg ketamine or 0.25 μg/kg remifentanil.
Keywords: Analgesic requirement, ketamine, post-operative pain score, preemptive analgesia, propofol, remifentanil
INTRODUCTION
Millions of people undergo a surgical operation every year and update data and modern surgical techniques are used for them. Effective pain control is essential during taking care of surgical patients. However, in spite of great improvements in pathophysiology and pharmacology of analgesics, many patients suffer from the debilitating post-operative pain. Obviously, the main goal of pain control is providing comfort to patients and decreasing the complications caused by inappropriate pain control.[1]
According to the results obtained in different studies, it is believed that analgesic activities are more effective if they are carried out prior to injury, compared to being carried out after injury.[2] Preemptive analgesia is an analgesic treatment preventing central sensitization and the central pain process, called hyperalgesia and limits the patients’ pain.[3]
Administration of opioids prior to the first phase could prevent the development of the late phase. Hence, preventing the early nervous cascade could lead to the elimination of central sensitization.[3]
Preemptive analgesia theoretically decreases post-operative pain and may prevent the development of chronic pain as well.[4]
Though the basis for “preemptive analgesia” is widely known, different reports are available about its effectiveness.[5] On the other hand, many different definitions have been proposed for preemptive analgesia. This has led to many ambiguities and controversies in this field. According to some studies, if active treatment is used, long and severe pain will develop and remain.[4,5,6,7,8,9,10]
Furthermore, different and controversial results have been reported in the studies that have evaluated the effect of non-steroidal anti-inflammatory drugs, systematic opioids, ketamine, dextromethorphan, local anesthesia and epidural anesthesia agents on the body.[10]
Ketamine anesthesia has been approved in post-operative pain control and it has been reported that low-dose ketamine significantly reduce the amount of morphine required and also nausea after abdominal surgery.[11]
Propofol is a short-acting intravenous (IV) hypnotic agent used for induction and maintenance of general anesthesia and sedation for surgical operation and mechanical ventilation in adults.[12] The mechanisms of action are as follows: Potentiation of gamma-aminobutyric acid type A (GABA-A) receptor activity by increasing channels block time and also playing the role as a sodium channel blocker.[13,14,15,16,17,18,19]
Remifentanil is a potent ultra-short-acting synthetic opioid analgesic drug, used during the surgical operation for pain relief and its anesthetic effects. Remifentanil is used for sedation. It is also combined with other medications in general anesthesia.[20]
We investigated the preemptive effects of propofol, remifentanil or ketamine on post-operative pain scores and analgesic requirements in elective lower abdominal surgeries under general anesthesia during the first 24 h of post-operative period due to lack of any studies regarding the comparison.
MATERIALS AND METHODS
This is a randomized double-blind clinical trial, approved by Research Committee of School of Medicine, Isfahan University of Medical Sciences in 2011.
Prior to study, all patients signed an informed written consent. The present clinical trial was carried out on 75 people, divided into three groups of 25 patients. The participants were in the age range of 18-65, with American Society of Anesthesiologists (ASA) physical status I-II (ASA I: Normal healthy patient, ASA II: Patient with mild systemic disease; no functional limitation) and were a candidate for lower abdomen elective surgery. All participants had normal values of blood urea nitrogen, Cr and liver function tests and were negative for history of psychological problems, convulsion or alcohol and opioid addiction. In addition, they had to be able to use verbal rating scale (VRS: 0 = no pain, 1 = mild pain, 2 = moderate pain, 3 = severe pain, 4 = extremely severe pain) or visual analog scale (VAS: Patient explain the severity of his pain with numerical 0-10 scale). The participants were randomly divided into three groups using sealed envelopes with 25 patients in each group respectively.
(The sample size was estimated based on a power calculation, which showed that at least 25 patients per group were necessary to achieve 80% power to detect a 20% difference between the two groups in the VAS scoring with α equal to 0.05).
In each group, one drug was used as the preemptive analgesic. One group received remifentanil (GSK Glaxosmith Kline Italy) (R) while the two other groups received propofol (Claris Lifescience Limited India) (P) and ketamine (Rotex Medica Germany) (K). Regarding the differences between colors and volumes of the drugs, the individual who injected the drugs was different from the one who evaluated and also all of our syringes were covered, to fulfill a double-blind study. The anesthesia method was the same in all three groups; thiopental 5 mg/kg, fentanyl 2 μg/kg combined with atracurium 0.5 mg/kg, followed by morphine 0.2 mg/kg.
Maintenance of anesthesia was accomplished by 50% N2O in O2 through orotracheal tube in conjunction with propofol 100 μg/kg/min IV infusions. In case of complicated operation or prolonged anesthesia (more than 3 h), the participant was excluded from the study. Immediately after induction of anesthesia, 0.3 mg/kg ketamine, 0.25 mg/kg propofol and 0.25 μg/kg remifentanil were administered in groups (K), (P) and (R) as preemptive anesthesia, respectively.
Systolic, diastolic and mean arterial blood pressure (BP), pulse and respiratory rate of patient with 15-min intervals were monitored and recorded during the operation. Furthermore, these variables were measured and recorded 2, 6, 12, 24 h post-operation. Post-recovery pain intensity of patients was measured and recorded in VAS and VRS tables in the recovery room immediately after the operation and also 2, 6, 12 and 24 h after the surgery. Furthermore, time interval between operation ending and first post-operative opioid dose received (pethidine 0.1 mg/kg or morphine 0.15 mg/kg, providing VAS reaches above 3) and recovery duration were recorded in three groups. The amount of opioid used was recorded in three groups in the first 24 h after the surgery. Sleep scores (according to the Ramsey Sedation Scale) were recorded in three groups 2, 6, 12 and 24 h after the operation as well. Meanwhile, complications which seemed to be caused by the drugs such as bradycardia (heart rate (HR) < 60), tachycardia (HR > 120), hypertension (BP ≥ 140/90 mmHg), hypotension (BP ≤ 90/60 mmHg) and hallucination were recorded. The data were analyzed by SPSS software version 20 and all dependent quantitative variables were compared and analyzed in three groups. The variables include sleep score, respiratory rate, HR, arterial BP, VAS, VRS, amount of post-operative opioid used and time interval between end of the operation and the first post-operative opioid dose received.
RESULTS
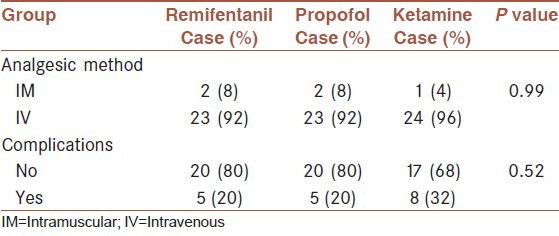
According to the Chi-square test results, the three groups were not significantly different in gender (P = 0.713), job (P = 0.419), ASA (P = 0.853), type of surgical operation (P = 0.126) and post-operative analgesic method (P = 0.284). According to fisher exact test, no statistical difference between 3 groups (P = 0.99).
However, the groups were significantly different with regard to the complications (bradycardia, tachycardia, hypertension, hypotension and hallucination) of anesthetic drugs (P = 0.026). Ketamine caused more complications than the two other drugs (tachycardia, hypertension and hallucination) [Table 1]. According to Chi-square test, no statistical difference between 3 groups (P = 0.52).
Table 1.
Frequency distribution of post-operative analgesic method and perioperative complications in the three groups
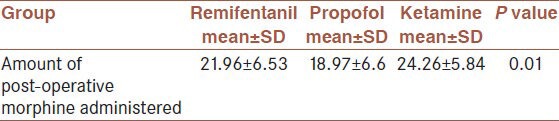
The analysis of variance test (ANOVA) demonstrated that there was no significant difference in the mean age (P = 0.66), mean weight (P = 0.279), time interval between the end of operation and the first opioid dose received (P = 0.552), duration of recovery (P = 0.856), duration of anesthesia (P = 0.623), duration of extubation (P = 0.617), mean dose of morphine during the operation (P = 0.994), mean VRS 24 h after recovery (P24 = 0.453), respiratory rate immediately after recovery (P0 = 0.793) and 2 h after recovery (P = 0.493), HR in all times (P15 = 0.417), (P30 = 0.382), (P45 = 0.275), (P0 = 0.225), (P2 = 0.249), (P6 = 0.10), (P12 = 0.225), (P24 = 0.250). Mean Arterial Pressure (MAP) at minutes 15, 30 and 45 during the operation (P15 = 0.541), (P30 = 0.309), (P45 = 0.337) and immediately after recovery (P0 = 0.621) and 2, 6 and 24 h after recovery (P2 = 0.610), (P6 = 0.322), (P24 = 0.323) in the three groups were not also significantly different [Table 2]. The three groups were significantly different in the amount of post-operative morphine (P = 0.01); such that the lowest amount was used in the propofol group (18.97 ± 6.6), followed by the remifentanil (21.96 ± 6.53); and the highest amount of morphine was administered in the ketamine group (24.6 ± 5.84) [Table 3].
Table 2.
Means MAP values in different times in the three groups
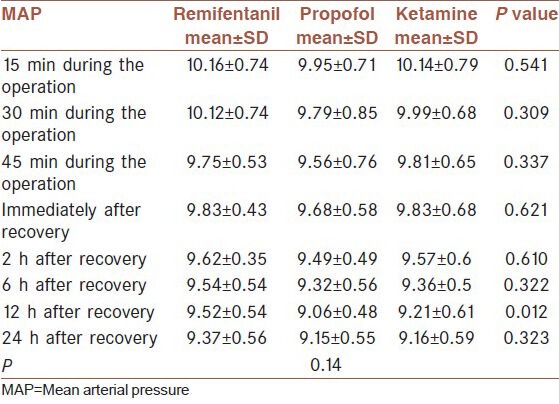
Table 3.
Mean amount of post-operative morphine administered in the three groups
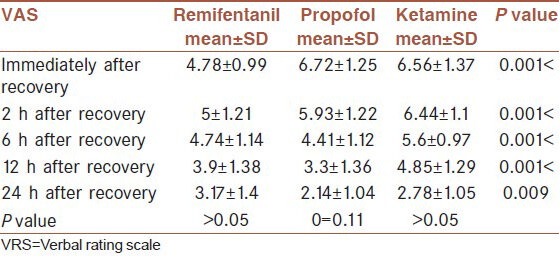
The three groups were significantly different in mean VAS in all the times (P0 < 0.001), (P2 < 0.001), (P6 < 0.001), (P12 < 0.001) and (P24 = 0.009). The lowest mean VAS immediately after recovery belonged to the remifentanil group (4.78 ± 0.99), the propofol group (6.72 ± 1.25) and then the ketamine group (6.56 ± 1.37). The lowest mean VAS 2 h after recovery was related to the remifentanil group (5 ± 1.21), followed by the propofol (5.93 ± 1.22) and then the ketamine groups (6.44 ± 1.1). However, the lowest mean VAS 6 h after recovery was related to the propofol group (4.41 ± 1.12) and then the remifentanil (3.9 ± 1.38) and the ketamine groups (4.85 ± 1.29). The lowest mean VAS 24 h after recovery belonged to the propofol group (2.17 ± 1.04), then the ketamine group (2.78 ± 1.05) and the highest mean value related to the remifentanil group (3.17 ± 1.4) [Table 4]. According to repeated measures ANOVA, there was no statistically difference between 3 groups (P = 0.11).
Table 4.
Means VAS values in the three groups in different times after recovery
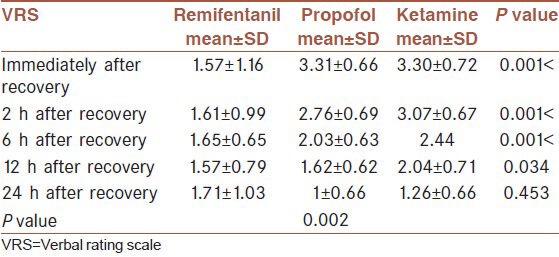
By comparing the mean values of VAS in the three groups using analysis of variance, it is observed that the groups were significantly different in the mean VAS score immediately, 2, 6 and 12 h after recovery (P0 < 0.001), (P2 < 0.001), (P6 < 0.001) and (P12 < 0.034). The lowest mean VAS immediately after recovery related to the remifentanil group (1.57 ± 1.16) and there was no significant difference between the ketamine (3.30 ± 0.72) and propofol groups (3.31 ± 0.66). However, the lowest mean VRS 2 h after recovery related to the remifentanil group (1.61 ± 0.99), followed by the propofol (2.76 ± 0.69) and ketamine groups (3.07 ± 0.67). The lowest mean VAS 6 h after recovery related to the remifentanil group (1.65 ± 0.65) and then the propofol (2.03 ± 0.63) and ketamine groups (2.44 ± 0.58). The lowest mean VAS 12 h after recovery related to the remifentanil group (1.57 ± 0.79), then the propofol group (1.62 ± 0.62) and the ketamine group (2.04 ± 0.71). With regard to the mean VAS 24 h after recovery, the lowest mean related to the propofol group (1 ± 0.66), after that the remifentanil group (1.17 ± 1.03) and the highest mean related to the ketamine group (1.26 ± 0.66) [Table 5]. According to repeated measures ANOVA, there is a statistically difference between 3 groups (P = 0.002).
Table 5.
Means VRS in the three groups 0, 2, 6, 12 and 24 h after recovery
Analysis of variance showed statistically significant difference among the groups with regard to the respiratory rate during the operation with 15 min intervals and also 6, 12 and 24 h after the operation (P15 = 0.001), (P30 = 0.002), (P45 < 0.001), (P6 = 0.01), (P12 = 0.001) and (P24 < 0.001). In most times, the ketamine group had the highest mean respiratory rate (RR15 = 14.22 ± 1.28), (RR30 = 14.26 ± 1.35), (RR45 = 14.37 ± 1.27), (RR6 = 15.33 ± 0.92), (RR12 = 14.89 ± 1.12), (RR24 = 15.07 ± 1.52) and there was no great different between the propofol group (12.97 ± 0.98), (13.03 ± 1.05), (13.3 ± 1.05), (14.76 ± 1.53), (13.69 ± 1.36), (12.45 ± 1.05) and the remifentanil group (13.70 ± 1.26), (13.43 ± 1.31), (13.43 ± 1.31), (14.22 ± 1.20), (13.70 ± 1.33), (13.39 ± 1.53). Analysis of variance indicated that the mean MAP values of the three groups 12 h after recovery were significantly different (P = 0.012). The mean value in the propofol group was the least (9.06 ± 0.48), followed by the remifentanil group (9.52 ± 0.54) and then the ketamine group (9.21 ± 0.61) [Table 2]. According to repeated measures ANOVA, no statistically difference between 3 groups (P = 0.14).
The results obtained from the Kruskal-Wallis test indicated that the groups were not significantly different with regard to the education level (P = 0.67), median sleep score immediately after recovery (P = 0.485), 12 h after recovery (P = 0.142) and 24 h after recovery (P = 1). However, the test showed that there is a significant difference among the median sleep scores 2 and 6 h after recovery in the three groups. In the ketamine group, 2 h after recovery, none of the patients were restless, anxious and agitated; 17 patients (68%) were cooperative and oriented; 3 people (12%) just obeyed the orders; and 5 patients (20%) just responded to glabella tap or loud auditory stimulus. In the propofol group, 25 patients (100%) were cooperative and oriented. In the remifentanil group, none were restless, anxious or agitated; 23 patients (92%) were cooperative and oriented; none of them obeyed the orders; and 2 people (8%) just responded to glabella tap and/or loud auditory stimulus. In the ketamine group, 6 h after recovery (P = 0.009), 20 patients (80%) were cooperative and oriented; 3 people (12%) obeyed the orders; and 2 patients (8%) just responded to glabella tap and/or loud auditory stimulus. In the propofol group, all patients (100%) were cooperative and oriented. In the remifentanil group, 23 patients (92%) were cooperative and oriented; 2 people (8%) just obeyed the orders; and none responded only to glabella tap and/or loud auditory stimulus.
DISCUSSION
No study has been carried out so far to compare ketamine, propofol and remifentanil. This is the first time to compare these drugs. Based on the results of a clinical trial carried out in 2006, single-dose ketamine (0/4 mg/kg) does not perform as preemptive analgesia in patients underwent hysterectomy.[21] Consistently, ketamine had the least effect in the present study.
In a trial carried out on mice in 1994, it was supposed that sedative agents such as IV administration of pentobarbitone and propofol may regulate phase 2 hyperalgesic responses by contributing to the development of central sensitization since it is known that both has GABA-A receptor activity effect. However, according to the results obtained in the current study, only propofol had preemptive analgesic effect.[22] Nevertheless, according to our findings, propofol had more desirable preemptive analgesic effect than remifentanil and ketamine in some cases. In another study, analgesic effect of propofol was reported.[23]
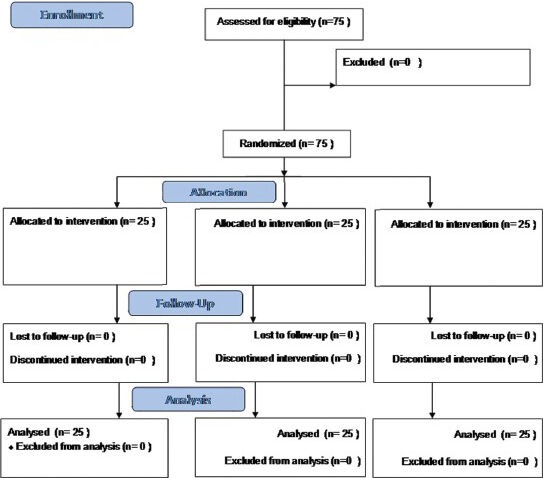
Consort flow diagram.
In a clinical trial in 2009, prescribing, ketamine combined with remifentanil had a great preemptive analgesic effect in post-operative pain control. This could be explained by combination of the two medications, especially for ketamine effect against opioid-induced hyperalgesia.[24] Based on the findings of the present study, preemptive analgesic effect of remifentanil is greater than that of ketamine.
In a clinical trial to compare the preemptive analgesic effect of sufentanil with remifentanil, it was reported that V.A.S was significantly higher in remifentanil 2 h after extubation. It was also observed that the time interval between recovery and administration of the first analgesic dose was longer in sufentanil while the post-operative opioid dose required was higher in remifentanil group.[25] In the current study, remifentanil, compared to the two other drugs has the least VAS 2 h after anesthesia. The three groups were not significantly different with regard to the time of administration of the first analgesic dose. However, the lowest post-operative opioid dose belonged to propofol and then to remifentanil.
In another clinical trial carried out in 2004, it was shown that performing preemptive analgesia during the surgical operation by ketamine leads to a desirable anesthesia after recovery. However, the duration is not sufficient (about 1 h); it also enhances the post-operative analgesic effect of tramadol.[26] Regarding the findings of the present study, ketamine had the lowest analgesic effect within the first 12 h after anesthesia.
In a clinical trial, combination of remifentanil and ketamine was administered in laparoscopic cystectomy. The most desirable preemptive analgesic effect was observed in patients who received 0.75 μg/kg remifentanil after anesthesia induction up to the suture time and 100 μg/kg ketamine from induction to 6 h after anesthesia.[27]
CONCLUSION
A low dose of remifetanil (0.25 μg/kg) immediately after induction of general anesthesia provide better post-operative pain relief in comparison with ketamine (0.3 mg/kg) or propofol (0.25 mg/kg) in lower abdominal surgery.
The principal limitation of this study was the specific setting for post-operative pain relief and the relatively low number of patients, so this observation should be replied in further surveys in a large number of patients and different setting and in our study median sleep score was different between three groups in some times after the recovery; therefore, our findings about VAS and VRS are reliable in patients who were cooperative.
ACKNOWLEDGMENT
We would like to express our gratitude to all those who gave us the possibility to complete this research. Furthermore, our special thanks are extended to the stuff of post-anesthesia care unit and the surgery ward for their assistance with the collection of our data.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ready L. Acute perioperative pain. In: Miller RD, editor. Anesthesia. 6th ed. Philadelphia, London: Churchill Livingstone; 2005. pp. 2323–40. [Google Scholar]
- 2.Woolf CJ. Central mechanisms of acute pain. In: Bond MR, Charlton JE, Woolf CJ, editors. Proc. 6th World Congr on Pain. Amsterdam: Elsevier; 1991. pp. 25–34. [Google Scholar]
- 3.Gottschalk A, Smith DS. New concepts in acute pain therapy: Preemptive analgesia. Am Fam Physician. 2001;63:1979–84. [PubMed] [Google Scholar]
- 4.Dahl JB, Møiniche S. Pre-emptive analgesia. Br Med Bull. 2004;71:13–27. doi: 10.1093/bmb/ldh030. [DOI] [PubMed] [Google Scholar]
- 5.Tverskoy M, Oz Y, Isakson A, Finger J, Bradley EL, Jr, Kissin I. Preemptive effect of fentanyl and ketamine on postoperative pain and wound hyperalgesia. Anesth Analg. 1994;78:205–9. doi: 10.1213/00000539-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ, Salter MW. Neuronal plasticity: Increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ, Chong MS. Preemptive analgesia: Treating postoperative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–79. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 8.Kissin I. Preemptive analgesia. Anesthesiology. 2000;93:1138–43. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 9.Dahl JB. The status of pre-emptive analgesia. Curr Opin Anaesthesiol. 1995;8:323–30. [Google Scholar]
- 10.Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: The role of timing of analgesia. Anesthesiology. 2002;96:725–41. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 11.George DL. Postoperative Ketamine Can Reduce Morphine Consumption and Nausea. Medscape. [Retrieved on 2008 Nov 04]. Available from: http://www.medscape.com/viewarticle/581018 .
- 12.Miner JR, Burton JH. Clinical practice advisory: Emergency department procedural sedation with propofol. Ann Emerg Med. 2007;50:182–7. doi: 10.1016/j.annemergmed.2006.12.017. 187.e1. [DOI] [PubMed] [Google Scholar]
- 13.Kotani Y, Shimazawa M, Yoshimura S, Iwama T, Hara H. The experimental and clinical pharmacology of propofol, an anesthetic agent with neuroprotective properties. CNS Neurosci Ther. 2008;14:95–106. doi: 10.1111/j.1527-3458.2008.00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanlersberghe C, Camu F. Propofol. Handb Exp Pharmacol. 2008;182:227–52. doi: 10.1007/978-3-540-74806-9_11. [DOI] [PubMed] [Google Scholar]
- 15.Trapani G, Latrofa A, Franco M, Altomare C, Sanna E, Usala M, et al. Propofol analogues. Synthesis, relationships between structure and affinity at GABAA receptor in rat brain, and differential electrophysiological profile at recombinant human GABAA receptors. J Med Chem. 1998;41:1846–54. doi: 10.1021/jm970681h. [DOI] [PubMed] [Google Scholar]
- 16.Krasowski MD, Jenkins A, Flood P, Kung AY, Hopfinger AJ, Harrison NL. General anesthetic potencies of a series of propofol analogs correlate with potency for potentiation of gamma-aminobutyric acid (GABA) current at the GABA(A) receptor but not with lipid solubility. J Pharmacol Exp Ther. 2001;297:338–51. [PubMed] [Google Scholar]
- 17.Krasowski MD, Hong X, Hopfinger AJ, Harrison NL. 4D-QSAR analysis of a set of propofol analogues: Mapping binding sites for an anesthetic phenol on the GABA(A) receptor. J Med Chem. 2002;45:3210–21. doi: 10.1021/jm010461a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haeseler G, Leuwer M. High-affinity block of voltage-operated rat IIA neuronal sodium channels by 2,6 di-tert-butylphenol, a propofol analogue. Eur J Anaesthesiol. 2003;20:220–4. doi: 10.1017/s0265021503000371. [DOI] [PubMed] [Google Scholar]
- 19.Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, Hecker H, et al. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155:265–75. doi: 10.1038/bjp.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoke JF, Cunningham F, James MK, Muir KT, Hoffman WE. Comparative pharmacokinetics and pharmacodynamics of remifentanil, its principle metabolite (GR90291) and alfentanil in dogs. J Pharmacol Exp Ther. 1997;281:226–32. [PubMed] [Google Scholar]
- 21.Karaman S, Kocabaş S, Zincircioğlu C, Firat V. Has ketamine preemptive analgesic effect in patients undergoing abdominal hysterectomy? Agri. 2006;18:36–44. [PubMed] [Google Scholar]
- 22.Goto T, Marota JJ, Crosby G. Pentobarbitone, but not propofol, produces pre-emptive analgesia in the rat formalin model. Br J Anaesth. 1994;72:662–7. doi: 10.1093/bja/72.6.662. [DOI] [PubMed] [Google Scholar]
- 23.Phillips WJ, Halpin J. Analgesic effect of propofol? Ann Emerg Med. 2008;51:331–2. doi: 10.1016/j.annemergmed.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Kitamura T, Imai Y, Ohno N, Muroya M, Ogawa M, Yamada Y. Effects of general anesthesia using ketamine and remifentanil on postoperative pain management for patients undergoing laparotomy. Masui. 2009;58:739–44. [PubMed] [Google Scholar]
- 25.Derrode N, Lebrun F, Levron JC, Chauvin M, Debaene B. Influence of peroperative opioid on postoperative pain after major abdominal surgery: Sufentanil TCI versus remifentanil TCI. A randomized, controlled study. Br J Anaesth. 2003;91:842–9. doi: 10.1093/bja/aeg263. [DOI] [PubMed] [Google Scholar]
- 26.Launo C, Bassi C, Spagnolo L, Badano S, Ricci C, Lizzi A, et al. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2004;70:727–34. [PubMed] [Google Scholar]
- 27.Truta E, Vatric M, Cristea AN. Clinical study regarding preemptive analgesic effect of ketamine and remifentanil in laparoscopic cholecystectomy. FARMACIA. 2011;59(2):239–45. [Google Scholar]


