Abstract
Background:
There have been few studies to examine the effect of magnesium (Mg) supplementation on liver enzymes. The aim of this study was to evaluate the effect of Mg supplementation and weight loss on liver enzymes, lipid profile, and fasting blood sugar in patients with nonalcoholic fatty liver disease (NAFLD).
Materials and Methods:
This study was a double-blind, placebo-controlled, randomized clinical trial. Ultrasonography was used to diagnose fatty liver in patients with alanine aminotransferase (ALT) ≥ 40 U/L and without other hepatic diseases. A total of 68 participants (18-59 years) with NAFLD were randomly divided into two groups to receive either Mg supplement (350 mg elemental Mg per day) or placebo for 90 days. At baseline and at the end of the intervention serum ALT, aspartate aminotransferase (AST), alkaline phosphatase (ALP), total cholesterol (TCHO), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), blood sugar and serum insulin, and Mg levels were measured in fasting state. Low-density lipoprotein cholesterol (LDL-C) and insulin resistance (IR) were calculated using Friedewald formula and homeostasis model assessment of insulin resistance (HOMA-IR), respectively. All participants received lifestyle recommendations including low calorie diet and physical activity.
Results:
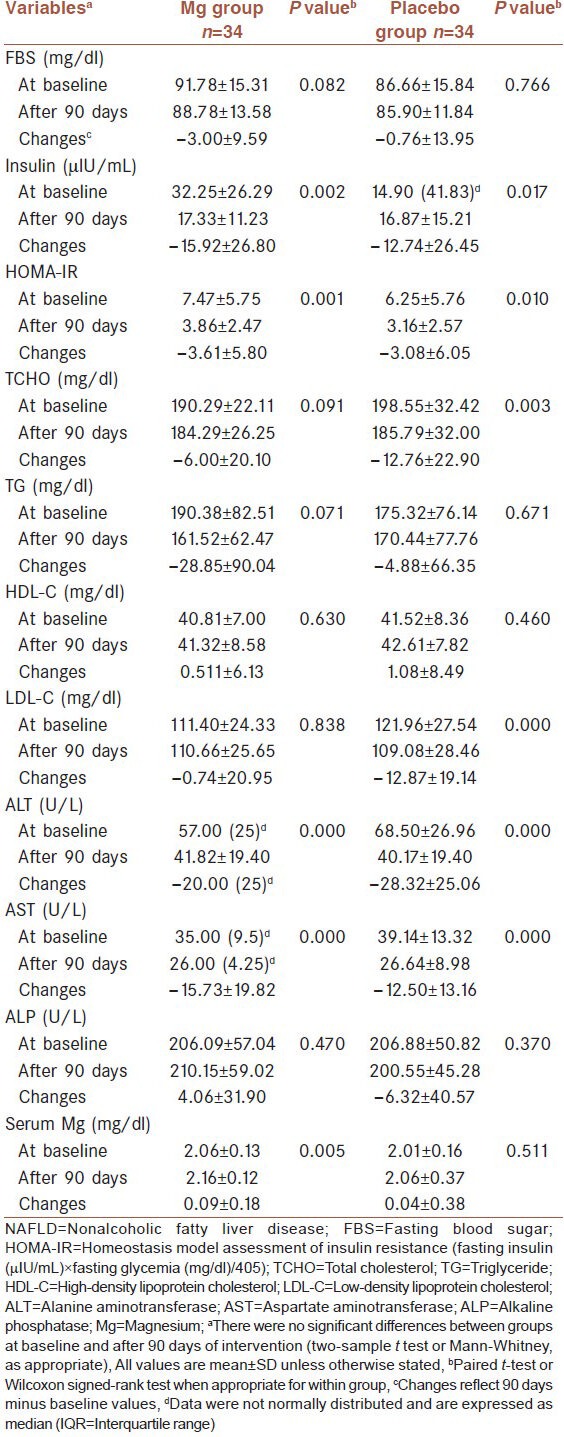
Significant decreases within the intervention and placebo groups were observed in ALT (57.00 (25) to 41.82 ± 19.40 U/L, P = 0.000; 68.50 ± 26.96 to 40.17 ± 19.40 U/L, P = 0.000 in Mg and placebo groups, respectively). Similar significant decreases were observed in AST and fasting serum insulin within the study groups. The decrease in weight was also significant in both groups (91.05 ± 13.77 to 87.60 ± 14.37 kg and 94.59 ± 16.85 to 91.45 ± 16.39 kg in Mg and placebo groups, respectively). LDL-C and TCHO were decreased significantly in placebo group but not in the intervention group. Serum Mg was increased significantly in the intervention group. No statistically significant differences were observed between the two study groups at baseline and after intervention.
Conclusion:
According to the findings of this study, Mg supplement does not affect liver enzymes but weight loss may have an important role in improving fatty liver disease.
Keywords: Alanine aminotransferase, aspartate aminotransferase, insulin, magnesium, nonalcoholic fatty liver disease
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is defined as a spectrum of disorders characterized predominantly by macrovesicular hepatic steatosis. The clinical relevance of NAFLD arises from the fact that a considerable proportion of these patients (20-30%) develop nonalcoholic steatohepatitis (NASH), and this condition, as opposed to simple fatty liver, is a potentially progressive hepatic disorder that can lead to end-stage liver disease and hepatocellular carcinoma.[1] Although the exact mechanism for development of NASH is not known, recent studies have shown that patients with NASH have a higher prevalence of insulin resistance.[2,3] Obesity is also strongly associated with hepatic steatosis.[3] In addition, some studies have reported a relationship between decreased serum magnesium (Mg) levels and NASH.[4]
Mg is a cofactor for more than 300 metabolic reactions in the body.[5] Mg plays a major role in overall cell functions, including DNA and protein synthesis, glucose and fat metabolism, and oxidative phosphorylation.[6] Mg deficiency is related to the triggering of inflammatory response, mitochondrial dysfunction, profibrogenic response, and decrease of the antioxidant system activity; which stimulates lipid peroxidation and cytokine induction, and well-known pathways for the development of steatohepatitis and hepatic fibrosis.[7]
It has been reported in a few studies that Mg supplementation is associated with improved inflammatory factors,[7] glucose metabolism, and insulin resistance. Such an association was not shown in other studies.[8,9,10,11] The benefits of Mg supplementation in patients with NAFLD have not been established. Therefore, the effect of Mg supplementation and weight loss on serum liver enzymes, lipid profile, and insulin resistance were investigated in this double-blind, placebo-controlled, randomized trial.
MATERIALS AND METHODS
Participants
This study was a double-blind, placebo-controlled, randomized clinical trial. Participants were recruited from Ahvaz, located in southwest of Iran. Written informed consent was obtained from all patients. The inclusion criteria were: Age between 18-59 years, abnormal liver function with alanine aminotransferase (ALT) ≥40 U/L, and fatty liver diagnosed by ultrasonography. The exclusion criteria were: Chronic diarrhea, alcohol intake (equal to or more than 30 g/day), consumption of diuretics or previous oral Mg supplement, hepatic disease, hemochromatosis, Wilson's disease, cirrhosis, history of any other hepatic, renal, or lung disorders.
Sample size was estimated based on a statistical power of 90%, α < 0.05, and 28% reduction in ALT levels, on the basis of a study conducted by Rodriguez-Hernandez et al.[7] Flow diagram of the participants’ enrolment and follow up is shown in Figure 1.
Figure 1.
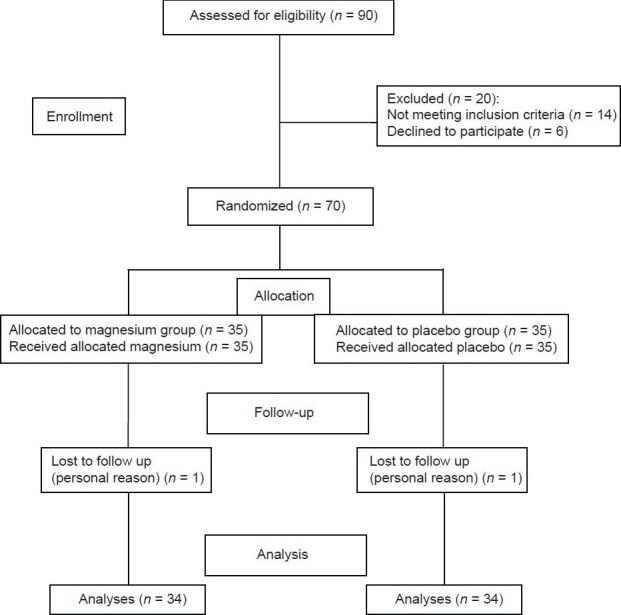
Flowchart of recruitment for the double-blind, placebo-controlled, randomized controlled trial of Mg supplementation in NAFLD
Seventy patients with NAFLD were randomly divided into two groups: The intervention group (n = 35) received 350 mg of elemental Mg per day in the form of magnesium oxide for 90 days, and the control group (n = 35) received placebo (lactose). The supplement and placebo capsules looked identical and were specially prepared for this study by Darou-Pakhsh Company (Tehran, Iran) and stored at 20-25°C. This dose of Mg was chosen according to tolerable upper intake levels (UL) of Mg and the report of Heriberto Rodriguez-Hernandez regarding the effect of Mg supplementation on ALT.[7]
Dietary habits, demographic, and anthropometric data were obtained by personal interview or measurement.
The daily nutrient intake including energy and Mg were estimated by three 24-h dietary recalls during the first month of the study by a trained dietitian. Dietary data were analyzed by Nutritionist IV software. Physical activity was assessed using the general practice physical activity questionnaire (GPPAQ) at baseline.[12]
A low calorie diet was calculated and the participants were asked to go under this diet. Energy requirement was calculated by the World Health Organization (WHO) formula and, then was reduced to 500 kcal/day (≥ 55% carbohydrate, 15-20% protein, and ≤30% fat). All participants were advised to perform at least 30 min of physical activity per day. Compliance and lifestyle intervention were assessed every 2 week by phone contact. Remaining capsules were received at the end of the study for calculating the compliance rate.
Measurements
Biochemical variables were measured at baseline and at the end of the study.
Height and weight were measured by using standard protocols with participants in light clothes and without shoes. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2), and body fat percentage was estimated with Omron body fat monitor (HBF-306) at baseline and at the end of the study. Blood samples were taken after 12-14 h of overnight fasting at baseline and after the intervention period. Serum samples were transferred into microtubes and were stored at -70°C until analysis. ALT and aspartate aminotransferase (AST) were determined by the method developed by International Federation of Clinical Chemistry (IFCC).[13,14] Alkaline phosphatase (ALP) was determined by Deutschen Gesellschaft für Klinische Chemie (DGKC);[15] total cholesterol (TCHO), high-density lipoprotein cholesterol (HDL-C), triglyceride (TG), and Serum Mg was measured by enzymatic colorimetric method. Fasting blood sugar (FBS) was measured by colorimetric method. Low-density lipoprotein cholesterol (LDL-C) was calculated by the Friedewald formula.[16] Insulin concentration was determined by immunoenzymometric method and insulin sensitivity was estimated by homeostasis model assessment of insulin resistance (HOMA-IR) by the following formula: Fasting insulin (μIU/mL) × fasting glycemia (mg/dl)/405.[17]
Statistical analysis
In order to compensate for the possible dropouts, almost 20% was added to the calculated sample size. Efficacy analyses were based on the intent-to-treat (ITT) number of participants who received at least one dose of prescribed Mg or placebo and had at least one assessment post baseline. Statistical analyses were conducted using SPSS software (version 15, SPSS Inc., Chicago, IL). The Kolmogorov-Smirnov test was used to test the normality of variable distribution. Results are shown as mean ± standard deviation (SD) when the data were normally distributed and as median (IQR; interquartile range) when the data had a non-normal distribution. χ2-test was used for analysis of nominal or ordinal variables between subgroups. The between-group comparisons for baseline characteristics and their changes after 90 days of intervention period were done by the two-sample t-test or Mann-Whitney, as appropriate. The within-group comparisons were done with paired t-test or Wilcoxon signed-rank test when appropriate. The P values less than 0.05 were considered as statistically significant. This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, (No. NRC-9011) and registered in Iranian Registry of Clinical Trials (IRCT number: IRCT201203128753N1) on March 12, 2012.
RESULTS
A total of 68 participants completed the trial. Two participants were lost to follow up (1 patient from each group). Because of personal reason they did not like to continue their participations. Equal numbers of lost to follow up in the study groups made the groups comparable regarding this variable. No adverse or side effects were reported by participants. Compliance rate were 85.2 and 84% in the Mg supplement and placebo groups, respectively. At the baseline, there were no significant differences in demographic characteristics and nutritional status between the intervention and placebo groups [Table 1]. The mean age of participants was 36 ± 7 years. Six participants from supplement and two participants from placebo groups were prescribed one tablet of Metformin (500 mg) per day during the study. However, there was no statistically significant difference between groups regarding this medication intake (P = 0.25). Mean ± SD of total Mg intake, including supplement, were 553.83 ± 54.38 and 188.25 ± 70.16 in the supplement and placebo groups, respectively.
Table 1.
Baseline demographic and nutritional data of 68 patients with NAFLD
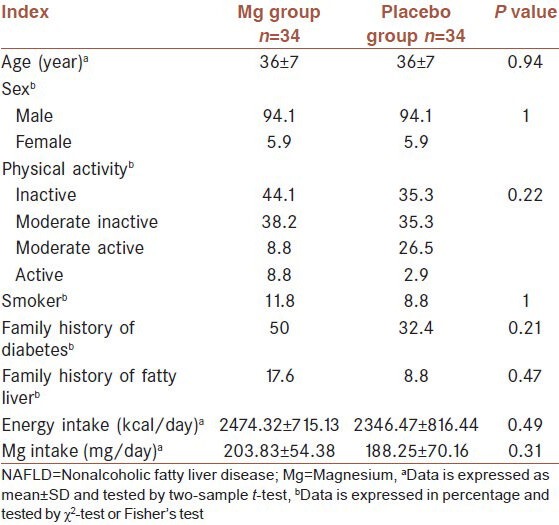
The anthropometric and biochemical characteristics of the 68 patients at baseline and at the end of the study are shown in Table 2 and 3, respectively. At baseline, there were no significant differences in all the measured variables between the two groups. After intervention, weight and BMI (P = 0.004 and P = 0.000 in Mg and placebo groups, respectively), percentage of body fat, insulin and insulin resistance (IR), ALT (P = 0.000 in both groups) and AST decreased significantly in intervention and placebo groups. At the end of intervention period, serum ALT levels were below 40 U/L in Mg (n = 19) and placebo groups (n = 22), respectively. FBS and TG decreased in both groups but not significantly (P > 0.05). A nonsignificant increase was observed in HDL-C in both groups. On the other hand TCHO and LDL-C decreased significantly in placebo group but not in the intervention group. ALP decreased in placebo group and increased in intervention group but not significantly. At the end of the study, no statistically significant differences were observed between the intervention and placebo groups.
Table 2.
Comparison of anthropometric characteristics at baseline and 90 days after intervention in 68 patients with NAFLD (Mean±SD)
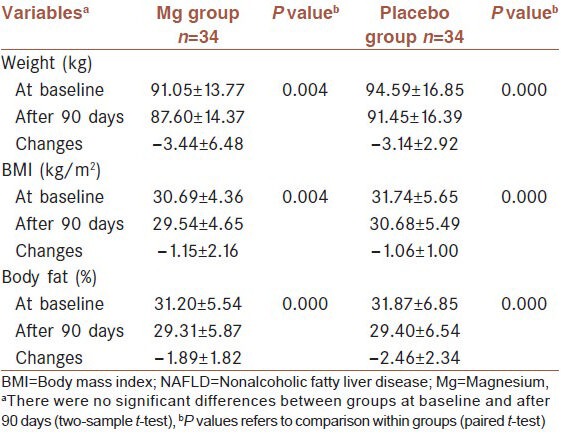
Table 3.
Comparison of biochemical characteristics at baseline and 90 days after intervention in 68 patients with NAFLD
DISCUSSION
This study showed that Mg supplementation does not affect liver enzymes in NAFLD. The benefits of Mg supplementation in patients with NAFLD have not been established yet.
Barbagallo et al.,[18] suggested that Mg deficiency may be involved in the pathogenesis of diabetes mellitus (DM). Some studies have showed the relationship between Mg depletion in serum and mononuclear cells in obese people with metabolic syndrome.[19,20] In addition, some studies have reported the relation between decreased serum Mg levels and NASH.[4] Data regarding the effects of Mg supplementation on the metabolic profile are inconsistent. Although, some clinical trials have showed the impact of Mg supplementation on improving fasting and postprandial glucose levels and insulin sensitivity in patients with type 2 DM with hypomagnesemia,[8,21] however some studies have reported no such association.[10] There is also evidence that Mg supplementation has a relatively small beneficial effect on reduction of insulin resistance in nondiabetic participants with hypomagnesemia.[9] Mg supplementation did not reduce blood pressure or enhance insulin sensitivity in normomagnesemic nondiabetic overweight people.[11] However, in this study Mg supplementation did not have significant effect on liver enzymes, lipid profile, and fasting glucose levels. Some of the differences among published studies may result from differences in the study designs. Song et al.,[22] carried out a meta-analysis of nine well-designed, randomized, controlled trials related to the effects of oral Mg supplementation and glycemic control in type 2 DM and found out such differences among the studies. For example, the doses of Mg supplements were 240-960 mg/day and also follow up durations were from 4 to 16 weeks. Type of Mg supplement (pidolate, chloride, citrate, aspartate, oxide) may be an additional reason for differences in the study results.
To our knowledge, till date only one control trial has been published about the effect of Mg supplement on ALT levels.[7] The significant effect of Mg supplement on ALT levels in that study was contrary to the finding of our study. There is a large difference between two studies. They classified the participants according to the hypomagnesemia and used a dose of Mg upper than tolerable UL (450 mg/day elemental Mg). Duration of that study was 4 month, which is a little longer than this study. Although Mg supplementation may be most beneficial for individuals with Mg deficiency,[7,8,9,21] but in our study participants were normomagnesemic.
In this study a significant reduction in ALT, AST, insulin, and IR in the intervention and placebo groups were observed and are thought to result from significant reductions of weight and BMI in both groups. Decrease of ALT, AST, TCHO, and TG levels in obese women who had received low carbohydrate diet (LCD) or low fat diet (LFD) for 6 month, led Heriberto Rodriguez-Hernandez et al.,[23] to suggest that irrespective of dietary pattern, the metabolic changes depend on weight reduction. Wang et al.,[24] showed that simple lifestyle interventions including dietary changes and increasing physical activity in obese children with NAFLD can lead to a significant improvement of liver function and insulin resistance. Wong et al.,[25] suggested that weight reduction is associated with reduced risk in progression of NAFLD and these patients should undergo lifestyle modifications.
In our study the patients of intervention and placebo groups reduced 3.78 and 3.31% of initial body weight, respectively. These reductions resulted from lifestyles modifications. Published literature shows that different degrees of weight loss, ranging from 1 to 10%, are associated with improvement of IR and liver enzymes.[26,27,28,29,30]
In our study significant reductions in TCHO and LDL-C were observed in the placebo group. These results were unexpected and there is no clear explanation for this. Since the average of changes was not large, it seems that there was statistical significance without any clinical importance and the mean of levels of LDL-C and TCHO were under 130 mg/dl and 200 mg/dl, respectively in both groups.
In two previous studies the effect of Mg supplementation on liver enzymes were shown but these studies had been carried out on alcoholic participants[31,32] and it is not similar to this study because as per our knowledge the pathogenesis of disordered liver enzymes in alcoholics is different from NAFLD. This study is the first randomized clinical trial that investigated the effect of Mg supplementation on NAFLD.
A few limitations existed in this study: Ultrasonography was used for determination of fatty liver but we did not repeat it at the end of the study. However, changes in grade of NAFLD were not the defined primary outcome of this study. The short duration of this study is another limitation, so it is not clear that changes in body weight as well as in the liver enzymes and insulin levels are sustained for long time. Other limitations of this study are lack of direct measurement of insulin homeostasis, potassium, and calcium. However, it has been established that the HOMA-IR is a useful method in determining insulin sensitivity in diabetic and nondiabetic participants and it can be reliably used in studies that only a fasting blood samples of glucose and insulin are measured.[17,33] Mg homeostasis is related to calcium and potassium status, and should be evaluated in combination with these two minerals.[5]
An important point is that, the results of this study are related to participants with normal serum Mg levels and these findings cannot be extrapolated to hypomagnesemic patients.
In conclusion, results of this randomized clinical trial show that Mg supplementation does not affect serum liver enzymes levels, lipid profile, and glycemic control in normomagnesemic patients with NAFLD. Lifestyles modifications, particularly weight reduction may improve the condition of this disease.
ACKNOWLEDGMENT
The source of data used in this paper was from MSc thesis of Mahtab Tamimi, student of Ahvaz Jundishapur University of Medical Sciences; and financial support was provided by Ahvaz Jundishapur University of Medical Sciences.
Footnotes
Source of Support: Arvand International Division, Ahvaz Jundishapur University of Medical Sciences, Abadan, Iran, No. NRC-9011 This study was approved by the Ethics Committee of Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran, and registered in Iranian Registry of Clinical Trials (IRCT number IRCT201203128753N1)
Conflict of Interest: None declared.
REFERENCES
- 1.Petta S, Muratore C, Craxì A. Non-alcoholic fatty liver disease pathogenesis: The present and the future. Dig Liver Dis. 2009;41:615–25. doi: 10.1016/j.dld.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 2.Toshimitsu K, Matsuura B, Ohkubo I, Niiya T, Furukawa S, Hiasa Y, et al. Dietary habits and nutrient intake in non-alcoholic steatohepatitis. Nutrition. 2007;23:46–52. doi: 10.1016/j.nut.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: An overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–89. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Hernandez H, Gonzalez JL, Rodriguez-Moran M, Guerrero-Romero F. Hypomagnesemia, insulin resistance, and non-alcoholic steatohepatitis in obese subjects. Arch Med Res. 2005;36:362–6. doi: 10.1016/j.arcmed.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Guerrera MP, Volpe SL, Mao JJ. Therapeutic uses of magnesium. Am Fam Physician. 2009;80:157–62. [PubMed] [Google Scholar]
- 6.Assadi F. Hypomagnesemia: An evidence-based approach to clinical cases. Iran J Kidney Dis. 2010;4:13–9. [PubMed] [Google Scholar]
- 7.Rodriguez-Hernandez H, Cervantes-Huerta M, Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnes Res. 2010;23:90–6. doi: 10.1684/mrh.2010.0204. [DOI] [PubMed] [Google Scholar]
- 8.Rodriguez-Moran M, Guerrero-Romero F. Oral magnesium supplementation improves insulin sensitivity and metabolic control in type 2 diabetic subjects: A randomized double-blind controlled trial. Diabetes Care. 2003;26:1147–52. doi: 10.2337/diacare.26.4.1147. [DOI] [PubMed] [Google Scholar]
- 9.Guerrero-Romero F, Tamez-Perez HE, González-González G, Salinas-MartÍnez AM, Montes-Villarreal J, Treviño-Ortiz JH, et al. Oral magnesium supplementation improves insulin sensitivity in non-diabetic subjects with insulin resistance. A double-blind placebo-controlled randomized trial. Diabetes Metab. 2004;30:253–8. doi: 10.1016/s1262-3636(07)70116-7. [DOI] [PubMed] [Google Scholar]
- 10.de Valk HW, Verkaaik R, van Rijn HJ, Geerdink RA, Struyvenberg A. Oral magnesium supplementation in insulin-requiring Type 2 diabetic patients. Diabet Med. 1998;15:503–7. doi: 10.1002/(SICI)1096-9136(199806)15:6<503::AID-DIA596>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Park HK, Son SP, Lee CW, Kim IJ, Kim HJ. Effects of oral magnesium supplementation on insulin sensitivity and blood pressure in normo-magnesemic nondiabetic overweight Korean adults. Nutr Metab Cardiovasc Dis. 2009;19:781–8. doi: 10.1016/j.numecd.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Puig Ribera A, Peña Chimenis O, Romaguera Bosch M, Duran Bellido E, Heras Tebar A, Solà Gonfaus M, et al. How to identify physical inactivity in Primary Care: Validation of the Catalan and Spanish versions of 2 short questionnaires. Aten Primaria. 2012;44:485–93. doi: 10.1016/j.aprim.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergmeyer HU, Hørder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 3. IFCC method for alanine aminotransferase (L-alanine: 2-oxoglutarate aminotransferase, EC. 2.6.1.2) J Clin Chem Clin Biochem. 1986;24:481–95. [PubMed] [Google Scholar]
- 14.Bergmeyer HU, Hørder M, Rej R. International Federation of Clinical Chemistry (IFCC) Scientific Committee, Analytical Section: Approved recommendation (1985) on IFCC methods for the measurement of catalytic concentration of enzymes. Part 2. IFCC method for aspartate aminotransferase (L-aspartate: 2-oxoglutarate aminotransferase, EC. 2.6.1.1) J Clin Chem Clin Biochem. 1986;24:497–510. [PubMed] [Google Scholar]
- 15.Recommendations of the German Society for Clinical Chemistry. Standardization of methods for the determination of enzyme activities in biological fluids. Z Klin Chem Klin Biochem. 1970;8:658–60. [PubMed] [Google Scholar]
- 16.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 17.Yokoyama H, Emoto M, Fujiwara S, Motoyama K, Morioka T, Komatsu M, et al. Quantitative insulin sensitivity check index and the reciprocal index of homeostasis model assessment in normal range weight and moderately obese type 2 diabetic patients. Diabetes Care. 2003;26:2426–32. doi: 10.2337/diacare.26.8.2426. [DOI] [PubMed] [Google Scholar]
- 18.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa L, et al. Role of magnesium in insulin action, diabetes and cardio-metabolic syndrome X. Mol Aspects Med. 2003;24:39–52. doi: 10.1016/s0098-2997(02)00090-0. [DOI] [PubMed] [Google Scholar]
- 19.Lima Mde L, Cruz T, Rodrigues LE, Bomfim O, Melo J, Correia R, et al. Serum and intracellular magnesium deficiency in patients with metabolic syndrome-evidences for its relation to insulin resistance. Diabetes Res Clin Pract. 2009;83:257–62. doi: 10.1016/j.diabres.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 20.Guerrero-Romero F, Rodríguez-Morán M. Hypomagnesemia, oxidative stress, inflammation, and metabolic syndrome. Diabetes Metab Res Rev. 2006;22:471–6. doi: 10.1002/dmrr.644. [DOI] [PubMed] [Google Scholar]
- 21.Guerrero-Romero F, Rodríguez-Morán M. Complementary therapies for diabetes: The case for chromium, magnesium, and antioxidants. Arch Med Res. 2005;36:250–7. doi: 10.1016/j.arcmed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, He K, Levitan EB, Manson JE, Liu S. Effects of oral magnesium supplementation on glycaemic control in Type 2 diabetes: A meta-analysis of randomized double-blind controlled trials. Diabet Med. 2006;23:1050–6. doi: 10.1111/j.1464-5491.2006.01852.x. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Hernandez H, Cervantes-Huerta M, Rodriguez-Moran M, Guerrero-Romero F. Decrease of aminotransferase levels in obese women is related to body weight reduction, irrespective of type of diet. Ann Hepatol. 2011;10:486–92. [PubMed] [Google Scholar]
- 24.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong VW, Wong GL, Choi PC, Chan AW, Li MK, Chan HY, et al. Disease progression of non-alcoholic fatty liver disease: A prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969–74. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 26.Elias MC, Parise ER, de Carvalho L, Szejnfeld D, Netto JP. Effect of 6-month nutritional intervention on non-alcoholic fatty liver disease. Nutrition. 2010;26:1094–9. doi: 10.1016/j.nut.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Park HS, Kim MW, Shin ES. Effect of weight control on hepatic abnormalities in obese patients with fatty liver. J Korean Med Sci. 1995;10:414–21. doi: 10.3346/jkms.1995.10.6.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hickman IJ, Jonsson JR, Prins JB, Ash S, Purdie DM, Clouston AD, et al. Modest weight loss and physical activity in overweight patients with chronic liver disease results in sustained improvements in alanine aminotransferase, fasting insulin, and quality of life. Gut. 2004;53:413–9. doi: 10.1136/gut.2003.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freedland ES. Role of a critical visceral adipose tissue threshold (CVATT) in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr Metab. 2004;1:12. doi: 10.1186/1743-7075-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer M, Schaffner F. Effect of weight reduction on hepatic abnormalities in overweight patients. Gastroenterology. 1990;99:1408–13. doi: 10.1016/0016-5085(90)91169-7. [DOI] [PubMed] [Google Scholar]
- 31.Poikolainen K, Alho H. Magnesium treatment in alcoholics: A randomized clinical trial. Subst Abuse Treat Prev Policy. 2008;3:1. doi: 10.1186/1747-597X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gullestad L, Dolva LO, Søyland E, Manger AT, Falch D, Kjekshus J. Oral magnesium supplementation improves metabolic variables and muscle strength in alcoholics. Alcohol Clin Exp Res. 1992;16:986–90. doi: 10.1111/j.1530-0277.1992.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 33.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]


