Abstract
Background:
Androgen deprivation is the basis of treatment for advanced stages of prostate cancer. Cardiovascular disease may be a risk factor for mortality in prostate cancer. Therefore, we decided to evaluate the effect of androgen deprivation therapy (ADT) on the cardiovascular risk factors in patients with prostate cancer.
Materials and Methods:
In a cross-sectional study on 2011, 35 patients suffering from metastatic prostate cancer as candidates for ADT were enrolled. Serum levels of fasting blood sugar (FBS), triglyceride (TG) and total cholesterol (TC) were measured at the beginning and after the 5th month of ADT.
Results:
The mean level of TG increased significantly from 130.82 ± 41.57 mg/dl to 150.05 ± 48.29 mg/dl (P < 0.012). Furthermore, serum level of TC increased from 197.62 ± 40.71 mg/dl to 212.54 ± 38.25 mg/dl, which is statistically significant (P < 0.001). A non-significant increase in the serum level of FBS from 96.74 ± 14.04 mg/dl to 99.17 ± 15.23 mg/dl was also seen (P = 0.27).
Conclusion:
ADT in prostate cancer may lead to an increase in TG and TC levels. In patients with a high risk of cardiovascular disease patient's lipid profile should be considered during ADT.
Keywords: Androgen antagonist, blood glucose, cholesterol, prostatic neoplasms, triglycerides
INTRODUCTION
Prostate cancer is one of the most prevalent cancers in male and the second most common cause of death due to cancer among males in the United States of America.[1,2] Men with a family history of prostate cancer are at increased risk of this disease.[3] About 86% of men who have prostate cancer are diagnosed in the localized stage. At 5 years survival of these patients is approximately 100% and 98.8% regardless of disease stage.[4,5] A great deal of mortality among these patients is not due to malignancy. Recent studies indicate that mortality from the malignancy itself is lesser than 40% in prostate cancer.[6]
Androgen deprivation therapy (ADT) is the basis of treatment for early and advanced stages of prostate cancer.[7,8,9,10] ADT in advanced localized prostate cancer increased the survival rate.[10,11]
ADT inhibits the growth of prostate tumors. In addition, it has some side-effects due to testosterone deprivation, including vasomotor symptoms, osteoporosis, anemia, gynecomastia and sexual dysfunction.[12,13] Affecting the metabolism and endocrine function of patients with prostate cancer, ADT can lead to metabolic syndrome.[14] Since much prostate cancer mortality is due to causes other than malignancy and also increased use of ADT for its boost to survival rates, we decided to evaluate the effect of ADT on cardiovascular risk factors in prostate cancer.
MATERIALS AND METHODS
This cross-sectional study was performed in 2011 in Seyyed-Al-Shohada hospital, Isfahan, Iran. All patients with documented metastatic prostate cancer[10] without documented cardiovascular diseases, referred for ADT to Seyyed-Al-Shohada Hospital, were included in the study. The patients were excluded from our study if they didn’t follow the treatment. This project was approved by the Ethical Committee of Isfahan University of Medical Sciences (project number 290145).
After obtaining written consent from the patients, 5 cc of fasting venous blood sample was taken from each to evaluate the serum levels of total cholesterol (TC), triglyceride (TG) and fasting blood sugar (FBS) at the beginning and after 5 months of therapy. All patients received intramuscularly 3.75 mg diphereline (PharmaSwiss SA, Albania) monthly. Patients were educated for the diet considering possible effects on lipid profile changes. They were also encouraged for regular walking as well.
Data was analyzed using the Statistical Package for the Social Sciences statistics version 20 software. SPSS 20 (SPSS Inc., Chicago, IL, USA) Paired t-tests were used for analysis. Data was considered to be significant at the level of P < 0.05.
RESULT
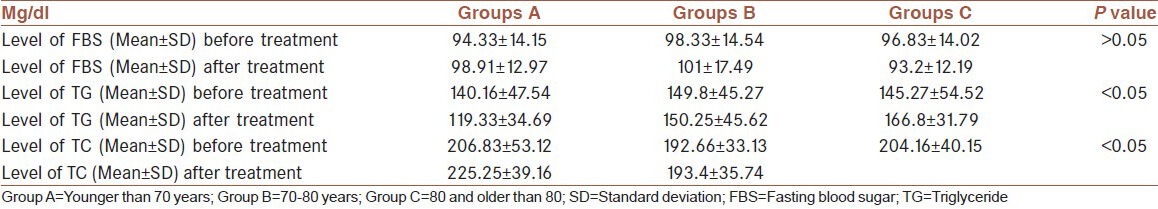
A total of 35 male patients suffering from metastatic prostate cancer (Stage 4) were enrolled in this study. The mean ± standard deviation (SD) of patient age was 71.77 ± 6.84 with a range of 60-88 years old. At the beginning of the study, the mean ± SD serum levels of FBS, TG and TC were 96.74 ± 14.04 mg/dl (normal range: 65-100), 130.82 ± 41.57 mg/dl (normal range < 200) and 197.62 ± 40.71 (normal range < 200) mg/dl respectively. The mean level of TG and cholesterol was increased significantly after 5 month follow-up [Figure 1]. The patients’ ages’ were categorized into three levels. Group A (n = 12) were males under 70 years old, Group B (n = 18) were males between 70 and 80 and Group C (n = 5) were males 80 and older. Level of FBS, TC and TG were compared based on the patients’ age range. In patient older than 80 years old, we don’t find any significant difference in FBS, TC and TG between the beginning and end of the study [Table 1]. No mortality was observed during the 5 months follow-up.
Figure 1.
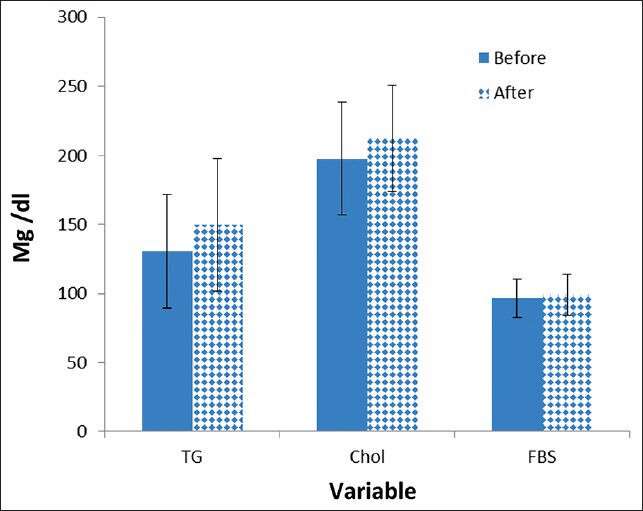
Mean of triglyceride, cholesterol and fasting blood sugar before and after intervention. TG = Triglyceride; Chol = Cholesterol; FBS = Fasting blood sugar
Table 1.
Comparison the level of FBS, TG and serum total cholesterol before and after treatment based on age's group
DISCUSSION
The result of our study indicates that the FBS level does not change significantly during the 20 weeks of treatment. Other studies in this field indicate that in patients with prostate cancer who are undertaking ADT, fasting plasma insulin levels increase.[15,16,17] Other studies have shown that ADT may reduce insulin sensitivity.[16,18] Therefore, it is expected that fasting plasma glucose levels increase during treatment. Smith et al. have shown that hemoglobin A1c levels increase in ADT; however, FBS is not affected by this kind of therapy.[19] Smith's findings are in agreement with our results.
In our study results, serum levels of TG and TC increased significantly during the 5 months of ADT, which is completely in agreement with other studies.[20,16,17,18]
Our findings showed that cholesterol levels in patients under 70 years of age (Group A) increased the most. Furthermore, blood TG levels in patients between 70 and 80 (Group B) increased significantly (P < 0.05). However, none of the parameters of metabolic syndrome in patients over 80 were subject to significant increase (P > 0.05). It is possible that in men who ages over 80 years old, the level of androgen are low enough that ADT doesn’t have any effect on level of androgen.
Aging reduces testicular function and thus may reduce plasma testosterone.[21] Some studies indicate that there is a significant correlation between serum testosterone levels and insulin sensitivity and that insulin injection for males suffering from hypogonadism, could maintain insulin sensitivity[22,23] and decrease insulin sensitivity over the time lead to impairing glucose tolerance.
CONCLUSION
ADT in prostate cancer may lead to an increase in TG and TC levels. In patients with a high risk of cardiovascular disease patient's lipid profile should be considered during ADT. We suggest performing another study with a larger sample size, to compare adverse effects of ADT based on patients’ age ranges.
Limitations of the study are (1) although the patients were educated for diet and exercise, the uncertainly is still present. (2) The small changes in lipid profile may be due to small sample size.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Saylor PJ, Smith MR. J Urol. 2009 May;181(5):1998–2006. doi: 10.1016/j.juro.2009.01.047. discussion 2007-8. Epub 2009 Mar 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faris JE, Smith MR. Metabolic sequelae associated with androgen deprivation therapy for prostate cancer. Curr Opin Endocrinol Diabetes Obes. 2010;17:240–6. doi: 10.1097/MED.0b013e3283391fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sprenkle PC, Fisch H. Pathologic effects of testosterone deprivation. Curr Opin Urol. 2007;17:424–30. doi: 10.1097/MOU.0b013e3282f0ebef. [DOI] [PubMed] [Google Scholar]
- 4.Leahy Y. Risk of metabolic syndrome, cardiovascular disease, and diabetes in androgen deprivation therapy. Clin J Oncol Nurs. 2008;12:771–6. doi: 10.1188/08.CJON.771-776. [DOI] [PubMed] [Google Scholar]
- 5.Saylor PJ, Smith MR. Metabolic complications of androgen deprivation therapy for prostate cancer. J Urol. 2009;181:1998–2006. doi: 10.1016/j.juro.2009.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MR, Lee H, McGovern F, Fallon MA, Goode M, Zietman AL, et al. Metabolic changes during gonadotropin-releasing hormone agonist therapy for prostate cancer: Differences from the classic metabolic syndrome. Cancer. 2008;112:2188–94. doi: 10.1002/cncr.23440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–9. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–24. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 9.Barry MJ, Delorenzo MA, Walker-Corkery ES, Lucas FL, Wennberg DC. The rising prevalence of androgen deprivation among older American men since the advent of prostate-specific antigen testing: A population-based cohort study. BJU Int. 2006;98:973–8. doi: 10.1111/j.1464-410X.2006.06416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Amico AV, Manola J, Loffredo M, Renshaw AA, DellaCroce A, Kantoff PW. 6-month androgen suppression plus radiation therapy vs radiation therapy alone for patients with clinically localized prostate cancer: A randomized controlled trial. JAMA. 2004;292:821–7. doi: 10.1001/jama.292.7.821. [DOI] [PubMed] [Google Scholar]
- 11.Roach M, 3rd, Bae K, Speight J, Wolkov HB, Rubin P, Lee RJ, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: Long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–91. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama T, Kanazawa S, Watanabe R, Terunuma M, Takahashi K. Influence of hot flashes on quality of life in patients with prostate cancer treated with androgen deprivation therapy. Int J Urol. 2004;11:735–41. doi: 10.1111/j.1442-2042.2004.00896.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharifi N, Gulley JL, Dahut WL. Androgen deprivation therapy for prostate cancer. JAMA. 2005;294:238–44. doi: 10.1001/jama.294.2.238. [DOI] [PubMed] [Google Scholar]
- 14.Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–7. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 15.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–8. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 16.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 17.Smith MR, Lee H, Fallon MA, Nathan DM. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–22. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eri LM, Urdal P, Bechensteen AG. Effects of the luteinizing hormone-releasing hormone agonist leuprolide on lipoproteins, fibrinogen and plasminogen activator inhibitor in patients with benign prostatic hyperplasia. J Urol. 1995;154:100–4. [PubMed] [Google Scholar]
- 19.Smith MR, Finkelstein JS, McGovern FJ, Zietman AL, Fallon MA, Schoenfeld DA, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 20.Vermeulen A. Ageing, hormones, body composition, metabolic effects. World J Urol. 2002;20:23–7. doi: 10.1007/s00345-002-0257-4. [DOI] [PubMed] [Google Scholar]
- 21.Pitteloud N, Mootha VK, Dwyer AA, Hardin M, Lee H, Eriksson KF, et al. Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care. 2005;28:1636–42. doi: 10.2337/diacare.28.7.1636. [DOI] [PubMed] [Google Scholar]
- 22.Traish AM, Saad F, Guay A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J Androl. 2009;30:23–32. doi: 10.2164/jandrol.108.005751. [DOI] [PubMed] [Google Scholar]
- 23.Medlinsky JT, Napier CD, Gurney CW. The use of an antiandrogen to further investigate the erythropoietic effects of androgens. J Lab Clin Med. 1969;74:85–92. [PubMed] [Google Scholar]


