Abstract
Introduction:
Filarial chyluria is a common problem in filarial endemic countries. Its management begins with medical therapy but some patients progress to require surgery. The present study aimed to determine factors affecting response to medical management in patients of filarial chyluria.
Materials and Methods:
This prospective study conducted between August 2008 and November 2012, included conservatively managed patients of chyluria. Demographic profile, clinical presentation, treatment history and urinary triglycerides (TGs) and cholesterol levels at baseline were compared between the responders and non-responders. Apart from the clinical grade of chyluria, hematuria was evaluated as an independent risk factor.
Results:
Out of the 222 patients (mean age, 37.99 ± 13.29 years, 129 males), 31 patients failed to respond while 35 had a recurrence after initial response; the overall success rate being 70.3% at a mean follow-up of 25 months. No difference was observed in demographics, clinical presentation, presence of hematuria, disease duration and mean urinary TGs loss between responders and non-responders. On multivariate analysis, patients with treatment failure were found to have a higher-grade disease (14.3% Grade-I, 36.6% Grades-II and 60% Grade-III), higher number of pretreatment courses (1.59 ± 1.08 vs. 1.02 ± 0.79) and heavier cholesterol (26.54 ± 23.46 vs. 8.81 ± 8.55 mg/dl) loss at baseline compared with responders (P < 0.05).
Conclusion:
Conservative management has a success rate in excess of 70%, not affected by the disease chronicity, previous episodes and recurrent nature. However, higher-grade disease, extensive pre-treatment with drugs and higher urinary cholesterol loss at baseline are the predictors of poor response. Hematuria is not an independent poor risk factor for conservative management.
Keywords: Cholesterol, chyluria, filariasis, triglycerides
INTRODUCTION
Parasitic or primary chyluria caused by Wuchereria bancrofti and other nematodes, is fairly common in Asia, Africa and parts of Central and South America where infections with these nematodes are endemic.[1,2,3] It continues to be a significant health problem, particularly in the rural and economically weaker populations.
Chyluria has a highly unpredictable clinical course, characterized by multiple recurrences and relapses.[4] Although it may resolve spontaneously with bed rest and/or use of abdominal binders that increase intra-abdominal pressure to stop lymph leakage, medical management is the usual initial treatment offered to all patients.[2] It consists of a fat-restricted, high-protein diet with the addition of fats containing medium chain triglycerides (TGs), anti-filarial drugs and high fluid intake.[2,5,6,7] However, a major limitation with medical management is limited response and frequent relapses after successful initial treatment. To the best of our knowledge, no study has attempted to determine the factors predictive of outcome of medical management in these patients. In this prospective study, we have analyzed various demographic, clinical and biochemical parameters to figure out the factors affecting response to medical management of chyluria.
MATERIALS AND METHODS
A prospective longitudinal study was conducted between August 2008 and December 2012 at our tertiary-care referral institute in India which has endemic filariasis. Ethical clearance was obtained from the Institutional Review Board and a written informed consent was signed by all patients or their guardians (in case of minors). Patients with primary or recurrent chyluria of filarial origin, presenting with the complaint of chyluria, hematochyluria or passage of chyle clots, were included in the study. Patients below 15 years of age, non-chyluric whitish-cloudy urine, non-parasitic chyluria, any malignancy, pregnancy, medical renal disease and uncontrolled diabetics were excluded from the study. A detailed history of the present illness was noted, including presenting symptoms, onset, duration, severity, aggravating/relieving factors and any seasonal variation. In patients with recurrent chyluria, detailed past history was also noted regarding the number and duration of previous attacks, treatment taken and response to treatment. Baseline evaluation included routine blood investigations including complete hemogram, renal function test, blood sugar, serum albumin levels and ultrasound abdomen to rule out any other pathology. Urinary investigations included routine urinalysis and culture, urine examination for chyle (gross assessment, ether dissolution test followed by microscopy for fat globules and lymphocytes), and quantitative analysis of urinary TGs and cholesterol levels using the biochemical auto-analyzer. TGs and cholesterol in urine samples were determined by an enzymatic kit using modified Trinder color reaction to produce a fast, linear and end point reaction.[8] The intensity of the color produced was directly proportional to the concentration of lipids, when measured at 500 nm in a random access analyzer (Selectra 2012). Urinary tests were performed using morning post-prandial samples, collected within 1-2 h of breakfast. Serial dilutions were made for high-lipid content samples with milky appearance, as the micelles formed over the top of the urine samples may interfere with the accuracy of the autoanalyzer in case of undiluted samples.[3]
The severity of chyluria was graded according to the prevalent clinical grading system,[9,10,11] incorporating slight modifications as per the severity of hematuria as mentioned below:
Grade-I: Single episode or ≤ 1-episode/year of hematuria of < 24 h duration, not associated with the passage of blood clots or anemia
Grade-II: Single episode or ≤ 1-episode/year of hematuria of ≥ 24 h duration, not associated with the passage of blood clots or anemia
Grade-III: ≥2 episodes/year or hematuria associated with the passage of blood clots or anemia.
All patients were initially managed with anti-filarial drugs, supportive medicines such as hematinics, bed-rest and dietary modifications. Anti-filarial drug regimen included two courses of diethylcarbamazine (DEC) at a dose of 6 mg/kg/day (three divided doses) for 3-weeks each, with 15-days interval between two courses. Prescribed dietary modifications included high protein diet, green-leafy vegetables, restricted fat intake (25-50 g/day) with the inclusion of fats containing medium-chain triglycerides (MCTs) and high fluid intake. A diet chart mentioning general guidelines for dietary modification was given to all patients as prescribed in previous studies.[5] However, no specific recommendations regarding precise amounts or particular foods items were given. Compliance with the dietary recommendations was ensured at each follow-up visit, by personal interview with a dedicated dietician.
Patients were initially followed at 3-weeks followed by 3-monthly visits thereafter. In case of symptoms relapse, patients were advised to visit immediately. Follow-up evaluation included history, examination and urinary investigations for the presence of chyle. Quantitative analysis of TGs and cholesterol levels in urine was performed at each follow-up visit. Successful treatment was defined as complete resolution of symptoms with stable remission until last follow-up. Patients not responding to prescribed regimen of two courses of DEC along with appropriate dietary and supportive measures or those presenting with recurrent symptoms after initial response were regarded as failures and were compared with the responders to determine factors predictive of response to conservative management of chyluria.
Data was entered in SPSS (Statistical Product and Service Solutions) version-15 statistical software package (International Business Machines Corporation, Chicago, USA). Continuous variables were expressed as mean ± standard deviation and discrete variables were expressed as proportions. Continuous variables were compared using independent t-test or Mann-Whitney U test, wherever applicable. Discrete variables were compared using Chi-square test or Fischer's exact t-test. The multivariate logistic regression analysis was used to find out the adjusted odds ratios for the outcome and P < 0.05 was considered as statistically significant.
RESULTS
222 patients fulfilled the study criteria, were medically managed and had complete follow-up details. Table 1 describes the demographic profile and clinical presentation of all patients.
Table 1.
Patient characteristics
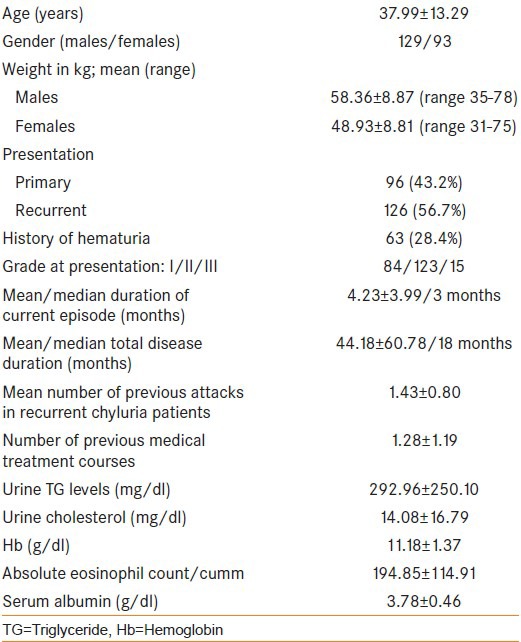
Out these 222 patients managed conservatively, 31 failed to respond while another 35 had a recurrence of symptoms after initial response. Hence, overall success rate for medical management was 70.3% (156/222) at a mean follow-up of 25 months. No treatment related adverse effects requiring discontinuation of treatment was noted in any of the patients. When 66 patients who failed medical management were compared with 156 successfully treated patients, no difference was observed in any of the demographic parameters, clinical presentation, total disease duration and duration of current episode [Table 2]. However, patients who failed were found to have a higher-grade disease, higher number of previous attacks and extensive pretreatment in the past (P < 0.05) as compared with responders.
Table 2.
Demography and clinical profile as predictors of response to conservative management of chyluria
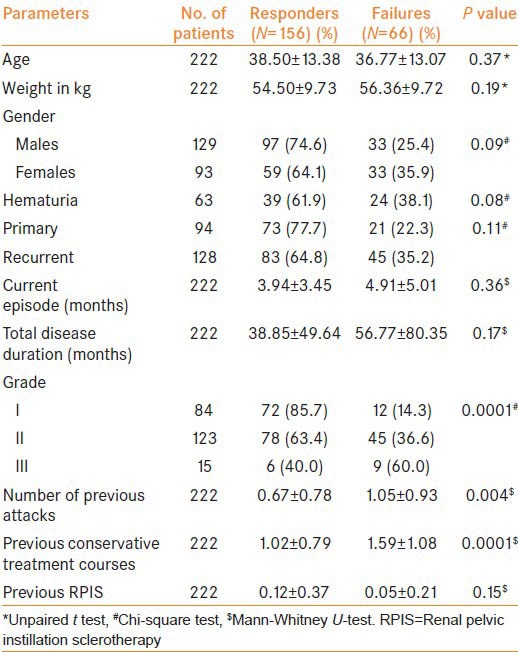
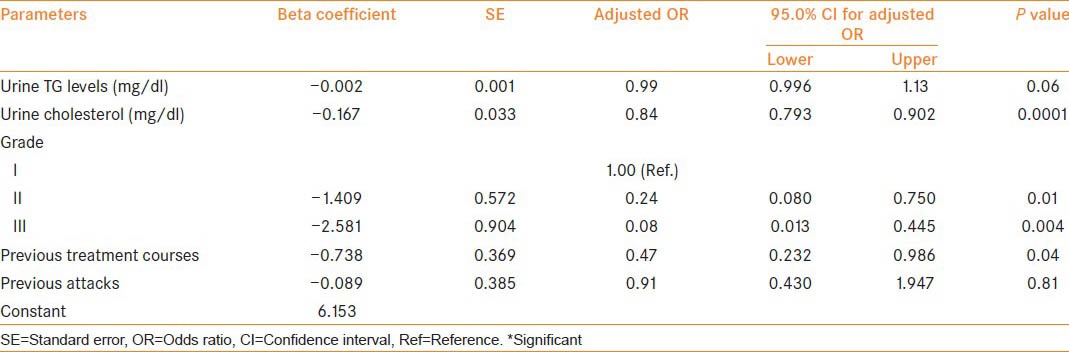
On comparison of pre-treatment urinary biochemical parameters, patients with treatment failure had significantly higher TGs and cholesterol loss as compared to responders [Table 3]. On multivariate logistic regression analysis, Grade-II/III disease (P = 0.01 and 0.004 respectively), higher urine cholesterol at baseline (P = 0.0001) and extensive prior treatment (P = 0.04) were found to be independent factors affecting the response to medical management [Table 4].
Table 3.
Biochemical parameters as predictors of response to conservative management
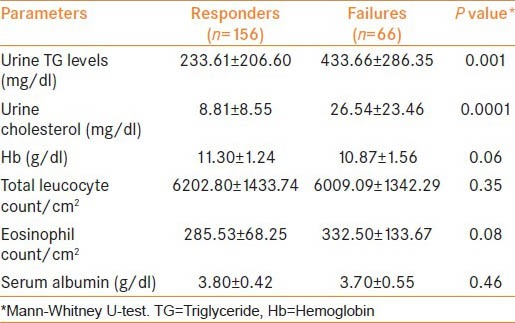
Table 4.
Associated factors with the outcome (response/failure): Multivariate logistic regression analysis
DISCUSSION
Chyluria is a very ancient disease with its mention in the literature as old as Hippocrates.[2] Although the exact pathogenesis of chyluria has been a subject of speculation, different hypotheses have been given from time to time. However, the role of diet on the pathogenesis and manifestation of chyluria is universally acknowledged. Hence dietary modifications with avoidance of fat intake, which is absorbed through the lymphatic system, is the cornerstone of any non-invasive treatment protocol for this disease. Medical management with anti-filarial drugs, bed-rest and dietary fat restriction is usually the first modality offered for treatment of filarial chyluria in patients with mild symptoms.[6,12,13] However, reported initial success rates for medical management have been highly variable associated with high relapse rates after successful initial treatment. The treatment of chyluria is also complicated by the unpredictability of the natural history of the disease with varying periods of spontaneous remission and variable response to different treatment modalities. Yu et al.,[14] studied 161 patients of chyluria, observing that 119 responded well to conservative treatment and only 42 patients required operative intervention over a period of 10-year with an overall success rate for medical management being 73.9%. Ohyama et al.[12] observed a cure rate of 61% for conservative management of chyluria using dietary modifications and anti-filarial drugs. Our study shows comparable results with overall success rate of 70.3% at mean follow-up of 25-months. Tandon et al.[15] have also reported similar results with a success rate of 62% at a mean follow-up of 2.2 years. On the contrary, Okamoto observed a cure rate of only 15% and partial improvement in 23% for conservatively treated patients of chyluria.[16] Apart from the variable results noted for conservative management, maintaining a fat free diet (an integral part of conservative management) remains a tough task.[15] While on fat-free diet, chylomicrons are no longer produced by the enterocytes and chyluria disappears. However, this may lead to severe malnutrition, weakness and weight loss. Hashim et al. advocated substitution of dietary fats with MCTs.[6] Instead of normal lipids, which are assembled in chylomicrons and delivered to thoracic duct, MCTs are rapidly absorbed from the gut and directly transported to the liver via the portal vein, bypassing the lymphatic circulation. However, most of the MCT oils are not very palatable, apart from being expensive.[15] Thus, poor compliance with dietary modifications remains an important factor responsible for failed medical management.
Studies have utilized clinical grading and duration of chyluria for therapeutic decision-making with mild to moderate severity patients being treated medically while patients with severe or prolonged chyluria being managed surgically.[2,9,15,17] Surgery has been proven to be highly effective with reported success rates in excess of 90%, in alleviating even the most severe disease. However, none of the published studies have discussed the basis for failure of conservative management in higher-grade disease patients. On extensive literature search, we could not find any study on the factors affecting the outcome of medical management chyluria.
In this prospective study, we have systematically analyzed all the demographic, clinical and biochemical parameters to find-out possible factors responsible for failure of medical management. No difference was observed in mean age, weight at presentation, gender and chronicity of disease being it primary or recurrent disease. Similarly, no difference was observed in the mean “duration of current episode” as well as “total disease duration” that were separately noted for recurrent disease patients. Disease severity was graded according to the prevalent clinical grading system, with some modification. Majority of the published studies have classified hematochyluria of any magnitude as Grade-III disease and advised surgical treatment for these patients.[9,10,11] However, from our experience of chyluria management, we hypothesize that occasional mild hematuria does not adversely affect the outcome of medical management. Therefore, mere presence of hematuria was not used to decide the disease as Grade-III in the current study. In patients with occasional/mild hematuria, not associated with blood clots or anemia, disease severity was graded depending upon other clinical features. Hematuria was evaluated as a separate risk factor also. On statistical analysis, hematuria itself was not a poor risk factor while higher clinical grade of disease was associated with poor response to medical management in both univariate and multivariate analysis. According to the prevalent grading system, Grade-III disease patients are offered invasive treatment in the form of renal pelvic instillation sclerotherapy or surgical pyelo-lymphatic disconnection. The present study suggests that grade-III disease responds poorly to conservative management, compared with low-grade disease, however 40% patients (n = 15) of even Grade-III disease were in stable remission after medical management. Secondly, just the presence of hematuria should not be an indication for invasive therapy as hematuria does not adversely affect the response to medical management. Chyluria does not have a predictable pattern of recurrence implying that total disease duration may not exactly represents the chronicity of disease. Therefore, number of previous attacks and treatment taken for that was also taken into account. Although no difference was observed in disease chronicity as already explained, “extensive pretreatment” in the past in the form of receiving higher number of treatment courses was determinant of poor outcome on multivariate analysis.
It is accepted that the TGs and cholesterol being lost in chyluria come from intestinal chylomicrons. Only one study has quantified the urinary lipids in patients with a history of chyluria and authors have utilized it only as a diagnostic tool for conformation of chyluria in a filarial endemic country.[3] In the current study, we have quantified the urinary lipids loss, i.e. urinary TGs and cholesterol levels at baseline. Patients who failed to respond to medical management were found to have significantly higher urinary TGs loss at baseline (433.66 ± 286.35 vs. 233.61 ± 206.60 mg/dl) compared with successfully managed patients. Similarly, patients with poor treatment response had significantly higher urinary cholesterol loss (26.54 ± 23.46 vs. 8.81 ± 8.55 mg/dl) compared with successfully managed patients. Although this difference was statistically significant for both urinary TG and cholesterol on univariate analysis, only urinary cholesterol loss was a significant parameter on multivariate analysis (P = 0.0001) [Table 4]. So, urinary cholesterol loss at baseline is a better predictor of response to medical management. No difference was observed in mean hemoglobin levels, total leucocyte count, absolute eosinophil count and serum albumin levels between the two groups.
The important limitations of the current study include short follow-up and a small number of patients to study a chronic disease like chyluria. Secondly, the number of patients in Grade-III disease was very small, which is partially explainable by the fact that the presence of hematuria alone was not used as criteria to define Grade-III disease, so many of the patients that would have been ascribed to Grade-III (according to the original system) were classified as Grade-I or II in the current study. As the amount of urinary lipids loss is dependent upon the quantity and quality of dietary fats consumed; the observed differences in urinary TGs and cholesterol levels at baseline might have been affected by the different dietary habits of patients from different cultural backgrounds. Finally, the clinical grading system and the modifications incorporated are subjective and prone to inter-observer variability.
CONCLUSION
Medical management of chyluria with dietary modifications, anti-filarial drugs, bed rest and supportive measures gives reasonable success rate in excess of 70%, which is not affected by the disease duration, chronicity and primary versus recurrent nature of the disease. Higher clinical grade at presentation and history of heavy pre-treatment with drugs are the predictors of poor response to conservative management. Higher urinary cholesterol loss at baseline is a poor-risk factor for response to medical management of chyluria. Hematuria itself does not adversely affect the response to medical management.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Ngan H, Leong CH. A lymphographic study of chyluria. Br J Radiol. 1977;50:863–70. doi: 10.1259/0007-1285-50-600-863. [DOI] [PubMed] [Google Scholar]
- 2.Hemal AK, Gupta NP. Retroperitoneoscopic lymphatic management of intractable chyluria. J Urol. 2002;167:2473–6. [PubMed] [Google Scholar]
- 3.Peng HW, Chou CF, Shiao MS, Lin E, Zheng HJ, Chen CC, et al. Urine lipids in patients with a history of filariasis. Urol Res. 1997;25:217–21. doi: 10.1007/BF00941986. [DOI] [PubMed] [Google Scholar]
- 4.Aye UT, Aung ST. Chyluria. Clin Radiol. 1975;26:237–42. doi: 10.1016/s0009-9260(75)80051-1. [DOI] [PubMed] [Google Scholar]
- 5.Ansari MS. Medical treatment of filariasis and chyluria. Indian J Urol. 2005;21:24–6. [Google Scholar]
- 6.Hashim SA, Roholt HB, Babayan VK, Vanitallie TB. Treatment of chyluria and chylothorax with medium-chain triglyceride. N Engl J Med. 1964;270:756–61. doi: 10.1056/NEJM196404092701502. [DOI] [PubMed] [Google Scholar]
- 7.Singh LK, Datta B, Dwivedi US, Singh PB. Dietary fats and chyluria. Indian J Urol. 2005;21:50–4. [Google Scholar]
- 8.Trinder P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann Clin Biochem. 1969;6:24–7. [Google Scholar]
- 9.Suri A, Kumar A. Chyluria – SGPGI experience. Indian J Urol. 2005;21:59–62. [Google Scholar]
- 10.Goel S, Mandhani A, Srivastava A, Kapoor R, Gogoi S, Kumar A, et al. Is povidone iodine an alternative to silver nitrate for renal pelvic instillation sclerotherapy in chyluria? BJU Int. 2004;94:1082–5. doi: 10.1111/j.1464-410X.2004.05108.x. [DOI] [PubMed] [Google Scholar]
- 11.Dhabalia JV, Pujari NR, Kumar V, Punia MS, Gokhale AD, Nelivigi G. Silver nitrate sclerotherapy for chyluria: Evaluation for the optimal instillation regime. Urol Int. 2010;85:56–9. doi: 10.1159/000296287. [DOI] [PubMed] [Google Scholar]
- 12.Ohyama C, Saita H, Miyasato N. Spontaneous remission of chyluria. J Urol. 1979;121:316–7. doi: 10.1016/s0022-5347(17)56767-1. [DOI] [PubMed] [Google Scholar]
- 13.Tan LB, Chiang CP, Huang CH, Chou YH, Wang CJ. Experiences in the treatment of chyluria in Taiwan. J Urol. 1990;144:710–3. doi: 10.1016/s0022-5347(17)39562-9. [DOI] [PubMed] [Google Scholar]
- 14.Yu HH, Ngan H, Leong CH. Chyluria – A 10 year follow-up. Br J Urol. 1978;50:126–33. doi: 10.1111/j.1464-410x.1978.tb03042.x. [DOI] [PubMed] [Google Scholar]
- 15.Tandon V, Singh H, Dwivedi US, Mahmood M, Singh PB. Filarial chyluria: Long-term experience of a University Hospital in India. Int J Urol. 2004;11:193–8. doi: 10.1111/j.1442-2042.2003.00761.x. [DOI] [PubMed] [Google Scholar]
- 16.Okamoto K, Ohi Y. Recent distribution and treatment of filarial chyluria in Japan. J Urol. 1983;129:64–7. doi: 10.1016/s0022-5347(17)51921-7. [DOI] [PubMed] [Google Scholar]
- 17.Dalela D, Rastogi M, Goel A, Gupta VP, Shankhwar SN. Silver nitrate sclerotherapy for ‘clinically significant’ chyluria: A prospective evaluation of duration of therapy. Urol Int. 2004;72:335–40. doi: 10.1159/000077689. [DOI] [PubMed] [Google Scholar]


