Abstract
Introduction:
Free to total prostate specific antigen ratio (f/t PSA) has been used to help improving specificity of PSA in the range of 4-10 ng/ml based on the data on population based screening. There is no data on test characteristics of f/t PSA in men presenting with clinical symptoms of benign prostatic hyperplasia (BPH). This study is aimed to determine the usefulness of f/t PSA in symptomatic men.
Methodology:
From January 2006 to June 2012, men of 50-75 years with lower urinary tract symptoms (LUTS), normal rectal examination and PSA between 4-20 ng/ml had free and total PSA assessment. Men with clinical evidence of prostatitis, retention, history of 5α blocker reductase inhibitors and those who had surgery or biopsy on the prostate in last 3 months were excluded. Receiver operating characteristic curves were derived for f/t PSA and total PSA. The effect of age, prostate volume and Gleason score on the f/t PSA was also analyzed. All statistical analyses were performed on SPSS 16 (Chicago, USA).
Results:
Out of 170 men with the mean age of 67.4 ± 6.6 years, 43 (25.3%) had cancer on biopsy. Area under the curve for predicting the presence or absence of prostate cancer in all the men with f/t ratio was 0.63 (confidence interval [CI]: 0.54-0.71). The median value of f/t PSA for men with cancer was 5.5% (1-25%) and 9.2% (1-63%) for those with no cancer. Cut-offs derived at 95% specificity at PSA between 4-10 ng/ml and 4-20 ng/ml were 0.5% and 1% respectively. The specificity of f/t PSA ratio at cut-off levels 7%, 10% and 15% was 73%, 60%, 45% for PSA range of 4-10 ng/ml and 63%, 47% and 35% for PSA range of 4-20 ng/ml PSA. Age, prostate volume and Gleason grade did not show any effect on f/t PSA.
Conclusion:
In men with LUTS the specificity of various f/t PSA ratio cut-offs; described for population based screening, is too low to be used as an aid to defer the decision of biopsy in PSA ranges of 4-20 ng/ml.
Keywords: Free to total prostate specific antigen ratio, prostate cancer, screening
INTRODUCTION
Prostate cancer screening for the population has been questioned by united states preventive service task force (USPSTF), where it has been recommended that regardless of age, men without symptoms should not routinely have their PSA tested.[1] Despite that prostate specific antigen (PSA) is being done world-wide to detect early prostate cancer.
PSA is not a cancer specific marker and its positive predictive value (PPV) to detect prostate cancer for the range of 4-10 ng/ml and normal rectal examination (RE) is around 30%.[2] With such a low PPV, 70% of men undergo unnecessary trans rectal ultrasonography (TRUS) guided biopsy to diagnose prostate cancer.[3] To reduce this percentage of unnecessary biopsy, f/t PSA has been described to improve specificity of total PSA in the PSA range of 4-10 ng/ml without affecting its sensitivity.[4]
There is a significant geographical variation in the incidence of prostate cancer with incidence rates varying by more than 25-fold world-wide with the lowest rates reported from Asian countries.[5] Therefore, PPV of PSA in the range of 4-10 ng/ml is lower in Asian countries than the west.[6] Our own data set on more than 4000 men presenting to the urology office with lower urinary tract symptoms (LUTS) has given a PPV of only 15.2% in PSA range of 4-10 ng/ml with normal RE (under publication in Indian Journal of Medical Research). In countries like India, where population based screening is not practiced; men seeking medical help for LUTS are often subjected to PSA testing.
Although data on diagnostic characteristics of free PSA in symptomatic men are not there, free PSA is often advised in men with raised PSA levels. This study has analyzed the test characteristics of free PSA and its ratio to the total PSA (f/t PSA) and its utility in screening men presenting with LUTS for benign prostatic hyperplasia (BPH).
METHODOLOGY
From January 2006 to June 2012, men with LUTS with American Urologic Association (AUA) symptom score of moderate to severe category, who had RE and PSA testing formed the cohort for this study. Men between the ages from 50 to 75 years were included for the analysis. Men with clinical evidence of prostatitis, positive urine culture, men on urethral catheter, 5 α reductase inhibitors and those who had surgery or biopsy on the prostate in last 3 months were excluded. Men with positive RE, i.e. asymmetry, induration or nodularity were excluded. Transrectal ultrasound guided 10-12 core systematic biopsy was performed in all cases. Institute ethical committee permission was sought.
Though the free PSA is recommended in general population with PSA of 4-10 ng/ml to reduce the unnecessary biopsy, here in due to low PPV of the PSA in Asians, we included men with PSA between 4-20 ng/ml.
After taking a difference of 5% of mean f/t PSA ratio between benign and malignant prostatic disease, a group sample size of 43 each was calculated to achieve 91% of power using two sided two sample t test.
Total and free Serum PSA was measured by an immunoenzymetric assay kits (DSI EIA PSA, Italy) that have minimum detectable value of <0.003 and 0.001 ng/ml from a single laboratory of the department of urology. These measurements were performed in stored serum sample of men presenting before May 2009 (N = 42). Two outcomes as cancer versus no cancer were considered. Sensitivity and specificity of f/t PSA ratio were derived on receiver operating characteristic curve for the PSA range of 4-10 ng/ml and 4-20 ng/ml. A cut-off of f/t PSA ratio was derived at 95% specificity. Specificity was also calculated at different cut-offs suggested in the literature to defer biopsy, i.e., 7%, 10% and 15%. The one-way ANOVA and Bonferroni test was used to compare free to total PSA ratio in patients with and without cancer in relation to age, prostate volume and Gleason score. P < 0.005 were considered to be significant. All statistical analyses were performed on SPSS 16 (Chicago, USA).
RESULTS
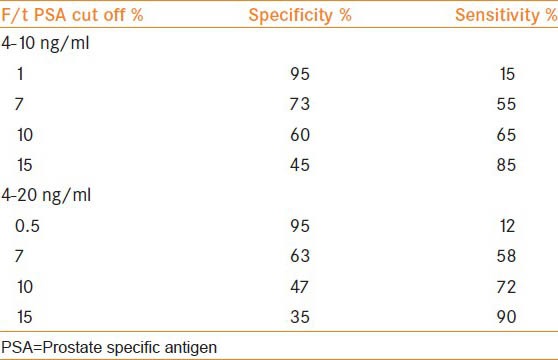
Out of 170 men with total PSA of 4-20 ng/ml and normal RE, 40 (24.7%) men had cancer on biopsy. The mean age was 67.5 ± 6.7 years. In the PSA range of 4-10 ng/ml and 10-20 ng/ml, PPV of cancer was 21.5 and 29.5% respectively. Area under the curve with f/t PSA ratio for predicting prostate cancer was 0.63 (confidence interval [CI]: 0.54-0.71) P value 0.010 in PSA levels of 4-20 ng/ml and 0.71 (CI: 0.59-0.82) P value 0.004 in the PSA range between 4 and 10 ng/ml [Figures 1 and 2]. The median value of f/t PSA for men with cancer was 5.6% (0.1-25%) and 9.4% (0.1-63%) for those with no cancer. Median values at different PSA range is given in Table 1. The cut-off of f/t PSA derived for all men at 95% specificity was 1% and 0.5% with PSA of 4-10 ng/ml and 4-20 ng/ml respectively. Cut-off of f/t PSA derived as 7%, 10% and 15% had a specificity of 73%, 60%, 45% and 63% 47% 35% respectively for PSA range of 4-10 ng/ml and 4-20 ng/ml [Table 2].
Figure 1.
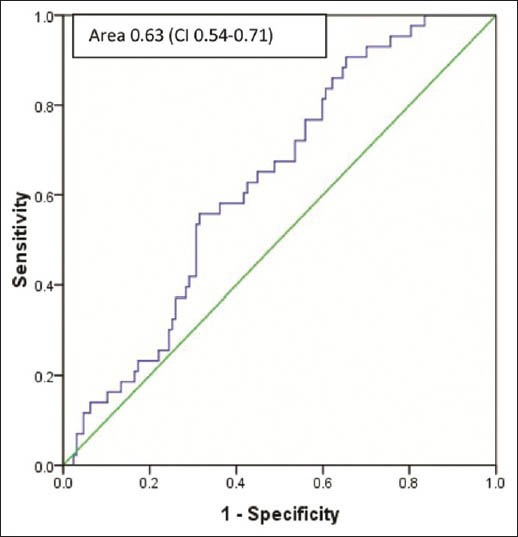
Receiver operating characteristic curve of percent f/t ratio in prostate specific antigen range of 4-20 ng/ml
Figure 2.
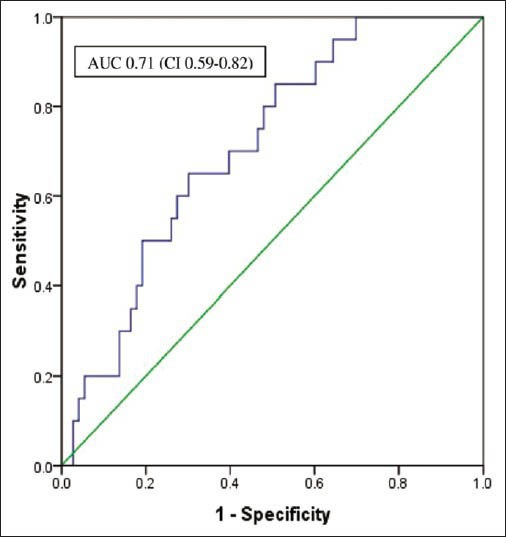
Area under the curve for f/t prostate specific antigen in men with PSA of 4-10 ng/ml
Table 1.
Median value of f/t PSA at different PSA levels
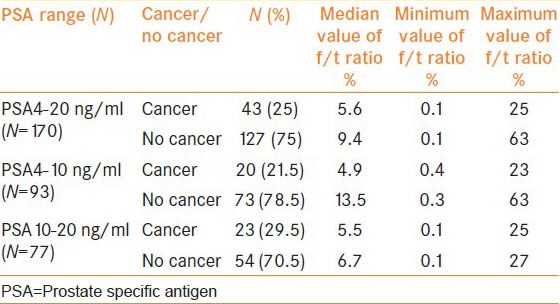
Table 2.
Sensitivity and specificity of f/t PSA at different cut off level and corresponding test characteristics of total PSA
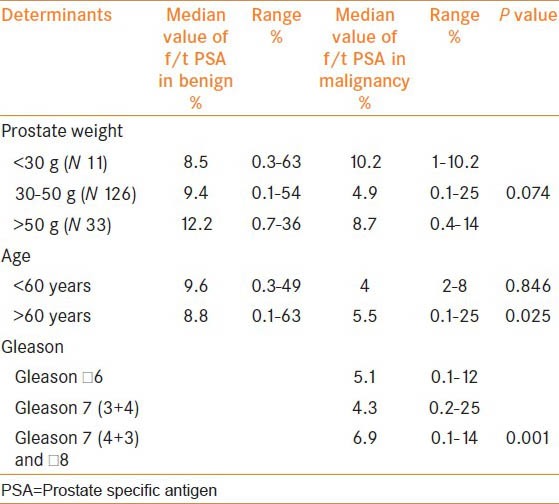
Nearly, 9% of men had prostate volume of less than 30 g, 70% with 30-50 g and 21% had volume of more than 50 g. Similarly, among the men who had cancer, 26% had Gleason of less than 6, 20% with Gleason 7 and 54% had a primary Gleason grade of four and above. The difference in the median value of f/t PSA among men with cancer or no cancer was not confounded by the age of the patients and prostate weight. Similarly, Gleason grade did not show a trend of lower values of f/t PSA ratio in relation with the increasing grades [Table 3].
Table 3.
Effect of age, prostate weight and Gleason grades on the f/t PSA levels
DISCUSSION
Following recommendation by USPSTF that regardless of age, men without symptoms should not routinely have their PSA tested, AUA has issued a new guideline on PSA screening, which recommends that shared decision-making based screening should be an appropriate option in men between 55 and 69 years of age (http://www.cancernetwork.com/prostate-cancer/content/article/10165/2143059). In India, where there is no such guideline available, most of the urologists are getting PSA done for their patients presenting with LUTS. In the western population, the probability of detecting prostate cancer, in the range of 4-10 ng/ml is around 30%, which increases to about 67% at PSA level of more than 10 ng/ml.[2] Data from our institute have demonstrated that cancer detection rate in Indians, based on screening for symptomatic men with normal RE was 15.2% and 24% for PSA between 4-10 ng/ml and 4-20 ng/ml respectively.
The low PPV, as seen in Indian men, has also been reported in another Asian country Korea, where PPV in PSA range of 4-10 ng/ml in symptomatic men was found to be 15.95, which is comparable with the rate in the present study.[6]
Low PPV of the total PSA results in an unnecessary biopsy in a significant number of patients. Total to free PSA has been described to reduce this rate of needless biopsies. Free PSA could differentiate between benign and malignant disease as it has been found to be in lower concentration in patients with carcinoma prostate.[7,8] Although prostate cancer cells do not produce more PSA than benign prostate epithelium, the PSA produced from malignant cells appears to escape proteolytic process resulting in more serum PSA complexed to Alpha chymotrypsin (ACT) and a lower percentage of total PSA that is in free form.[9]
Currently, f/t PSA ratio is approved by the Food and Drug Administration for men with normal RE and PSA within the diagnostic gray zone of 4-10 ng/ml. Data on various cut-off levels of f/t PSA for improving the specificity are mostly from population based screening and extrapolating this information for screening symptomatic men for prostate cancer would not be correct. There is no literature on the cut-off levels of f/t PSA for screening for symptomatic men.
Largest ever experience on f/t PSA is from a multi-center trial, where subjects were primarily enrolled through prostate cancer screening centers and had not received any treatment for prostate symptoms. In that trial, a cut-off to avoid biopsy was suggested as 25% in men with PSA range of 4-10 ng/ml regardless of age and prostate volume.[4]
In one meta-analysis on 19,643 patients, area under the curve for f/t PSA in the PSA range between 4-10 ng/ml, was 0.68, which would denote a weak test to discriminate between benign and diseased states.[10] To make use of f/t PSA, one would expect a cut-off with a higher specificity to avoid unnecessary biopsy. In that meta-analysis, a cut-off of 10% gave specificity of 72% and at a cutoff of 7%, specificity derived was 92%.[10] In the present series, the area under the curve in the same PSA range of 4-10 ng/ml was 0.71 (CI: 0.59-0.82). At 95% of specificity, which ideally should be the case, cut-off derived from present data was 0.5%, which is much lower than the reported cut-off from asymptomatic men.
Why would symptomatic men have such a low specificity of f/t PSA? The possible reason could be that symptomatic men have relatively higher percentage of benign prostate tissue with or without subclinical inflammation. It has been shown that PSA level (which is a denominator) in men with BPH is higher than asymptomatic men and moreover, subclinical inflammation has also been associated with lower levels of free PSA than in men without inflammation.[11] Hence, overall f/t PSA ratio is coming out to be very low.[11]
The highest probability for cancer was observed in men greater than 70 years of age who had a f/t PSA of less than 7%.[12] However, we did not find any relation of age with the f/t PSA. Similarly, f/t PSA did not show a trend of reduction in the percentage with the increasing Gleason grades.
Free PSA was measured in samples from men before 2009 from the stored sera. To contain the possibility of degradation of free PSA in stored sera, we compared the readings between samples from stored sera and the ones done in fresh samples. In both kinds of samples, a value of free PSA was lower than reported in the literature. Regarding storage of serum affecting the true value of free PSA, it has been seen that sera stored at temperatures of − 70°C did not demonstrate worse test performance than the samples analyzed prospectively.[13] In another study, it was seen that serum sample stored at − 70°C for 2-5 years, there was no significant decline in the values of free PSA.[14]
It is evident from our study that f/t PSA ratio adds modest clinical value in symptomatic men with PSA range of 4-10 ng/ml and 4-20 ng/ml. With accepted cut-offs of 7% and 10%, the specificity has been found to be too low to be taken as a parameter to defer biopsy. There are weaknesses in the present study. Men with negative initial biopsy did not have a second set of biopsy. Secondly, it is a retrospective study. Despite these weaknesses, this study opens up an interesting debate, that cellular and molecular understanding of free PSA to differentiate malignant from benign disease of the prostate gland needs further elucidation.
CONCLUSIONS
Percent f/t PSA ratio has been used in the practice to improve PPV for detecting cancer in the PSA range of 4-10 ng/ml. Most of information on f/t PSA is based on the population based screening of men for prostate cancer. This study highlights the value of f/t PSA in symptomatic men and found a very limited value to improve specificity of total PSA or in other words to reduce unnecessary biopsy in the PSA range of 4-20 ng/ml. There is a significant overlap of f/t PSA ratio in men with cancer and BPH. In places where symptomatic men are screened for prostate cancer, the use of “free PSA” should be reconsidered.
ACKNOWLEDGMENT
The authors are thankful to the funding agency Indian Council of Medical Research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Moyer VA. U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. preventive services task force recommendation statement. Ann Intern Med. 2012;157:120–34. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- 2.Cooner WH, Mosley BR, Rutherford CL, Jr, Beard JH, Pond HS, Terry WJ, et al. Prostate cancer detection in a clinical urological practice by ultrasonography, digital rectal examination and prostate specific antigen. J Urol. 1990;143:1146–52. doi: 10.1016/s0022-5347(17)40211-4. [DOI] [PubMed] [Google Scholar]
- 3.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: Results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 4.Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, Patel A, et al. Use of the percentage of free prostate-specific antigen to enhance differentiation of prostate cancer from benign prostatic disease: A prospective multicenter clinical trial. JAMA. 1998;279:1542–7. doi: 10.1001/jama.279.19.1542. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 6.Yang WJ, Lee DH, Chung BH, Cho JS, Choi YD, Kim SJ, et al. Detection rate of prostate cancer on biopsy according to serum prostate-specific antigen in Korean men: A multicenter study. Urology. 2006;67:333–6. doi: 10.1016/j.urology.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 7.Christensson A, Laurell CB, Lilja H. Enzymatic activity of prostate-specific antigen and its reactions with extracellular serine proteinase inhibitors. Eur J Biochem. 1990;194:755–63. doi: 10.1111/j.1432-1033.1990.tb19466.x. [DOI] [PubMed] [Google Scholar]
- 8.Stenman UH, Leinonen J, Alfthan H, Rannikko S, Tuhkanen K, Alfthan O. A complex between prostate-specific antigen and alpha 1-antichymotrypsin is the major form of prostate-specific antigen in serum of patients with prostatic cancer: Assay of the complex improves clinical sensitivity for cancer. Cancer Res. 1991;51:222–6. [PubMed] [Google Scholar]
- 9.Christensson A, Björk T, Nilsson O, Dahlén U, Matikainen MT, Cockett AT, et al. Serum prostate specific antigen complexed to alpha 1-antichymotrypsin as an indicator of prostate cancer. J Urol. 1993;150:100–5. doi: 10.1016/s0022-5347(17)35408-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee R, Localio AR, Armstrong K, Malkowicz SB, Schwartz JS Free PSA Study Group. A meta-analysis of the performance characteristics of the free prostate-specific antigen test. Urology. 2006;67:762–8. doi: 10.1016/j.urology.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 11.Jung K, Meyer A, Lein M, Rudolph B, Schnorr D, Loening SA. Ratio of free-to-total prostate specific antigen in serum cannot distinguish patients with prostate cancer from those with chronic inflammation of the prostate. J Urol. 1998;159:1595–8. doi: 10.1097/00005392-199805000-00050. [DOI] [PubMed] [Google Scholar]
- 12.Chen YT, Luderer AA, Thiel RP, Carlson G, Cuny CL, Soriano TF. Using proportions of free to total prostate-specific antigen, age, and total prostate-specific antigen to predict the probability of prostate cancer. Urology. 1996;47:518–24. doi: 10.1016/s0090-4295(99)80487-7. [DOI] [PubMed] [Google Scholar]
- 13.Woodrum D, York L. Two-year stability of free and total PSA in frozen serum samples. Urology. 1998;52:247–51. doi: 10.1016/s0090-4295(98)00156-3. [DOI] [PubMed] [Google Scholar]
- 14.Scaramuzzino DA, Schulte K, Mack BN, Soriano TF, Fritsche HA. Five-year stability study of free and total prostate-specific antigen concentrations in serum specimens collected and stored at-70°C or less. Int J Biol Markers. 2007;22:206–13. doi: 10.1177/172460080702200308. [DOI] [PubMed] [Google Scholar]


