Abstract
Management of ureteric strictures is a challenging task. Subtle presentation, silent progression and complex aetiology may delay diagnosis. A wide range of available treatment options combined with the lack of adequate randomised trials has led to the introduction of personal bias in the management of this difficult group of patients. Metallic ureteric stents offer an alternative to the conventional treatment modalities. A review of the currently available metallic stents and their role in the long-term management of ureteric strictures is presented. Materials used in the manufacture of indwelling urological devices are evolving all the time. Improved endo-urological techniques combined with new devices made from better compounds will continue to improve patient experience.
Keywords: Metallic stricture, stent, ureter
INTRODUCTION
Ureteric strictures are difficult to treat. The diagnosis may be delayed in the presence of two functioning renal units as one kidney may silently lose its function due to progressive asymptomatic obstruction. The late presentation may therefore result in a significant atrophy of renal parenchyma. The etiology differs between patients. A wide range of treatment options from long-term stenting to open or minimally invasive correction can be considered. The relative rarity of this condition has resulted in limited published data on its management. Most studies are single arm and retrospective, based on personal experience. Randomized trials are sparse.
Metallic stents have been considered as an alternative to long-term JJ stents in the management of ureteric strictures. A wide variety of such stents have become available during the past two decades. Their resilience to compression can intuitively lead to long-term patency compared with conventional JJ stents.[1] This may reduce the need for frequent stent changes. It may also offer an improvement in the quality of life for patients with chronic ureteric obstruction caused either by malignancy or recurrent benign conditions.[2,3]
This article provides an overview of the current literature on the role of metallic stents in the management of ureteric strictures.
Methodology
A review of the published articles on the subject of metallic stents from 1991 to 2013 has been used to assess their current place in the long-term management of ureteric strictures. Pubmed and Medline databases were searched for relevant articles published in peer review journals. Studies with an adequate number of patients and follow-up of over 12 months have been selected.
The author's personal experience with the Memokath 051 stent over the past 16 years has also been included.
Location of Strictures
Ureteric strictures can develop at different locations. While data on the location of such strictures is scarce in the world literature, in my personal experience, they are more frequent in the lower third of the ureter. However, they can be located at multiple sites, may extend over a long segment and can be bilateral. They may also develop at uretero–ileal anastomosis following cystectomy (in the conduit or following an orthotopic reconstruction), after an uretero–neo–cystostomy following implantation during renal transplantation or repair of severe ureteric trauma. Ureteric strictures can also develop following trans-uretero–ureterostomy, uretero–calycostomy, uretero–sigmoidostomy[4] and following pyeloplasty for PUJ obstruction.
Classification
Ureteric strictures can be broadly sub-divided into two groups based on the etiology – recurrent benign and malignant. This division is important as the management options may differ in each group. Issues such as quality of life, longevity, salvage treatment options and prolonged duration of follow-up would determine the choice of the treatment modality.
Etiology
Benign ureteric strictures
Benign ureteric strictures usually result from trauma or inflammatory conditions. The most frequent cause of ureteric injury is iatrogenic. The exponential increase in the number of diagnostic as well as therapeutic ureteroscopies led to an initial rise of ureteric strictures. Ureteric trauma had been reported in about 2% of procedures.[5,6] However, the incidence is falling with the advent of smaller scopes and improved training.[7] However, risk factors such as stone impaction may increase this risk.[8] This may be compounded if the procedure is prolonged or is associated with ureteric trauma.[9]
Gynecological, obstetric and colo-rectal procedures can lead to ureteric trauma, which leads to the formation of strictures.[10,11] Chronic ureteric obstruction can also be caused by retroperitoneal fibrosis, recurrent UPJ obstruction, operations on the ureter such as repairs following trauma or implantation following renal transplantation.[12]
Ureteric strictures may also be caused by other benign conditions such as endometriosis, tuberculosis[13,14] and bilharziasis.[15]
Malignant ureteric obstruction
Ureteric obstruction may occur due to malignancy, arising from the urothelium of the ureter itself or by the invasion or extrinsic compression by other malignancies arising from the bladder, prostate, colon, ovary or the endometrium.
Retroperitoneal lymphadenopathy caused by lymphomas, malignancies of the prostate, bladder and other organs can also lead to extrinsic ureteric compression.
Endorological Management of Ureteric Strictures
Initial treatment
The first step in the management of any ureteric stricture is the decompression of the obstructed kidney. This may be achieved by insertion of a conventional JJ stent or per-cutaneous nephrostomy tube.
The selection of the most appropriate method of decompression is based on the clinical condition of the patient, morbidities, severity of renal impairment, electrolyte abnormalities and presence of sepsis. It may be modified by the local protocols and availability of an interventional radiologist, severity of renal impairment and electrolyte abnormalities.
Although the published literature suggests little difference between the outcome and costs involved, the choice between insertion of a nephrostomy tube and JJ stenting is often based on clinical judgment, characteristics of obstruction and logistical factors.[16]
Definitive treatment
There is a lack of consensus regarding the definitive long-term management of ureteric strictures. The choice of treatment ranges from indwelling JJ stents, nephrostomy tube drainage, metallic stents, extra-anatomic stenting, open, laparoscopic or robotic corrections, urinary diversions or nephrectomy.
Principles of Management
The long-term management of ureteric strictures needs careful planning. This requires a full understanding of the underlying pathology that led to the stricture, longevity of the life and the impact of treatment on the quality of life. The latter is more significant in benign strictures as they recur and the negative impact on patient's quality of life due to JJ stents cannot be underestimated.
Strictures caused by malignancy need additional considerations.[17] The decision to consider the initial decompression needs to be taken with care as renal failure due to obstructive uropathy may be a terminal event in some patients with advanced malignancy. It could perhaps be a more appropriate form of palliation in a small cohort of patients.
Consideration should be given to the patient co-morbidity, performance status and the nature of the underlying malignancy and salvage therapy options. Longevity of life and patients’ own views too must to be taken into account.
Urological Considerations
It is essential to get comprehensive information about the stricture as well as the patient before planning the management of a ureteric stricture. The exact location and the length of the stricture must be delineated with the use of appropriate imaging. A combination of a conventional intravenous urogram, computed tomography or magnetic resonance imaging scans will yield valuable information. A retrograde study, which can be combined with an antegrade pyelogram in the presence of a nephrostomy, is complimentary.
Multiple strictures at different levels can pose a problem if discovered unexpectedly while undertaking a definitive procedure. The reasons behind previous (failed) attempts to correct the stricture should be studied as this may alter the choice of treatment.
Access
Access to the strictured segment needs to be planned. Although retrograde access through a cystoscope is more conventional, it may not be suitable for a small cohort of patients. Tight strictures, especially located in the upper ureter, can be difficult to negotiate from below. A proximal access through a nephrostomy tube is often useful. This will also allow the urologist to undertake the rendezvous procedure if necessary. This form of access is essential in patients with urinary diversions, especially ileal conduits. Approaching the uretero–ileal anastomosis through the ileal conduit can be exceedingly difficult. Placement of a guide wire via a nephrostomy tube down the ureter, past the stricture, into the conduit and its retrieval with the help of a cystoscope passed through the conduit helps to establish a safe access (a “skin-to-skin” guide wire). This enables the surgeon to place a suitable stent across the obstructed segment safely without the loss of access.
Choice of Stents
Conventional options
Insertion of a single or a double J stent over a guide wire placed across the obstructed segment of the ureter is expected to maintain upper tract decompression.
However, the commitment of the urologist does not end here. A complete resolution of ureteric strictures is rarely achieved by the use of a JJ stent. Recurrence is inevitable after removal of the stent, as the maturation of scar tissue will result in re-obstruction. Strictures caused by malignancy rarely resolve. The patient therefore is rendered “stent dependent.” This leads to the inevitable morbidity, which includes encrustation, irritative lower urinary tract symptoms, hematuria, urinary sepsis, migration and an overall reduction in the quality of life. Loss of earnings due to absence from work may be an important consideration.
Drainage across JJ stents is often inadequate. Extrinsic compression of a conventional poly tertra fluoro ethylene (PTFE) stent may occur in malignancy[18] and can lead to re-obstruction.
Alternative Options
The use of two JJ stents
The use of two JJ stents in the same ureter (tandem stents) has been described.[19] The concept is based on providing additional drainage with the lumen of the second stent as well as the extra space between the two stents and the ureteric wall. However, this procedure can be technically difficult and worsen the stent-related morbidity.
Metallic stents
Metallic stents have been considered as an alternative to the JJ stents for several reasons. Their strength resists the compressive force of the stricture and prevents collapse of the stent lumen. They are purported to have a longer “life span” due to their prolonged patency rates. Segmental stents are designed to not enter the bladder, and may be of additional benefit.
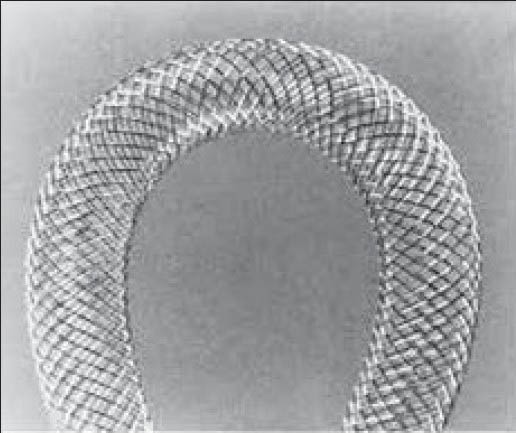
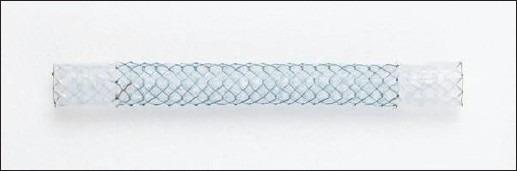
Wallstent (Wallstent, Schneider, Bulach, Switzerland)
Segmental metallic stents have been in vogue for a long time. The first such successful stent was the Wallstent[20] [Figure 1]. Made from a stainless steel wire mesh, it is inserted either in a retrograde or an antegrade manner and left across the obstructed segment. Patency rates up to 45% were achieved.[19,21] However, stent occlusion due to tumor in-growth as well as “overgrowth” was observed.[22] Removal of these stents is difficult.
Figure 1.
Wallstent
Several other metallic ureteric stents have been reported since. The currently available stents include Memokath 051,[2] Resonance,[23] Allium[24] and Uventa[25] The common feature of these stent is the metal used in the manufacture of all these devices. It is the alloy of nickel and titanium - NiTinol. NiTinol has been shown to be biocompatible. It is soft, yet strong. Therefore, the stents made from this alloy can be packed in a small shape, which is easy to introduce in the ureter. Its strength resists compression. Both these characteristics make it ideal in the design of ureteric stents.
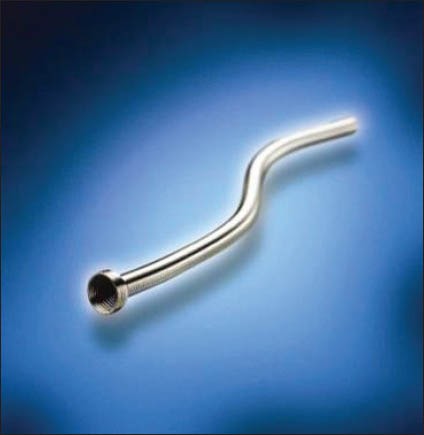
Memokath 051 (PNN [formerly Engineers and Doctors], Copenhagen, Denmark)
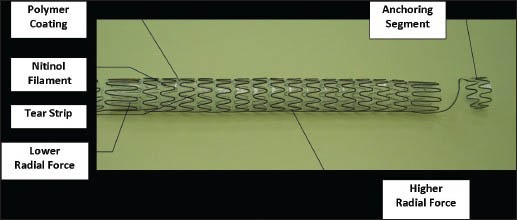
The Memokath 051 stent [Figure 2] is made from a single wire of NiTinol and has a funnel shape. Packed in a cylindrical shape, the shaft expands to 10.5 FG while its wide proximal end expands to 22 FG when exposed to 55°C. The duel-expansion variety has expanded ends on either side that reach a diameter of 22FG. It can be inserted in a retrograde manner through a cystoscope or ante-gradely through a nephrostomy tube after dilatation of the tract to 14FG. These stents are available in fixed lengths of 3, 6, 10 and 15 cm. Tailor-made longer lengths can be manufactured for individual patients.
Figure 2.
Memokath 051 stent
Allium (Allium Medical, 2 Ha’Eshel St, Caesarea Industrial Park South, 38900 Israel)
The Allium stent [Figure 3] is cylindrical in shape and has a distal coil that is retained in the bladder. It is constructed from a “ribbon,” which in itself is a sandwich of the NiTinol wire placed between two layers of PTFE. Packed in a narrow cylindrical shape, it is inserted through its dedicated delivery catheter. Once the catheter is withdrawn, the stent expands to its pre-determined shape with a diameter between 24 and 30FG. These are available in 8 and 10 cm lengths.
Figure 3.
Allium stent
Uventa (Gojeong-ro, Wolgot-myeon, Gyeonggi-do, South Korea)
The Uventa stent [Figure 4] is based on a similar concept. A NiTinol mesh design is sandwiched between two layers of PTFE sheets. Its cylindrical shape has a diameter of 7, 8 or 10 mm and is available in lengths between 6 and 12 cm. It is designed to stay in the ureter, with its distal end protruding in the bladder. This stent too is packed in a compressed form and is delivered through a dedicated sheath.
Figure 4.
Uventa stent
Resonance (Cook Medical Inc, Bloomington, IN, USA)
The Resonance stent [Figure 5] in reality is a solid JJ stent. As it has no lumen, it is delivered into the renal pelvis and the ureter through the lumen of a wide ureteric catheter. As the catheter is withdrawn, the stent coils into a JJ stent. The urine is expected to drain by the side of the stent.
Figure 5.
Resonance stent
Results with Metallic Ureteric Stents
Patency rates from 45% to 55% have been reported with the Wallstent.[1,19] However, most studies are limited by a small number of patients and a short-term follow-up.[1,19,22] Re-obstruction due to tumor in-growth has been reported, which makes additional procedures necessary.
The published literature on Resonance stent in the management of ureteric strictures suggests that it is moderately effective.[26,27,28,29,30] However, it has been shown to be ineffective in pediatric practice.[31] Tight occlusion of the ureteric lumen by malignant process, coupled with the lack of a stent lumen, is the most likely cause of failure in patients with malignant ureteric obstruction. A high incidence of migration has been reported when placed in the ileal conduits.[32]
There is limited published data on the Allium and Uventa stents, which precludes adequate comparison of these devices with other stents. Patency rates from 28% to 100% have been reported. However, longevity of this cannot be ascertained.[23,24] These studies are retrospective, short-term and single center.
The Memokath 051 stent has been in use from 1996. Patency rates from 90% to 100% have been achieved. Migration rates between 14% and 20% have been reported. An improvement in quality of life has been maintained in both benign as well as malignant ureteric strictures.[2,3,33,34]
The Memokath 051 stent appears to be effective in maintaining upper tract decompression, and offers an improved quality of life in patients with malignant ureteric obstruction. It also seems to offer a good alternative to JJ stents or open surgery in selected benign strictures.[33,34]
Author's Experience with the Memokath 051 Ureteric Stent
Between 1996 and 2013, a total of 130 stents were inserted (94 patients) [Tables 1 and 2] in the management of ureteric strictures. The majority of the strictures were in the lower ureter.
Table 1.
Experience with Memokath stents: Indications
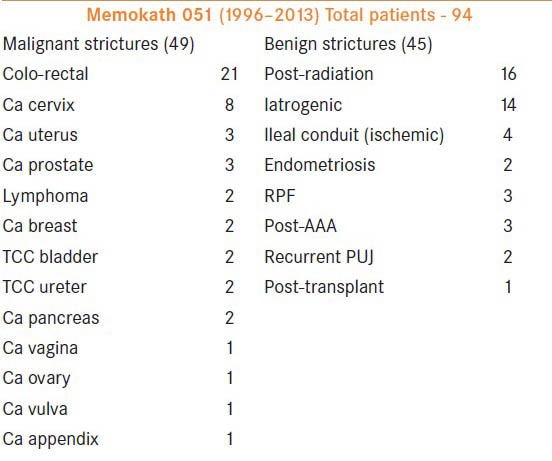
Table 2.
Memokath stent locations
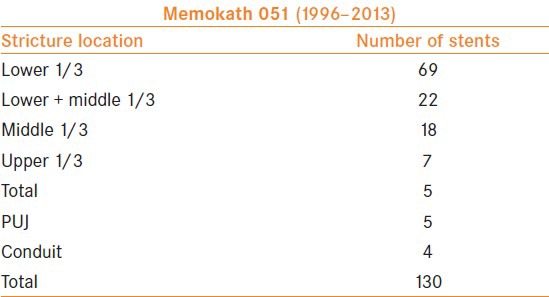
Initial satisfactory experience with malignant ureteric obstruction led to the introduction of these stents in the management of recurrent benign strictures. As shown in the tables, 49 patients underwent Memokath stent insertion for malignant and 45 for benign pathology. Forty-two males and 52 females between the ages of 12 and 90 years (median, 60.4 years) were included. The stricture criteria for inclusion in the study were failure of conventional open or endo-urological a procedures and the need for improved quality of life. In nine patients, the duel expansion Memokath stent was used.
A standard protocol was followed for the stent insertion, anesthesia, antibiotic prophylaxis and follow-up.
Pre-operative assessment including renal function tests, urine culture and cardio-respiratory functional evaluation was undertaken. All procedures were performed under general anesthesia, with the patient in a lithotomy position. Intravenous Gentamicin was injected at the beginning of the procedure. The patients were sent home with oral Amoxicillin for 5 days. With the exception of two patients who were discharged on the day of the procedure, all other patients were sent home the next day. A urethral catheter was left overnight. The imaging protocol included a plain abdominal X-ray on the day after the procedure, followed by an IVU at 6 weeks. Subsequent imaging was combined with the requirement of the oncologists and included CT or MRI scans as necessary and MAG III renography at 3 monthly intervals. A urine culture and renal function was assessed at each visit.
With a median follow-up of 18 months (range 3–69 months), all patients reported an improvement of their quality of life. This was due to the lack of lower tract symptoms, reduced need for re-admission for change of stents and an un-interrupted life away from the hospital. Migrations were noted in eight patients (8.7%) and re-insertions were necessary in 18 patients (19%) due to progression of the disease proximal or distal to the stent. Re-insertions were also necessary in five patients (5%) due to other reasons such as incorrect placement, encrustation and re-obstructions.
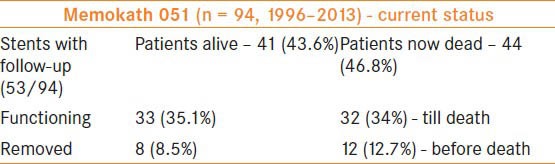
The current status of the stents inserted to date is included in Table 3. Stent migration was noted in two patients with UPJ obstruction, one patient with retro-peritoneal fibrosis, two patients with post-radiotherapy strictures and two patients with uretero–ileal anastomoses. The stent migrated in one patient with non-Hodgkin's lymphoma after the lymph nodal mass shrank following chemotherapy. He did not require further stenting.
Table 3.
Patient follow-up
Re-insertion in previously well-placed stents was necessary due to progression of malignancy in three patients with prostate cancer. The remaining patients who required re-insertions due to the progression of the tumors had colo-rectal, gynecological, breast and pancreatic carcinomas.
The development and the severity of encrustation of this stent in the author's series varied from 6 to 156 months. Table 3 shows the current results of our experience.
THE TECHNIQUE OF MEMOKATH STENT REMOVAL
The NiTinol used in the manufacture of the Memokath stents is unique. It has a thermal shape memory - it deforms and softens when cooled below 10°C and recovers its original, undeformed shape at the body temperature (which is above its “transformation temperature”).
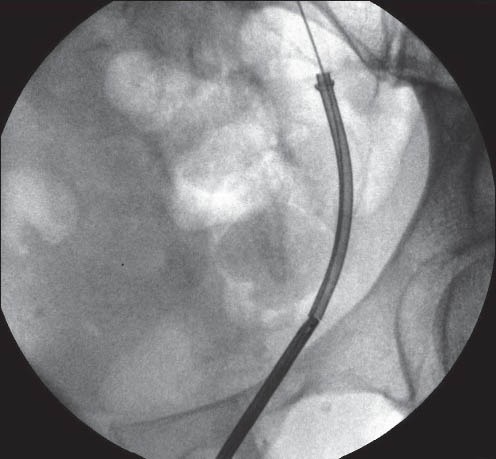
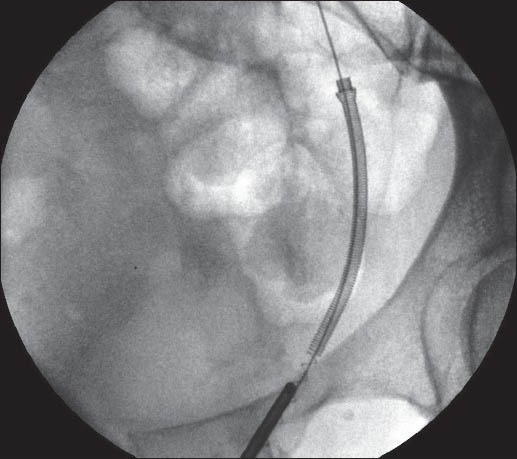
Ureteroscopy is performed with pre-cooled irrigation fluid [Figure 6]. Therefore, the irrigant fluid kept in a conventional refrigerator overnight is adequate, as it will bring the temperature down to 4°C. The lower end of the stent is held in a grasping forceps and gently withdrawn. The metal will soften and the stent coil will un-ravel [Figure 7]. The entire stent can be removed through the urethra as an uncoiled wire.
Figure 6.
Technique of removal of Memokath 051 (a)
Figure 7.

Technique of removal of Memokath 051 (b)
It is vital to maintain the temperature of the entire stent below 10°C to ensure softening. Re-warming and rigidity of the material is very rapid due to the body temperature. Hence, a ureteric catheter may need to be inserted through the lumen of the stent to its upper end to allow injection of cold saline during removal. This technique is especially important in longer versions of this stent as the upper end is likely to get warm and re-expand while withdrawing the distal end. This can lead to ureteric trauma.
The entire process of softening and hardening is very rapid and takes a few seconds.
CURRENT INDICATIONS FOR METALLIC URETERIC STENTS
Because of the paucity of published data and the lack of randomized trials, there is a degree of reluctance to consider metallic stent in the management of ureteric strictures. The other factors that limit the use of these stents include lack of availability, the cost of these devices as well as the complexity of management.
The current literature suggests that these stents are suitable for ureteric obstruction caused by malignancy. The improvement in quality of life, reduction in the need to re-admit patients for repeated stent changes and effective maintenance of upper tract decompression are some of the benefits of metallic stents. They are more cost-effective if the patient has a life expectancy of over 6 months (personal experience).
Metallic stents may be suitable as a part of palliative care in patients with advanced malignancy and a short life expectancy, as there is a significant reduction in the stent-related morbidity.
These stents also have an important place in the management of recurrent benign strictures that have failed conventional treatments. Open (or minimally invasive) reconstructive techniques too have associated morbidity, which may not be acceptable to a patient. The option of metallic ureteric stents should be considered as an alternative in this group of patients.
TIPS, TRICKS AND PERSONAL OBSERVATIONS
The techniques of metallic stent insertions are broadly similar to those applied during conventional stenting. However, one needs to be aware of a few variations and specific technical considerations during the insertion and removal of these stents. Some clinical settings require special attention.
There is a presumed reduction in the rate of encrustation associated with segmental metallic stents due to the lack of stasis. However, poor renal function with reduced glomerular filtration rate in the kidney of a stented ureter has been shown to increase the chance of encrustation (personal experience). The author prefers to avoid insertion of metallic stents if the split function is reduced below 15%.
Metallic stents are more likely to encrust in patients with concurrent or previous uro-lithiasis. Hypercalicuria is a likely cause for this phenomenon (personal observation).
Memokath 051 stents have a higher probability of migration in patients with retroperitoneal fibrosis and uretero–pelvic junction obstruction. Perhaps failure of the scar tissue to “grip” the stent effectively results in early migration. This complication group can be reduced with the use of the duel expansion Memokath 051 stent.
Ureteric strictures following cystectomy pose a difficult problem. These develop at the site of the uretero–ileal anastomosis in the conduit or the orthotopic reconstruction. The stent needs to protrude into the conduit or the neo-bladder to be effective. The incidence of encrustations and migrations of metallic stents is much higher (personal experience).
Some of these stents (Allium and Uventa) are available in fixed lengths, which precludes their use in patients with longer strictures.
Removal and re-insertion may become necessary due to migration, encrustation, tumor progression or patient intolerance. It is important to be familiar with the technique and feasibility of removal of these stents.
Limitations of Metallic Ureteric Stents
As one can expect, metallic ureteric stents are not a panacea for the treatment of ureteric strictures. They provide a useful alternative to long-term JJ stents and open or minimally invasive corrective surgery. However, complications and failures have been reported.
Metallic ureteric stents are not entirely free from migration and encrustation.
Re-obstruction can occur due to these complications. Recurrence or progression of malignancy can also necessitate revision surgery. Lack of randomized studies comparing metallic stents with conventional JJ stents or indeed with another metallic stent restricts their scientific evaluation. There is a wide variation in the cost of these devices. The benefit gained in the quality of life and reduction in the need to hospitalize the patient for repeated stent changes needs to be compared.
Indications for Metallic Ureteric Stents
The literature suggests that metallic ureteric stents are superior to conventional JJ stents in the long-term management of ureteric strictures. They provide a longer duration of upper tract decompression, reduce the need for frequent changes thus reducing the cost of health care and an improvement in the quality of life in patients with malignant ureteric strictures. They are cost-effective if the patient has a reasonable life expectancy.
They also have a place in selected recurrent benign ureteric strictures that are refractory to conventional treatments, especially in patients with severe co-morbidity or those who are unwilling to undergo reconstructive surgery.
CONCLUSION
Metallic stents offer an alternative to conventional JJ stenting in patients with recurrent benign and malignant ureteric obstruction. They also provide an alternative to corrective open or minimally invasive surgery in patients who are unfit, unwilling or unsuitable to undergo these procedures. Segmental metallic stents also lead to a genuine improvement in the patients symptoms and hence have a role to play in patients with advanced malignancy.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pauer W, Lugmayr H. Self-expanding permanent endoluminal stents in the ureter. 5 years results and critical evaluation. Urologe A. 1996;35:485–9. doi: 10.1007/s001200050057. [DOI] [PubMed] [Google Scholar]
- 2.Kulkarni R, Bellamy E. Nickel–Titanium shape memory alloy Memokath 051 ureteral stent for managing long-term ureteral obstruction: 4 year experience. J Urol. 2001;166:1750–4. [PubMed] [Google Scholar]
- 3.Maan Z, Patel D, Moraitis K, El-Husseiny T, Papatsoris AG, Buchholz NP, et al. Comparison of stent-related symptoms between conventional Double-J stents and a new-generation thermoexpandable segmental metallic stent: A validated-questionnaire-based study. J Endourol. 2010;24:589–93. doi: 10.1089/end.2009.0318. [DOI] [PubMed] [Google Scholar]
- 4.Safak M, Saglam R, Baltaci S, Adsan O, Sanlidilek U, Bedük Y. Management of ureterosigmoidal anastomotic stricture by balloon dilatation. Urol Int. 1991;47:103–4. doi: 10.1159/000282199. [DOI] [PubMed] [Google Scholar]
- 5.Al-Awadi K, Kehinde EO, Al-Hunavan A, Al-Khayat A. Iatrogenic ureteric injuries: Incidence, aetiological factors and the effect of early management on subsequent outcome. Int Urol Nephrol. 2005;37:235–41. doi: 10.1007/s11255-004-7970-4. [DOI] [PubMed] [Google Scholar]
- 6.Alapont Alacreu JM, Broseta Rico E, Pontones Moreno JL, Oliver Amorós F, Palmero Martí JL, Boronat Tormo F, et al. Complications of uretero-renoscopy. Actas Urol Esp. 2003;27:692–9. doi: 10.1016/s0210-4806(03)72998-2. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DB, Pearle MS. Complications of ureteroscopy. Urol Clin North Am. 2004;31:157–71. doi: 10.1016/S0094-0143(03)00089-2. [DOI] [PubMed] [Google Scholar]
- 8.Roberts WW, Cadeddu JA, Micali S, Kavoussi LR, Moore RG. Ureteral stricture formation after removal of impacted calculi. J Urol. 1998;159:723–6. [PubMed] [Google Scholar]
- 9.Taş S, Tuğcu V, Mutlu B, Karadağ S, Bitkin A, Yücel M, et al. Incidence of ureteral stricture after ureterorenoscopic pneumatic lithotripsy for distal ureteral calculi. Arch Ital Urol Androl. 2011;83:141–6. [PubMed] [Google Scholar]
- 10.de la Rosette, Skrekas T, Segura J. Handling and prevention of complications in stone basketing. Eur Urol. 2006;50:991–8. doi: 10.1016/j.eururo.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 11.Reyes JM, Canter DJ, Sirohi M, Simhan J, Smaldone MC, Teper EM, et al. Delayed proximal ureteric stricture formation after complex partial nephrectomy. BJU Int. 2012;109:539–43. doi: 10.1111/j.1464-410X.2011.10395.x. [DOI] [PubMed] [Google Scholar]
- 12.Pereira H, Buchler M, Brichart N, Haillot O, d’Arcier BF, Braguet R, et al. Ureteral stenosis after renal transplantation: Risk factors and impact on survival. Prog Urol. 2011;21:389–96. doi: 10.1016/j.purol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Zarrabi AD, Heyns CF. Clinical features of confirmed versus suspected urogenital tuberculosis in region with extremely high prevalence of pulmonary tuberculosis. Urology. 2009;74:41–5. doi: 10.1016/j.urology.2008.12.083. [DOI] [PubMed] [Google Scholar]
- 14.Singh JP, Priyadarshi V, Kundu AK, Vijay MK, Bera MK, Pal DK. Genito-urinary tuberculosis revisited--13 years’ experience of a single centre. Indian J Tuberc. 2013;60:15–22. [PubMed] [Google Scholar]
- 15.Badmos KB, Popoola AA, Buhari MO, Abdulkadir AY. Ureteric schistosomiasis with obstructive uropathy. J Coll Physicians Surg Pak. 2009;19:456–8. [PubMed] [Google Scholar]
- 16.Pearle MS, Pierce HL, Miller GL, Summa JA, Mutz JM, Petty BA, et al. Optimal method of urgent decompression of the collecting system for obstruction and infection due to ureteral calculi. J Urol. 1998;160:1260–4. [PubMed] [Google Scholar]
- 17.Misra S, Coker C, Richenberg J. Percutaneous nephrostomy for ureteric obstruction due to advanced pelvic malignancy: Have we got the balance right? Int Urol Nephrol. 2013;45:627–32. doi: 10.1007/s11255-013-0458-3. [DOI] [PubMed] [Google Scholar]
- 18.Allen DJ, Longhorn SE, Philp T, Smith RD, Choong S. Percutaneous urinary drainage and ureteric stenting in malignant disease. Clin Oncol (R Coll Radiol) 2010;22:733–9. doi: 10.1016/j.clon.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Rotariu P, Yohannes P, Alexianu M, Rosner D, Lee BR, Lucan M, et al. Management of malignant extrinsic compression of the ureter by simultaneous placement of two ipsilateral ureteral stents. J Endourol. 2001;15:979–83. doi: 10.1089/089277901317203047. [DOI] [PubMed] [Google Scholar]
- 20.Lugmayr H, Pauer W. Self-expanding metal stents for palliative treatment of malignant ureteral obstruction. AJR Am J Roentgenol. 1992;159:1091–4. doi: 10.2214/ajr.159.5.1384298. [DOI] [PubMed] [Google Scholar]
- 21.Wang HJ, Lee TY, Luo HL, Chen CH, Shen YC, Chuang YC, et al. Application of resonance metallic stents for ureteral obstruction. BJU Int. 2011;108:428–32. doi: 10.1111/j.1464-410X.2010.09842.x. [DOI] [PubMed] [Google Scholar]
- 22.Lang EK, Winer AG, Abbey-Mensah G, Anne R, Allaei A, Friedman F, et al. Long-term results of metallic stents for malignant ureteral obstruction in advanced cervical carcinoma. J Endourol. 2013;27:646–51. doi: 10.1089/end.2012.0552. [DOI] [PubMed] [Google Scholar]
- 23.Pauer W, Kerbl K. Self-expanding permanent endoluminal stents in the ureter: Technical considerations. Tech Urol. 1995 Summer;1:67–71. [PubMed] [Google Scholar]
- 24.Moskovitz B, Halachmi S, Nativ O. A new self-expanding, large-caliber ureteral stent: Results of a multicenter experience. J Endourol. 2012;26:1523–7. doi: 10.1089/end.2012.0279. [DOI] [PubMed] [Google Scholar]
- 25.Kim JH, Song K, Jo MK, Park JW. Palliative care of malignant ureteral obstruction with polytetrafluoroethylene membrane-covered self-expandable metallic stents: Initial experience. Korean J Urol. 2012;53:625–31. doi: 10.4111/kju.2012.53.9.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang HJ, Lee TY, Luo HL, Chen CH, Shen YC, Chuang YC, et al. Application of resonance metallic stents for ureteral obstruction. BJU Int. 2011;108:428–32. doi: 10.1111/j.1464-410X.2010.09842.x. [DOI] [PubMed] [Google Scholar]
- 27.López-Huertas HL, Polcari AJ, Acosta-Miranda A, Turk TM. Metallic ureteral stents: A cost-effective method of managing benign upper tract obstruction. J Endourol. 2010;24:483–5. doi: 10.1089/end.2009.0192. [DOI] [PubMed] [Google Scholar]
- 28.Gayed BA, Mally AD, Riley J, Ost MC. Resonance metallic stents do not effectively relieve extrinsic ureteral compression in pediatric patients. J Endourol. 2013;27:154–7. doi: 10.1089/end.2012.0263. [DOI] [PubMed] [Google Scholar]
- 29.Goldsmith ZG, Wang AJ, Bañez LL, Lipkin ME, Ferrandino MN, Preminger GM, et al. Outcomes of metallic stents for malignant ureteral obstruction. J Urol. 2012;188:851–5. doi: 10.1016/j.juro.2012.04.113. [DOI] [PubMed] [Google Scholar]
- 30.Abbasi A, Wyre HW, Ogan K. Use of full-length metallic stents in malignant ureteral obstruction. J Endourol. 2013;27:640–5. doi: 10.1089/end.2012.0448. [DOI] [PubMed] [Google Scholar]
- 31.Gayed BA, Mally AD, Riley J, Ost MC. Resonance metallic stents do not effectively relieve extrinsic ureteral compression in pediatric patients. J Endourol. 2013;27:154–7. doi: 10.1089/end.2012.0263. [DOI] [PubMed] [Google Scholar]
- 32.Garg T, Guralnick ML, Langenstroer P, See WA, Hieb RA, Rilling WS, et al. Resonance metallic ureteral stents do not successfully treat ureteroenteric strictures. J Endourol. 2009;23:1199–201. doi: 10.1089/end.2008.0454. [DOI] [PubMed] [Google Scholar]
- 33.Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: An 11-year follow-up. BJU Int. 2009;103:372–6. doi: 10.1111/j.1464-410X.2008.08018.x. [DOI] [PubMed] [Google Scholar]
- 34.Zaman F, Poullis C, Bach C, Moraitis K, Junaid I, Buchholz N, et al. Use of a segmental thermoexpandable metal alloy stent in the management of malignant ureteric obstruction: A single centre experience in the UK. Urol Int. 2011;87:405–10. doi: 10.1159/000326081. [DOI] [PubMed] [Google Scholar]


