Abstract
Drugeluting metal stents (DESs) have been extensively used in coronary and vascular disease. This type of stents has been proven to provide significantly lower restenosis rates due to the reduction of neo-intimal hyperplasia in comparison to the traditionally used bare metal stents (BMSs). The latter stents have been evaluated for more than a decade in urological practice in an attempt to provide permanent relief of urethral or ureteral obstruction. Although the initial results were promising, long-term experience revealed significant complications, which are mainly attributed to stent-related hyperplastic reaction compromising stent patency. The favorable experience of vascular DESs led to the application of DESs in both the urethra and ureter of animal models. These experimental results demonstrated a reduction of hyperplastic reaction of DESs in comparison to BMSs. Nevertheless, clinical data are currently not available. Considering the fact that DESs are under continuous development, the use of DESs in urology holds promise for the future and seems to be an intriguing field.
Keywords: Drug-eluting, metal stent, optical coherence tomography, paclitaxel, sirolimus, ureteral stent, zotarolimus
INTRODUCTION
Bare metal stents (BMSs) are an invaluable tool for the management of coronary and vascular disease in the fields of interventional cardiology and radiology.[1,2] In fact, the use of these stents represents the cornerstone for the above specialties as they are inserted in the arteries during the performance of percutaneous transluminal angioplasty. BMSs are small, tubular, wire mesh devices, which are loaded in a collapsed form onto the catheter balloon. The system composed of the stent and catheter are inserted over a guidewire through a vascular lesion, the balloon is inflated to dilate the obstruction and the stent is released on the vascular wall. After the expansion of the stent, the BMS acts as a mechanical scaffold, preventing elastic recoil and maintaining vessel luminal patency. Eventually, the stent is incorporated to the vascular wall after a period of epithelization.[3] The main complication of this process is lumen restenosis (20-30% of the cases), which is mainly attributed to neo-intimal hyperplasia.[2,3]
THE CONCEPT OF DRUG-ELUTING STENTS (DESs)
In an attempt to minimize lumen restenosis, DESs were introduced. The latter stents aim to reduce the risk of neo-intimal hyperplasia induced by stent insertion. DESs have the potential of endoluminal release of pharmacological anti-proliferative substances in a controlled fashion into the vessel lumen. Specifically, the anti-proliferative drug is coated onto the stent surface and is slowly released. These substances reduce the hyperplastic reaction by inhibiting the smooth muscle cell cycle and their proliferation.[4,5] The clinical evaluation of DESs proved their superiority of DES over BMS in terms of stent restenosis. In fact, significantly lower risk of lesion revascularization and significantly lower mortality in comparison to those receiving BMSs was observed. In fact, the need for lesion revascularization was decreased from 20% to 23.5% of the cases treated by BMSs to 7,5-10% by DESs.[3,6,7]
THE EVOLUTION OF DES DESIGN
The first generation of DESs was exemplified by stents such as the Cypher (sirolimus-eluting stent) and the Taxus (paclitaxel). Although these stents were a significant improvement over BMSs, they were associated with late stent thrombosis.[3,6] Thus, the second generation of DESs was introduced and included the Endeavor (zotarolimus) and XIENCE V (everolimus). The second generation had differences in stent structure, polymer layer and anti-proliferative agent resulting in improved safety outcomes even after a long follow-up period.[3,6] Currently, intensive research efforts by a large number of investigators and companies are underway in an attempt to improve the design and efficacy of the DESs. Since a DES consists of three components-stent platform, stent coating, and pharmaceutical agent-the aforementioned research extends to all these components.
Stent platform
Regardless to the generation of the stent, the design of the stent platform should result in minimum shortening, should follow the vessel geometry and provide high radial strength with minimal radial recoil. The biocompatibility of the stent is also very important. Thus, the stents are composed of biologically inert materials (i.e.,stainless steel). Metal alloys such as the nickel-titanium and cobalt-chromium have been proven to be advantageous over stainless steel in terms of biocompatibility. The issues with the long-term safety of the first generation of DESs led to the development of fully biodegradable stents and the introduction of biomimetic and biodegradable polymer stent platforms.[3,7] Even the concept of biocorrible iron platform has been proposed in an attempt to avoid the permanent presence of the stent platform and the associated complications in the treated organ.[8]
Stent coating
The stent coating surrounds the metallic structure of the DES. It is usually made of polymeric material, which provides controlled release of pharmaceutical agents. The biocompatibility of these agents is important and these polymers should have specific properties including suppression of intimal proliferation, should be non-thrombotic, non-inflammatory, non-toxic and hemocompatible (when referring to vascular stents). These features prevent thromboembolic events and facilitate the endothelization process of the stent.[2,3] The stent coating requires elastic properties, which undergo flaking or delaminating during the expansion of the stent by the balloon. The controlled and predictable rate of drug release is another factor when considering stent coating selection.
The coatings of DESs could be categorized to biostable (polyurethane, silicone, polyethylenecopolymers), to biodegradable (polyglycolic and polylactic polymers)and to biological polymers (i.e.,phosporylcholine, hyaluronicacid). The initial experience with the firstgeneration of DESs showed that permanent (biostable) material such as poly-n-butylmethacrylate, polyethylene-co-vinylacetate and the copolymer poly (styrene-b-isobutylene-b-styrene) were efficient in drug delivery.[9] The first stent coatings were substituted by more biocompatible permanent materials, such as phosphorylcholine copolymers. More recently, advanced DESs use bioabsorbable polymers with promising results.[3]
The pharmaceutical agents
Four categories of pharmaceutical agents could possibly be used on DESs: Anti-inflammatory, anti-thrombogenic, anti-proliferative and immunosuppressive drugs. The decision for the use of any of the above drug classes is based on the issues to be managed. Specifically, the selection of the most appropriate agent is based on the dose for local delivery and the determination of an appropriate biocompatible vehicle for local delivery.[10] The drugs used on DESs usually inhibit one or more biochemical pathways related to the hyperplastic reaction and platelet aggregation and eventually prevent intraluminal restenosis.
Sirolimus (rapamycin) and its analogs (zotarolimus, everolimus, biolimusA9, tacrolimus, pimecrolimus) bind to cytosolic proteins (FK-506 bindingprotein-12) and inhibit cell proliferation. The actions of sirolimus also include inhibition of several pathways such as inflammation, neointimalhyperplasia formation, synthesis of collagen and protein as well as the migration of smooth muscle cells.[11] Zotarolimus and everolimus inhibit smooth muscle cell and T-cell proliferation by binding on FK-506 binding protein-12. The chemical properties of these agents (noctanol/water partition coefficient and lipophilic features) allow the slow release rate of the drugs and favors efficient drug distribution.[12] Tacrolimus is an immunosuppressive agent which inhibits calcineurin and T cell signal transduction. Experimental studies show that tacrolimus facilitates earlier endothelization, but is less potent in the inhibition of smooth muscle cell proliferation.[13] BiolimusA9 has similar actions to sirolimus and also exerts potent anti-inflammatory action.[14]
Paclitaxel stabilizes the microtubules, which are necessary for the transition of the cell cycle from phase G2 to M-phase. The above event takes place by the polymerization of the subunits of tubulin by the action of paclitaxel. Moreover, paclitaxel inhibits the proliferation and migration of smooth muscle cells.[11] Actinomycin D is another anti-proliferative drug, which inhibits ribonucleic acid synthesis by forming as table complex with deoxyribonucleic acid. As a result, cell proliferation is efficiently inhibited. The clinical results of the respective DES were unfavorable.[11,15]
Dexamethasone has potent anti-inflammatory properties. The systematic administration of the drug is useful in the management of inflammatory diseases and inhibits the proliferation of fibroblasts, smooth muscle cells and macrophages. The use of dexamethasone in DES is based on the concept to reduce the inflammatory reaction induced by the implantation of the stent,which results to restenosis of the stent lumen. The clinical efficacy of the dexamethasone eluting stent was not promising since its efficiency was not comparable to the first generation of DESs.[3]
Antibodies
A significant issue of vascular stent restenosis is the formation of thrombus in the lumen. The prevention of the above complication is achieved by the administration of systematic antiplatelet regimen to stented patients. The use of an anti-thrombogenic agent on a DES would probably render the administration of antiplatelet drugs as unnecessary. Currently, experimental research showed promising results with the use of an antiplatelet glycoprotein IIb/IIa antiboby eluting stent in rabbit model.[3,16] A stainless steel stent covered by antibodies specific to surface antigens, which facilitate there-endothelialization of the stent and result in reduced stent restenosis.[17] A combination of antibodies for inhibition of the thrombotic process and cytotoxic drugs has been recently proposed. In fact, sirolimus-eluting stents received antihuman-CD34 antibodies on their surface in an attempt to improve the endothelialization process.[17,18] The combination of drugs and antibodies represents an interesting concept for the enhancement of the effects of the currently available DESs.
UROLOGICAL METAL STENTS
The urological application of metal stents (MSs) in the ureter and urethra has been investigated for more than a decade.[19,20] The use of MSs in the urethra aimed to relieve bladder outlet obstruction due to benign prostatic hyperplasia and urethral strictures. Placement of MSs in the urethra was promising but significant problems such as the frequent presence of stent encrustation, infection, migration and lower urinary tract symptoms limited the use of urethral MSs.[19,21] Infact, long-term experience showed high failure rates and significant difficulty in the management of failed urethral MSs since the removal of the stent may require complex reconstructive surgery.[22,23,24]
In the ureter, short-term experience with MSs had favorable outcomes, especially in the case of malignant ureteral obstruction, which was proven to be efficiently alleviated by their insertion.[20,25] Unfortunately, these initial promising results were followed by controversial results during the long-term evaluation of the ureteral MSs.[25,26] Malignant ureteral obstruction due to extrinsic tension by metastatic retroperitoneal tumor is probably the most common indication for MS insertion since the use of double-J polymeric stents is related to high failure rates.[27,28] The latter cases could be also managed by the placement of nephrostomy tubes,which have been proven to have a significant psychological impact to the patients and diminish their quality-of-life.[29] These patients usually have a limited life expectancy and the placement of a nephrostomy is avoided by the use of MSs. If the patency of the MS is compromised in the above cases, a double-J stent could be inserted through the stented ureter with excellent results.[26] Benign cases treated by MS insertion are very limited due to the MS related complications.[20] The most common cause of stent restenosis was the hyperplastic reaction developed through the stent struts. Encrustation, migration and infection were also complications of ureteral MSs.[19,26]
UROLOGICAL DESs
The use of DESs in the ureter or urethra is based on the same concept as the use of DESs in coronaryvessels; the DES releases cytostatic drugs in a controlled fashion. These drugs limit the hyperplastic reaction and potentially minimize restenosis of the stented ureter or urethra.[2,30] Nevertheless, the cell biology, physiology and histology of both ureter and urethra are significantly different in comparison to the artery. In fact, the urothelium comprises a special barrier,which minimizes crossing of substances and the efficiency of the drug of the DESs may be compromised.[31,32] Drug-eluting polymeric and biodegradable stents have been tested in experimental and clinical trials to minimize long-term problems related to the permanent MSs in the urinary tract and have shown favourable results.[33,34,35] Specifically, the insertion of a MS in the urinary tract is usually associated with significant difficulty in its removal when necessary. In a number of cases, removal requires challenging reconstructive procedures due to the significant local scarring. In view of these problems, use of DESs in the urethra and ureter should be carefully evaluated in experimental models before clinical use. Currently, only experimental animal studies are available on the use of DESs in the urinary tract.[30,36,37]
Shin et al. inserted paclitaxel eluting metallic stents and polyurethane covered stents alternately between the proximal and distal urethra of dogs.[36] 20 covered MSs (controls) and 20 DESs were custom made by the authors and placed in 20 male dogs. Specifically, the DESs were made by covering the metal structure of the stent with polyurethane solution containing 0.4% paclitaxel while the control stents were covered by polyurethane alone. The dogs were divided into two groups according to the follow-up period (4 or 8 weeks). Retrograde urethrography and histologic evaluation of the stented urethras was performed. Stent migration was observed in two cases. At 4 weeks, significantly lower hyperplasia was observed in the case of DESs in the proximal urethra in comparison to those in the distal urethra. No significant difference was observed in the hyperplastic reaction in the DESs compared to the polyurethane covered MSs, regardless of the stent position. There was less hyperplasia in the distal urethra than in the proximal urethra irrespective of the stent type. Moreover, hyperplastic reaction was never statistical significant when the DESs were compared to the covered MSs in the different urethral sites at 8 weeks. The authors concluded that the use of DESs in the urethra may be an interesting option for clinical application, especially when the drug-elution feature is combined with a more advanced retrievable design of the stent.
In an attempt to evaluate the effect of DESs in the ureter, Liatsikos et al. used a commercially available paclitaxel-eluting stent (Taxus, BostonScientific, Natick, MA, USA) in a porcine model.[37] A DES was inserted into one ureter and a bare MS (R-Stent, Orbus Medical Technologies, Hoevelaken, Netherlands) in the other ureter of each animal. Follow-up imaging included intravenous (IV) pyelography, nephrotomography and virtual endoscopy at 24h and 21 days. During the follow-up period, the majority of MSs were occluded while the remaining stents were stenosed by hyperplastic reaction. DESs were all patent during the follow-up period.
Recently, Kallidonis et al. inserted zotarolimus eluting MSs (Endeavor, Medtronics, USA) in porcine and rabbit ureters.[30] A BMS (R-Stent, Orbus Medical Technologies, Hoevelaken, Netherlands) was placed in the one ureter (control) and a DES in the other ureter of the same animal. The follow-up period was 4 weeks for the pigs (n = 10) and 8 weeks for the rabbits (n = 6). The use of two animal species was decided for the extension of the follow-up period. Pigs undergo rapid growth and their care represents a practical challenge even during a period of a few weeks. The inclusion of rabbits allowed the extension of the follow-up period and the investigation of the effect of the DES in the ureter for a longer period. Computerized tomography was performed for the evaluation of the porcine ureters every week and IV pyelography weekly for the rabbit ureters. Optical coherence tomography was used for the first time for the evaluation of the intraluminal patency and the wall of the stented ureter. The latter method has been well-documented for the evaluation of metallic stents in vessels.[38] Diuretic renogram evaluated renal function in conjunction with stent patency. Histologic evaluation of the stented ureters was performed by embedding the ureter and stent en bloc in polymeric resin (glycol-methylmethacrylate, GMA), which is considered the most appropriate method for the pathologic evaluation of stented vessels.[39] Hyperplastic tissue was observed by the computed tomography scans or IV pyelography in both stent types. BMSs in seven porcine ureters were completely occluded. The DESs were associated with hyperplastic tissue that did not result in obstruction. Two rabbit ureters stented by BMS were obstructed while ureters stented by ZES were never occluded. Diuretic scintigrams revealed that the function of the seven porcine renal units and one rabbit unit with obstructed stented ureter was significantly compromised.
Optical coherence tomography was performed in 23 stented ureters and revealed increased hyperplastic reaction in the ureters stented by BMSs in comparison to ZESs [Figure 1a and b]. Pathologic assessment of the MSs showed significantly more hyperplastic reaction in MSs in comparison to ZESs.
Figure 1.
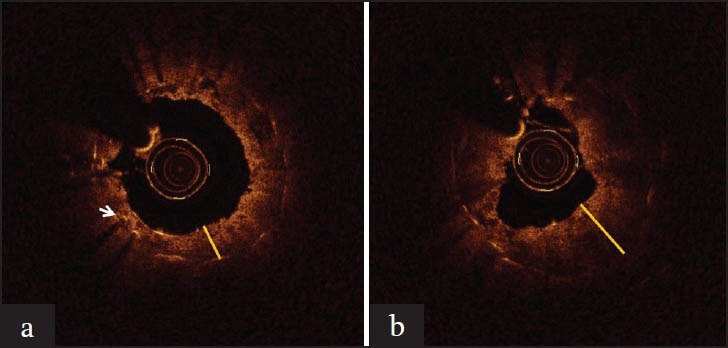
(a) Optical coherence tomography image of rabbit ureter stented by drug-eluting stent. Notice the presence of the stent struts which appear as an illuminating point accompanied by a backshadow. The yellow line shows the thickness of the urothelium.(b) Optical coherence tomography image of the other ureter of the same animal, stented by bare metal stent of. The yellow line shows the thickness of the urothelium, which in this case is obviously higher
CONCLUSION AND FUTURE PERSPECTIVES
DESs have been a field with extensive progress in interventional cardiology and radiology.[2,3] A variety of different stent designs, pharmacological agents and coatings have been introduced and are under experimental as well as clinical evaluation. Specifically, polymer-free and bioabsorbable DESs are under development with promising perspectives for the future.[2,3] The use of DESs in the ureter and urethra has been limited to few experimental studies. Further experimental investigation will decide the potential use of DESs in clinical trials. It remains to be proven if the promising benefit of DESs will eventually become a part of urological practice.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Macdonald S. Carotid artery stenting trials: Conduct, results, critique, and current recommendations. Cardiovasc Intervent Radiol. 2012;35:15–29. doi: 10.1007/s00270-011-0223-x. [DOI] [PubMed] [Google Scholar]
- 2.Kukreja N, Onuma Y, Daemen J, Serruys PW. The future of drug-eluting stents. Pharmacol Res. 2008;57:171–80. doi: 10.1016/j.phrs.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 3.Khan W, Farah S, Domb AJ. Drug eluting stents: Developments and current status. J Control Release. 2012;161:703–12. doi: 10.1016/j.jconrel.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Alahmar AE, Grayson AD, Andron M, Egred M, Roberts ED, Patel B, et al. Reduction in mortality and target-lesion revascularization at 2 years: A comparison between drug-eluting stents and conventional bare-metal stents in the “real world”. Int J Cardiol. 2009;132:398–404. doi: 10.1016/j.ijcard.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 5.McGinty S, McKee S, Wadsworth RM, McCormick C. Modelling drug-eluting stents. Math Med Biol. 2011;28:1–29. doi: 10.1093/imammb/dqq003. [DOI] [PubMed] [Google Scholar]
- 6.Räber L, Windecker S. Current status of drug-eluting stents. Cardiovasc Ther. 2011;29:176–89. doi: 10.1111/j.1755-5922.2010.00144.x. [DOI] [PubMed] [Google Scholar]
- 7.Martin DM, Boyle FJ. Drug-eluting stents for coronary artery disease: A review. Med Eng Phys. 2011;33:148–63. doi: 10.1016/j.medengphy.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Waksman R, Pakala R, Baffour R, Seabron R, Hellinga D, Tio FO. Short-term effects of biocorrodible iron stents in porcine coronary arteries. J Interv Cardiol. 2008;21:15–20. doi: 10.1111/j.1540-8183.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore JE., Jr Biomechanical issues in endovascular device design. J Endovasc Ther. 2009;16(Suppl 1):I1–11. doi: 10.1583/08-2605.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teomim D, Fishbien I, Golomb G, Orloff L, Mayberg M, Domb AJ. Perivascular delivery of heparin for the reduction of smooth muscle cell proliferation after endothelial injury. J Control Release. 1999;60:129–42. doi: 10.1016/s0168-3659(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 11.Vander Hoeven BL, Pires NM, Warda HM, Oemrawsingh PV, vanVlijmen BJ, Quax PH, et al. Drug-eluting stents: Results, promises and problems. Int J Cardiol. 2005;99:9–17. doi: 10.1016/j.ijcard.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 12.Burke SE, Kuntz RE, Schwartz LB. Zotarolimus (ABT-578) eluting stents. Adv Drug Deliv Rev. 2006;58:437–46. doi: 10.1016/j.addr.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 13.Matter CM, Rozenberg I, Jaschko A, Greutert H, Kurz DJ, Wnendt S, et al. Effects of tacrolimus or sirolimus on proliferation of vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2006;48:286–92. doi: 10.1097/01.fjc.0000248233.22570.8b. [DOI] [PubMed] [Google Scholar]
- 14.Grube E, Buellesfeld L. Bio Matrix Biolimus A9-eluting coronary stent: A next-generation drug-eluting stent for coronary artery disease. Expert Rev Med Devices. 2006;3:731–41. doi: 10.1586/17434440.3.6.731. [DOI] [PubMed] [Google Scholar]
- 15.Serruys PW, Ormiston JA, Sianos G, Sousa JE, Grube E, denHeijer P, et al. Actinomycin-eluting stent for coronary revascularization: A randomized feasibility and safety study: The ACTION trial. J Am Coll Cardiol. 2004;44:1363–7. doi: 10.1016/j.jacc.2004.03.084. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal RK, Ireland DC, Azrin MA, Ezekowitz MD, deBono DP, Gershlick AH. Antithrombotic potential of polymer-coated stents eluting platelet glycoprotein IIb/IIIa receptor antibody. Circulation. 1996;94:3311–7. doi: 10.1161/01.cir.94.12.3311. [DOI] [PubMed] [Google Scholar]
- 17.Miglionico M, Patti G, D’Ambrosio A, DiSciascio G. Percutaneous coronary intervention utilizing a new endothelial progenitor cells antibody-coated stent: A prospective single-center registry in high-risk patients. Catheter Cardiovasc Interv. 2008;71:600–4. doi: 10.1002/ccd.21437. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa G, Granada JF, Alviar CL, Tellez A, Kaluza GL, Guilhermier MY, et al. Anti-CD34 antibodies immobilized on the surface of sirolimus-eluting stents enhance stent endothelialization. JACC Cardiovasc Interv. 2010;3:68–75. doi: 10.1016/j.jcin.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 19.Oosterlinck W. Treatment of bulbar urethral strictures a review, with personal critical remarks. ScientificWorldJournal. 2003;3:443–54. doi: 10.1100/tsw.2003.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liatsikos E, Kallidonis P, Stolzenburg JU, Karnabatidis D. Ureteral stents: Past, present and future. Expert Rev Med Devices. 2009;6:313–24. doi: 10.1586/erd.09.5. [DOI] [PubMed] [Google Scholar]
- 21.Donnell RF. Urethral stents in benign prostate hyperplasia. Curr Urol Rep. 2003;4:282–6. doi: 10.1007/s11934-003-0085-0. [DOI] [PubMed] [Google Scholar]
- 22.Chapple CR, Bhargava S. Management of the failure of a permanently implanted urethral stent-a therapeutic challenge. Eur Urol. 2008;54:665–70. doi: 10.1016/j.eururo.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 23.Eisenberg ML, Elliott SP, McAninch JW. Management of restenosis after urethral stent placement. J Urol. 2008;179:991–5. doi: 10.1016/j.juro.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Palminteri E, Gacci M, Berdondini E, Poluzzi M, Franco G, Gentile V. Management of urethral stent failure for recurrent anterior urethral strictures. Eur Urol. 2010;57:615–21. doi: 10.1016/j.eururo.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: An 11-year follow-up. BJU Int. 2009;103:372–6. doi: 10.1111/j.1464-410X.2008.08018.x. [DOI] [PubMed] [Google Scholar]
- 26.Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009;182:2613–7. doi: 10.1016/j.juro.2009.08.040. [DOI] [PubMed] [Google Scholar]
- 27.Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004;172:592–5. doi: 10.1097/01.ju.0000130510.28768.f5. [DOI] [PubMed] [Google Scholar]
- 28.Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005;174:2125–8. doi: 10.1097/01.ju.0000181807.56114.b7. [DOI] [PubMed] [Google Scholar]
- 29.Aravantinos E, Anagnostou T, Karatzas AD, Papakonstantinou W, Samarinas M, Melekos MD. Percutaneous nephrostomy in patients with tumors of advanced stage: Treatment dilemmas and impact on clinical course and quality of life. J Endourol. 2007;21:1297–302. doi: 10.1089/end.2006.0104. [DOI] [PubMed] [Google Scholar]
- 30.Kallidonis P, Kitrou P, Karnabatidis D, Kyriazis I, Kalogeropoulou C, Tsamandas A, et al. Evaluation of zotarolimus-eluting metal stent in animal ureters. J Endourol. 2011;25:1661–7. doi: 10.1089/end.2011.0308. [DOI] [PubMed] [Google Scholar]
- 31.Khandelwal P, Abraham SN, Apodaca G. Cell biology and physiology of the uroepithelium. Am J Physiol Renal Physiol. 2009;297:F1477–501. doi: 10.1152/ajprenal.00327.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kreft ME, Hudoklin S, Jezernik K, Romih R. Formation and maintenance of blood-urine barrier in urothelium. Protoplasma. 2010;246:3–14. doi: 10.1007/s00709-010-0112-1. [DOI] [PubMed] [Google Scholar]
- 33.Krambeck AE, Walsh RS, Denstedt JD, Preminger GM, Li J, Evans JC, et al. A novel drug eluting ureteral stent: A prospective, randomized, multicenter clinical trial to evaluate the safety and effectiveness of a ketorolac loaded ureteral stent. J Urol. 2010;183:1037–42. doi: 10.1016/j.juro.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 34.Mendez-Probst CE, Goneau LW, MacDonald KW, Nott L, Seney S, Elwood CN, et al. The use of triclosan eluting stents effectively reduces ureteral stent symptoms: A prospective randomized trial. BJU Int. 2012;110:749–54. doi: 10.1111/j.1464-410X.2011.10903.x. [DOI] [PubMed] [Google Scholar]
- 35.Kotsar A, Nieminen R, Isotalo T, Mikkonen J, Uurto I, Kellomäki M, et al. Preclinical evaluation of new indomethacin-eluting biodegradable urethral stent. J Endourol. 2012;26:387–92. doi: 10.1089/end.2011.0327. [DOI] [PubMed] [Google Scholar]
- 36.Shin JH, Song HY, Choi CG, Yuk SH, Kim JS, Kim YM, et al. Tissue hyperplasia: influence of a paclitaxel-eluting covered stent — preliminary study in a canine urethral model. Radiology. 2005;234:438–44. doi: 10.1148/radiol.2342040006. [DOI] [PubMed] [Google Scholar]
- 37.Liatsikos EN, Karnabatidis D, Kagadis GC, Rokkas K, Constantinides C, Christeas N, et al. Application of paclitaxel-eluting metal mesh stents within the pig ureter: An experimental study. Eur Urol. 2007;51:217–23. doi: 10.1016/j.eururo.2006.05.054. [DOI] [PubMed] [Google Scholar]
- 38.Tahara S, Chamié D, Baibars M, Alraies C, Costa M. Optical coherence tomography endpoints in stent clinical investigations: Strut coverage. Int J Cardiovasc Imaging. 2011;27:271–87. doi: 10.1007/s10554-011-9796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cerri PS, Sasso-Cerri E. Staining methods applied to glycol methacrylate embedded tissue sections. Micron. 2003;34:365–72. doi: 10.1016/S0968-4328(03)00098-2. [DOI] [PubMed] [Google Scholar]


