Abstract
Background:
Sulfur mustard (SM) is an incapacitating chemical warfare agent, which has been widely employed in particular regions including Iran. We investigated and reported delayed biochemical and hematological complications of SM in severely toxic Iranian veterans 23 years after exposure.
Materials and Methods:
Forty-two Iranian veterans, residents of Khorasan Razavi, poisoned by SM, and suffering from clinical complications were investigated. A total of 30 healthy male volunteers were also selected as a control group. Biochemical and hematological variables were measured for the case and control groups. Data were analyzed using a Student's t-test by InStat software (GraphPad Inc., San Diego, CA) to determine significant differences between the data from the two groups.
Results:
The percentages of reticulocytes were significantly higher in patients (0.82 ± 0.04, P < 0.05). Total protein and albumin levels were significantly lower in veterans (total protein: 7.58 ± 0.07 g/dL, albumin: 4.97 ± 0.04 g/dL, P < 0.01). In addition, we observed a significant increase in serum cholesterol (226.74 ± 5.23 mg/dL, P < 0.01), triglyceride (173.53 ± 17.05 mg/dL, P < 0.05), and gamma-glutamyl transferase (GTT) activity of the patients (44.04 ± 3.35 IU/L, P < 0.05).
Conclusion:
Results showed that SM can cause long-term effects on some biochemical factors of veterans. As many of the functional tests of liver and kidney between two groups were statistically unchanged, it seems that the observed biochemical changes may be secondary to delayed respiratory complications of the patients.
Keywords: Albumin, complications, Khorasan Razavi, sulfur mustard, veterans
INTRODUCTION
Sulfur mustard (SM) is a vesicant chemical warfare agent because it causes skin and mucous membrane blisters on contact. Iraq used it as a chemical agent against Iranian combatants during the Iraq–Iran war from 1983 to 1988. SM readily forms a very reactive cyclic sulfonium ion. This intermediate permanently alkylates the guanine nucleotide of deoxyribonucleic acid (DNA) strands, impairing cellular division and function. This compound generally leads directly to apoptosis, mutations, and disturbance in the natural repair mechanisms of DNA. Increased frequency of cancer and complications after exposure to SM could be the reason for this claim.[1] Thus, it can theoretically affect all the human organs and cause long-term complications. SM readily accumulates in the lipid component of contacted tissues due to its lipophilic nature. The volume of distribution at the steady state is 74.4 L.[2,3] Upon exposure with the skin, about 20% of SM is absorbed by the skin and is distributed to other tissues. Only about 10% of the absorbed SM remains in the skin; it mostly enters the circulatory system. Therefore, even after the first contact, skin storages continue to distribute SM via the blood stream to the other tissues. It should be indicated that although skin is the primary accumulator of SM, its toxic effect is also important in distal organs. Therefore, the effects of SM after dermal contact are not limited to skin tissues alone.[4,5] Mustard has significant toxic effects on many organs such as the skin, eyes, and lungs, as well as the gastrointestinal, endocrine, and hematopoietic systems.[5,6,7,8] The presence of SM in the metabolizing systems, specifically in sites such as the liver and kidney has been reported by the whole-body autographic studies with labeled SM after percutaneous or intravenous administration.[9] In terminally ill patients with cancer, the majority of the radioactivity present in the injected[10] C-labeled SM disappeared after a few minutes from the blood and was secreted mainly in the urine within 24 hours.[11] Drasch and colleagues observed a high accumulation of SM in the kidneys following acute exposure.[12] In addition, SM can be found in the spleen, liver, and bone marrow.[13] Therefore, although SM is metabolized and excreted, it may cause liver and kidney damage.
SM also disorganizes the DNA of hematopoietic cells.[10,14] Toxicity and pancytopenia of hematopoietic stem cells have been seen in Iranian combatants due to high-dose exposure to SM.[15] Long-term effects on hematopoiesis may appear years later and follow-up studies are required to determine these side effects.[16]
Long-term effects of SM have been documented. The first report of long-term effects in Iranian victims was made in 1986. Several papers on the late toxic effects and complications of SM in Iranian veterans have been published since then.[17,18,19,20,21,22,23,24,25] As the toxic effects of SM on cells, especially on cellular DNA, are progressive and the clinical outcome of veterans can worsen over time, follow-up studies are crucial to determine these side effects.[16,19] To our knowledge, this is the first time that the late toxic effects of SM on biochemical factors of veterans have been independently evaluated, as in the present study.
We carried out the present study to evaluate delayed complications of SM on some biochemical factors associated with liver and renal functions of Iranian veterans 23 years after exposure. The study also evaluated long-term hematological effects (bone marrow) of SM poisoning.
MATERIALS AND METHODS
Study design
A case control model study was performed from March 2011 to November 2011. According to the medical association of the Khorasan Razavi Veteran Foundation, 76 veterans with more than 40% disability had severe SM complications. For the research study, 42 of 76 male patients volunteered and signed the consent form. The patients ranged in age from 45 to 65 years, with a mean of 50.6 years. As a control group, a total of 30 healthy male volunteers, with no past history of exposure to SM, were selected. They were matched in terms of sexuality and smoking. The subjects of the control group were excluded from the study if they had abnormal complete blood count (CBC) reports or infectious and noninfectious diseases. All experiments were done in the clinical laboratory of Imam Reza hospital, Mashhad, Iran. The present study was approved by the medical research ethics committee of Mashhad University of Medical Sciences, Mashhad, Iran.
Chemicals
Commercial colorimetric kits for fasting blood sugar (FBS), urea nitrogen, creatinine, uric acid, total and direct bilirubin (T and D Bili), total protein, albumin, alkaline phosphatase (ALP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), triglyceride, lactate dehydrogenase (LDH), and gamma-glutamyl transferase (GGT) were purchased from Pars Azmoon (Tehran, Iran). Reticulocyte stains were obtained from Sigma — Aldrich.
Blood sampling
After completion of the clinical examination, 10 mL blood samples were taken from the brachial vein of fasting patients, as well as from the control group. Blood samples were collected into sterile tubes with anticoagulant [ethylenediaminetetraacetic acid (EDTA)] for evaluation of hematological parameters and nonanticoagulant tubes for biochemical tests. Blood samples in nonanticoagulant tubes were centrifuged at 3000 rpm for five minutes and serum samples were harvested and kept at −70°C until testing.
Biochemical evaluation
All biochemical tests were determined by using commercially available kits from Pars Azmoon (Tehran, Iran). Serum samples were analyzed with a BT 3000 Plus biochemical analyzer (Biotecnica, Italy). The BT 3000 analyzer is a commercial colorimetric assay kit using the spectrophotometric method.
Determination of the hematological parameters
CBC was performed with a hematology cell counter (Sysmex KX-21, Japan). Routine hematological parameters were determined including hemoglobin content (Hb), hematocrit (Hct%), red blood cell (RBC) count, white blood cell (WBC) count, and platelet (Plt) count. Other indices such as mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), and mean platelet volume (MPV) were also reported. A blood smear was stained with Giemsa for each sample and the slides were observed under a light microscope. At least 100 cells were seen for differential analysis. The reticulocyte count was done using supravital staining (new methylene blue). An equal volume of stain was added to EDTA-anticoagulated blood, the dilution mixture was incubated for 10 minutes at room temperature, and a smear was prepared. The smears were examined to measure the number of reticulocytes. An erythrocyte containing two or more particles of blue-stained material was considered as a reticulocyte.
Statistical analysis
Data were analyzed using a Student's t-test to determine significant differences between the data from the two groups. Statistical tests were conducted using InStat software (GraphPad Inc., San Diego, CA). A P value <0.05 was considered significant. The values of the data presented are expressed as means ± standard error (SE).
RESULTS
Biochemical analysis
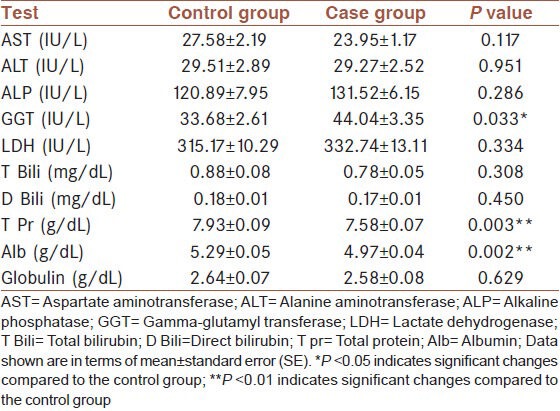
The assessments of serum blood urea nitrogen (BUN), creatinine, and uric acid levels did not show any significant differences between the case and control group [Table 1]. The results of the blood glucose and lipid profile are shown in Table 2. The levels of the lipids (cholesterol and triglycerides) were significantly higher in the case group compared to control. Despite this, however, we did not find any significant changes in serum HDL and LDL cholesterol. The liver function test results are shown in Table 3. There were no significant changes in AST, ALT, ALP, LDH, and Bili (T and D) between the two groups. Total protein and albumin levels decreased significantly in the serum of the case group in comparison with the control group. However, we observed a significant increase in serum GGT activity of the case group.
Table 1.
Comparison of kidney markers between case and control groups
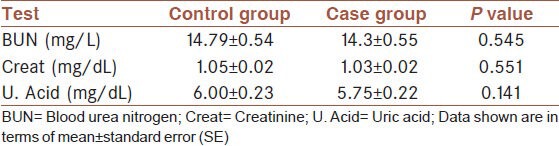
Table 2.
Determination of blood sugar and lipid profile status in case and control groups
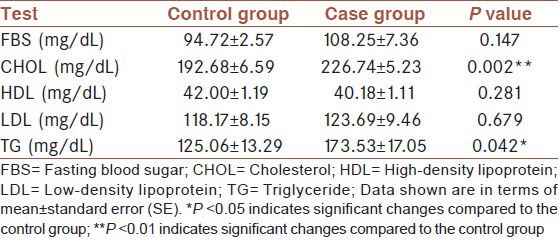
Table 3.
Comparison of liver function tests between case and control groups
Hematological parameters
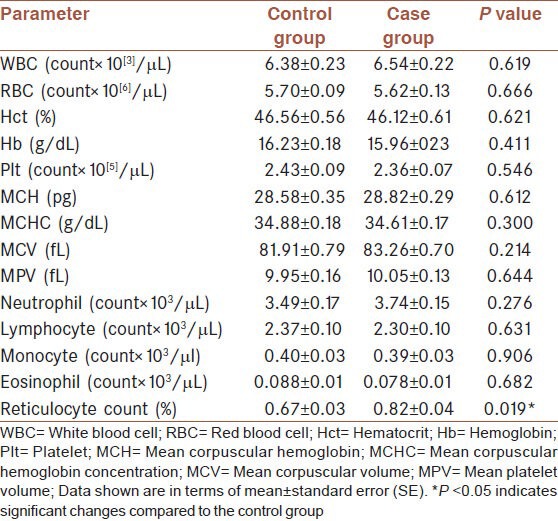
The hematological indices of 42 patients and 30 control subjects are shown in Table 4. Measures of the parameters (except the reticulocyte count) did not show any significant difference between the two groups. However, a significant increase in the percentage of the case group reticulocytes was seen (P < 0.05).
Table 4.
Hematological parameters between control and case groups
DISCUSSION
Despite international restrictions on the use of SM, it has been overtly produced, reserved, and employed in certain regions of the world.[26] SM has short- and long-term effects on various organs. It has been observed that a depression of leukocyte-producing centers is among the first changes in the circulating blood cells of victims exposed to SM.[27,28] Leukocytosis occurs during the first two to three days after exposure. Then, WBC counts begin to fall four days after exposure and reach their minimum level around the ninth day.[29] Bone marrow biopsies have shown atrophy involving all of the elements.[30,31] In a study performed, all Iranian victims with severe leukopenia (WBC <200 cells/mL) died during initial admissions due to fatal exposure.[29]
Although leukopenia and anemia are considered to be major acute hematological variations following SM exposure,[29,31] long-term follow-up of our veterans showed a significant increase in the percentage of the reticulocyte counts. Reticulocytes are immature RBCs. They leave the bone marrow and then circulate in the blood stream for about one day before promotion as mature RBCs. Reticulocytes typically comprise about 1% of the total RBCs. Hematologists use them as indicators of hematopoiesis (Wikipedia).
The significant increase in the reticulocyte percentage is probably due to the hypoxemic status of the patients as a result of their chronic respiratory problems. Both ‘increase in reticulocyte percentage’ and ‘unchanged RBC counts’ indicate that the RBC lifespan of the veterans may be less than normal. This status is similar to hemolytic anemia.
Although the majority of liver tests were normal, a significant decrease in serum albumin and total protein was observed in the case group. The main causes of hypoalbuminemia are undernourishment, decreased synthesis by the liver, renal losses, and chronic inflammation. Hypoalbuminemia is often attributed to malnutrition; however, albumin levels remain almost unchanged even in the presence of violent protein calorie malnutrition until about severe starvation.[32] Albumin levels are also reduced as a direct result of the loss of plasma proteins via the kidneys, such as in nephrotic syndrome or as a consequence of reduced synthesis in patients with liver disease.[33,34,35] Significantly decreased albumin in our patients may have been caused by the high frequency of acute and chronic respiratory infections present in these patients rather than direct toxic effects of SM on the liver.
Serum albumin is a negative acute-phase protein. Therefore, it is easily used as a marker of inflammation.[34] The inflammation process leads to the release of cytokines including interleukin-6 (IL-6), which operates through specific hepatic receptors to prevent the synthesis of albumin.[34] On the other hand, IL-6, through a positive autocrine feedback loop, stimulates the proliferation of lung fibroblasts.[36,37]
In our study, defined renal function tests of the patients were statistically unchanged. However, the decrease in serum total protein and albumin of our veterans could also have been caused by renal losses.
Our findings revealed that serum triglycerides and cholesterol of the patients were higher than the control group. This may be due to the lower level of daily physical activity among the veterans in comparison to the control group. The search for the factors causing an increase in serum lipid provided a clue that physical activity is directly associated with levels of serum lipid profile.[38,39,40]
In this study, a significant increase in the serum GGT activity of the patients was observed. GGT is an enzyme which is found in hepatocytes and the biliary tract. GGT may be high in liver disease. In particular, it is a characteristic of hepatobiliary obstruction rather than hepatocellular injury. GGT serum determination provides a very sensitive biomarker of the presence of hepatobiliary disease.[41] As there were no significant differences in AST, ALT, ALP, LDH, and Bili (T and D) between the two groups, the increased level of GGT activity in our patients seems not to be related to hepatobiliary damages.
GGT has been detected at low levels in bronchial discharges from normal cases and at higher levels in purulent sputum from patients with chronic bronchitis.[42] Raised levels of GGT have been reported in chronic obstructive pulmonary disease (COPD).[41] COPD is one of the major complications of SM poisoning.[43] Therefore, the increased serum GGT of our patients may be associated with their delayed respiratory complications.
In conclusion, it seems that SM has no direct long-term toxic effects on the function of the liver. Decreased albumin and total protein, increased GGT activity, and increased percentage of reticulocytes might have been caused secondary to delayed respiratory complications of the patients. Also, elevated serum lipids among the veterans could be due to the low level of their daily physical activities. However, SM has proved to have long-lasting toxic effects. Further studies are needed to assess the long-term toxic effects of mustard gas on the biochemical findings of veterans.
ACKNOWLEDGMENT
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences for the financial support.
Footnotes
Source of Support: Vice Chancellor of Research, Mashhad University of Medical Sciences, Mashhad, Iran.
Conflict of Interest: None declared.
REFERENCES
- 1.OPCW. Mustard agents: Description, physical and chemical properties, mechanism of action, symptoms, antidotes and methods of treatment. 2010. [Accessed June 8]. http://www.opcw.org/aboutchemical-weapons/types-of-chemicaal-agent/mustard-agents .
- 2.Roberts JJ, Warwick GP. Studies of the mode of action of alkylating agents. VI. The metabolism of bis-2-chlorethylsulphide (mustard gas) and related compounds. Biochem Pharmacol. 1963;12:1329–34. doi: 10.1016/0006-2952(63)90202-8. [DOI] [PubMed] [Google Scholar]
- 3.Kehe K, Szinicz L. Medical aspects of sulphur mustard poisoning. Toxicology. 2005;214:198–209. doi: 10.1016/j.tox.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 4.Sinclair DC. Disability produced by exposure of skin to mustard-gas vapour. Br Med J. 1950;1:346–9. doi: 10.1136/bmj.1.4649.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somani SM, Babu SR. Toxicodynamics of sulfur mustard. Int J Clin Pharmacol Ther Toxicol. 1989;27:419–35. [PubMed] [Google Scholar]
- 6.Moradi A. Clinical presentation of chemical warfare injuries. Iran J Med Sci. 1986;13:1–5. [Google Scholar]
- 7.Azizi F, Keshavarz A, Roshanzamir F, Nafarabadi M. Reproductive function in men following exposure to chemical warfare with sulphur mustard. Med War. 1995;11:34–44. doi: 10.1080/07488009508409195. [DOI] [PubMed] [Google Scholar]
- 8.Case RA, Lea AJ. Mustard gas poisoning, chronic bronchitis and lung cancer; investigation into the possibility that poisoning by mustard gas in 1914-18 war might be factor in production of neoplasia. Br J Prev Soc Med. 1995;9:62–72. doi: 10.1136/jech.9.2.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clemedson CJ, Kristoffersson H, Soerbo B, Ullberg S. Whole body autoradiographic studies of the distribution of sulphur 35- labelled mustard gas in mice. Acta Radiol Ther Phys Biol. 1963;1:314–20. doi: 10.3109/02841866309134109. [DOI] [PubMed] [Google Scholar]
- 10.Watson AP, Griffin GD. Toxicity of vesicant agents scheduled for destruction by the chemical stock pile disposal program. Environ Health Perspect. 1992;8:250–80. doi: 10.1289/ehp.9298259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davison C, Rozman RS, Smith PK. Metabolism of bis-beta-chloroethyl sulfide (sulfur mustard gas) Biochem Pharmacol. 1961;7:65–74. doi: 10.1016/0006-2952(61)90127-7. [DOI] [PubMed] [Google Scholar]
- 12.Drasch G, Kretschmer E, Kauert G, Von Meyer L. Concentrations of mustard gas [bis (2-chloroethy l) sulfide] in the tissues of a victim of a vesicant exposure. J Forensic Sci. 1987;32:1788–93. [PubMed] [Google Scholar]
- 13.Vijayaraghavan R, Kulkarni A, Pant SC, Kumar P, Rao PV, Gupta N, et al. Differential toxicity of sulfur mustard administered through percutaneous, subcutaneous, and oral routes. Toxicol Appl Pharmacol. 2005;202:180–8. doi: 10.1016/j.taap.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Mis JR, Kunz BA. Influence of DNA repair defects (rad 1, rads 2) on nitrogen mustard mutagenesis in yeast. Mol Gen Genet. 1992;235:304–10. doi: 10.1007/BF00279374. [DOI] [PubMed] [Google Scholar]
- 15.Tabarestani M. Mashhad: Proc 1st Int Med Cong Chem Warfare Agents; 1988. Stem cell and erythroid precursors disorders inthree patients with sulfur mustard poisoning. [Google Scholar]
- 16.Ghanei M. Delayed haematological complications of Mustard Gas. J Appl Toxicol. 2004;24:493–5. doi: 10.1002/jat.1006. [DOI] [PubMed] [Google Scholar]
- 17.Balali-Mood M, Hefazi M, Mahmoudi M, Jalali E, Attaran D, Maleki M, et al. Long-term complications of sulphur mustard poisoning in severely intoxicated Iranian veterans. Fundam Clin Pharmacol. 2005;19:713–21. doi: 10.1111/j.1472-8206.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- 18.Balali-Mood M, Hefazi M. The clinical toxicology of sulfur mustard. Arch Iran Med. 2005a;8:162–79. [Google Scholar]
- 19.Balali-Mood, Hefazi M. The pharmacology, toxicology, and medical treatment of sulphur mustard poisoning. Fundam Clin Pharmacol. 2005b;19:297–315. doi: 10.1111/j.1472-8206.2005.00325.x. [DOI] [PubMed] [Google Scholar]
- 20.Balali-Mood M, Hefazi M. Comparison of early and late toxic effects of sulfur Mustard in Iranian veterans. Basic Clin Pharmacol Toxicol. 2006;99:273–82. doi: 10.1111/j.1742-7843.2006.pto_429.x. [DOI] [PubMed] [Google Scholar]
- 21.Hefazi M, Maleki M, Mahmoudi M, Tabatabaee A, Balali-Mood M. Delayed complications of sulfur mustard poisoning in the skin and the immune system of Iranian veterans 16-20 years after exposure. Int J Dermatol. 2006;45:1025–31. doi: 10.1111/j.1365-4632.2006.03020.x. [DOI] [PubMed] [Google Scholar]
- 22.Balali-Mood M, Mousavi SH, Balali-Mood B. Chronic health effects of sulphur mustard exposure with special reference to Iranian veterans. Emerg Health Threats J. 2008;1:e7. doi: 10.3134/ehtj.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balali-Mood M. Sulphur Mustard Poisoning and its Complications in Iranian Veterans. Iran J Med Sci. 2009;34:155–71. [Google Scholar]
- 24.Shabestari MM, Jabbari F, Gohari B, Moazen N, Azizi H, Moghiman T. Coronary artery angiographic changes in veterans poisoned by Mustard Gas. Cardiology. 2011;119:208–13. doi: 10.1159/000331436. [DOI] [PubMed] [Google Scholar]
- 25.Balali-Mood M, Afshari R, Zojaji R, Kahrom H, Kamrani M, Mousavi SR, et al. Delayed toxic effects of sulfur mustard on respiratory tract of Iranian veterans. Hum Exp Toxicol. 2010;30:1141–9. doi: 10.1177/0960327110389501. [DOI] [PubMed] [Google Scholar]
- 26.Meselson M, Robinson JP. Chemical warfare and chemical disarmament. Sci Am. 1980;242:38–47. [Google Scholar]
- 27.Krumbhaar EB. Role of the blood and the bone marrow in certain forms of gas poisoning. JAMA. 1919a;72:39–41. [Google Scholar]
- 28.Krumbhaar EB. Bone marrow changes in mustard gas poisoning. JAMA. 1919b;73:715. [PMC free article] [PubMed] [Google Scholar]
- 29.Willems JL. Clinical management of mustard gas casualties. Ann Belg Med Mil. 1989;3:1–61. [Google Scholar]
- 30.Tabarestani M, Balali-Mood M, Farhoodi M. Hematological findings of sulfur mustard poisoning in Iranian combatants. Med J Islam Repub Iran. 1990;4:185–90. [Google Scholar]
- 31.Balali-Mood M, Tabarestani M, Farhoodi M, Panjvani FK. Study of clinical and laboratory findings of sulfur mustard in 329 war victims. Med J Islam Repub Iran. 1991;34:7–15. [Google Scholar]
- 32.Rigaud D, Hassid J, Meulemans A, Poupard AT, Boulier A. A paradoxical increase in resting energy expenditure in malnourished patients near death: The king penguin syndrome. Am J Clin Nutr. 2000;72:355–60. doi: 10.1093/ajcn/72.2.355. [DOI] [PubMed] [Google Scholar]
- 33.Rothschild MA, Oratz M, Schreiber SS. Serum albumin. Hepatology. 1988;8:385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- 34.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265:621–36. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Don BR, Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin Dial. 2004;17:432–7. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
- 36.Tabata C, Kadokawa Y, Tabata R, Takahashi M, Okoshi K, Sakai Y, et al. All-trans-retinoic acid prevents radiation- or bleomycin-induced pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:1352–60. doi: 10.1164/rccm.200606-862OC. [DOI] [PubMed] [Google Scholar]
- 37.Tabata C, Tabata R, Kadokawa Y, Hisamori S, Takahashi M, Mishima M, et al. Thalidomide prevents bleomycin-induced pulmonary fibrosis in mice. J Immunol. 2007;179:708–14. doi: 10.4049/jimmunol.179.1.708. [DOI] [PubMed] [Google Scholar]
- 38.Leon AS, Sanchez OA. Response of blood lipids to exercise training alone or combined with dietary intervention. Med Sci Sports Exerc. 2001;33:502–15. doi: 10.1097/00005768-200106001-00021. [DOI] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG. Physical activity in the prevention of cardiovascular disease: An epidemiological perspective. Sports Med. 2001;31:101–14. doi: 10.2165/00007256-200131020-00003. [DOI] [PubMed] [Google Scholar]
- 40.Mbalilaki JA, Hellenius ML, Masesa Z, Hostmark AT, Sundquist J, Stromme SB. Physical activity and blood lipids in rural and urban Tanzanians. Nutr Metab Cardiovasc Dis. 2007;17:344–8. doi: 10.1016/j.numecd.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 41.Goldberg DM, Martin JV. Role of gamma-glutamyl transpeptidase activity in the diagnosis of hepatobiliary disease. Digestion. 1975;12:232–46. doi: 10.1159/000197682. [DOI] [PubMed] [Google Scholar]
- 42.Barton AD, Powers JL, Lourenco RV. Gamma glutamyl transpeptidase in chronic obstructive pulmonary disease. Proc Soc Exp Biol Med. 1974;146:99–103. doi: 10.3181/00379727-146-38051. [DOI] [PubMed] [Google Scholar]
- 43.Attaran D, Lari SM, Towhidi M, Ghotbi Marallu H, Ayatollahi H, Khajehdaluee M, et al. Interleukin-6 and airflow limitation in chemical warfare patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2010;5:335–40. doi: 10.2147/COPD.S12545. [DOI] [PMC free article] [PubMed] [Google Scholar]


