Abstract
Backround:
We aimed to evaluate analgesic efficacy, opioid-sparing, and opioid-related adverse effects of intravenous paracetamol and intravenous dexketoprofen trometamol in combination with iv morphine after total abdominal hysterectomy.
Materials and Methods:
Sixty American Society of Anesthesiologist Physical Status Classification I-II patients scheduled for total abdominal hysterectomy were enrolled to this double-blinded, randomized, placebo controlled, and prospective study. Patients were divided into three groups as paracetamol, dexketoprofen trometamol, and placebo (0.9% NaCl) due to their post-operative analgesic usage. Intravenous patient controlled analgesia morphine was used as a rescue analgesic in all groups. Pain scores, hemodynamic parameters, morphine consumption, patient satisfaction, and side-effects were evaluated.
Results:
Visual Analog Scale (VAS) scores were not statistically significantly different among the groups in all evaluation times, but decrease in VAS scores was statistically significant after the evaluation at 12th h in all groups. Total morphine consumption (morphine concentration = 0.2 mg/ml) in group paracetamol (72.3 ± 38.0 ml) and dexketoprofen trometamol (69.3 ± 24.1 ml) was significantly lower than group placebo (129.3 ± 22.6 ml) (P < 0.001). Global satisfaction scores of the patients in group placebo was significantly lower than group dexketoprofen trometamol after surgery and the increase in global satisfaction score was significant only in group placebo.
Conclusion:
Dexketoprofen trometamol and Paracetamol didn’t cause significant change on pain scores, but increased patients’ comfort. Although total morphine consumption was significantly decreased by both drugs, the incidence of nausea and vomiting were similar among the groups. According to results of the present study routine addition of dexketoprofen trometamol and paracetamol to patient controlled analgesia morphine after hysterectomies is not recommended.
Keywords: Hysterectomy, multimodal treatment, NSAIDs, paracetamol
INTRODUCTION
Today, using multimodal analgesia has become more important to enhance effectiveness of post-operative pain therapy and to minimize side-effects of the drugs used.[1,2] For this purpose, combining the opioids with non-steroidal anti-inflammatory drugs or with techniques that apply local anesthetics.[3] Paracetamol is a centrally acting inhibitor of cyclooxygenases and also inhibits the peripheral synthesis of prostaglandins.[4] Dexketoprofen trometamol is an active enantiomer of racemic ketoprofen.[5]
As oral intake is not advisable, paranteral analgesic usage is preferred in post-operative period. Intravenous paracetamol, which has been marketed for last 2 years in our country, is suitable drug in the treatment of mild and moderate post-operative pain without having adverse effects of non-steroidal anti-inflammatory drugs. On the other hand, intravenous form of dexketoprofen trometamol has been marketed recently in our country. It is a non-steroidal anti-inflammatory drug and it has analgesic activity, which is started tremendous after parenteral usage. We aim to compare these drugs after total abdominal hysterectomy for their efficiencies and reducing effects on adverse effects of opioids.
SUBJECTS AND METHODS
A total of 60 patients of ASA I-II group, prepared for total abdominal hysterectomy operation and between 20 years and 70 years of age included in this placebo controlled, randomized and double-blind study after obtaining the approval of the Local Ethical Committee and patient's written informed consent. Patients without severe renal, hepatic and cardiac disease, peptic ulcer and gastrointestinal bleeding, crohn's disease or colitis ulcerosa, coagulation disorder, known allergic reaction to one of these drugs, chronic pain and routine analgesic usage, occurrence of complication during the operation were included into the study. All the patients were informed about Visual Analog Scale (VAS) and patient controlled analgesia (PCA) device during the pre-operative visits. Patients who did not take the premedication administered thiopental (3-5 mg/kg), vecuronium bromide (0.1 mg/kg), fentanyl (1-2 mcg/kg) in induction. Anesthesia maintained by using 50%/50% O2-N2O and sevoflurane (1-2%). During the operation, patients monitored by non-invasive blood pressure measurement, Electrocardiogram, SpO2 and end tidal CO2, and also ventilated by tidal volume of 10 ml/kg and 12 respirations/min.
Patients then randomly divided into three groups as Group I (paracetamol, n = 20), Group II (dexketoprofen, n = 20) and Group III (placebo, n = 20) for post-operative analgesia administration. Randomization was performed using a sealed opaque envelope with a computer-generated block random allocation. One gr of paracetamol (Perfalgan 10 mg/ml 100 ml Flacon Bristol-Myers Squibb Labarotories, Renaudin Itxassou France) administered to Group I by intravenous infusion in 15 min during the closure, and 1 gr of intravenous paracetamol administered at every 6 h during the following 24 h. Dexketoprofen trometamol 50 mg/2 mL (Arveles 50 mg/2 ml Menarini International Italy) diluted in 100 mL of saline and administered in 15 min by intravenous infusion during the closure, and dose repeated at every 8 h of post-operative period. 100 ml of saline was administered by intravenous infusion in 15 min to Group III during the closure and at every 6 h of post-operative period. The researcher who knows the group of the patient prepared the test drug was blind to the evaluation of pain relief, whereas the person evaluating the analgesic effects was blind to the treatment drug. Patients then taken to recovery room and pain was assessed by VAS. If the VAS ≥3, then 1 mg of morphine was administered as an additional analgesic in 10 min intervals until the VAS decreased below 3. PCA equipment (Abbott Pain Management Provider, North Chicago, USA) used for all patients PCA device was programmed as bolus dose of 1 mg morphine on demand and 10 min locked-out intervals. Intravenous morphine was used for PCA in all groups. (Morphine concentration is 0.2 mg/ml). VAS and additional analgesic consumptions of all patients were evaluated at 0th, 1st, 2nd, 4th, 8th, 12th, 16th, 20th, and 24th h of post-operative period.
Mean arterial pressures (MAPs) and heart rate (HR), were evaluated at 5 min intervals during the operation and also at 0th, 1st, 2nd, 4th, 8th, 12th, 16th, 20th, and 24th h in the post-operative period during the evaluation of VAS and morphine consumptions. Probable post-operative side-effects (nausea, vomiting, urine retention, gastric pain, itching, dizziness, visual impairment, sedation, and respiratory depression) and patient satisfaction were noted while monitoring the pain. Nausea, vomiting, urine retention, gastric pain, itching, dizziness, and visual impairment were assessed as 0: None, 1: Mild, 2: Moderate and 3: Severe. Sedation was assessed by Ramsay Sedation Scale (RSS) (1: Fully awake and oriented, 2: Drowsy, 3: Eyes closed, but rousable with command, 4: Eyes closed, but rousable with mild physical stimulation, 5: Eyes closed, not rousable with physical stimulation).
Statistical analyzing
The primary aim of this study was to compare by means of differences in cumulative 24-h morphine consumption among groups. A total sample size of 57 (19/group) was required to detect at least 15.5 mg difference between any of two groups with a power of 90% at the 5% significance level. The difference of 15.5 mg was taken from the literature.[6] Sample size estimation was performed by using the NCSS and PASS 2000 software.
Data analyzed with SPSS for Windows 11.5 software. Normal distribution of continuous variables was assessed by Shapiro Wilk test. Descriptive statistics were presented as mean±standard deviation or median (minimum-maximum) for continuous variables, medians (25th -75th percentiles) for ordinal variables, and case count and (%) for nominal variables. Significance of differences between groups was analyzed with one way analysis of variances for means, and with Kruskal Wallis for medians. If the results of ANOVA or Kruskal Wallis was found to be significant then post hoc Tukey or nonparametric multiple comparisons were utilized for determining the distinct group. Nominal data were evaluated by Pearson's Chi-square test. Significance of differences for repeated hemodynamic and analgesic use within groups were measured with repeated measures variance analysis; changes in VAS, RSS, and Global Satisfaction Scale scores were assessed by Friedman test; significance of differences of changes of incidence of nausea and vomiting was evaluated with Cochrane's Q. If the results of repeated measures ANOVA or Friedman were found to be significant then Bonferroni adjusted multiple comparison tests or Wilcoxon Signed Ranks test were used for determining the distinct follow-up times. P values lover than 0.05 were considered as statistically significant. Bonferroni correction was used in all multiple comparisons for controlling the Type I error.
RESULTS
There were no statistically significant differences between the groups for the demographic data and durations of anesthesia and surgery (P > 0.05) [Table 1].
Table 1.
Patient characteristics
There were no statistically significant differences between groups for the average blood pressure levels and HRs at all follow-up times (P > 0.006).
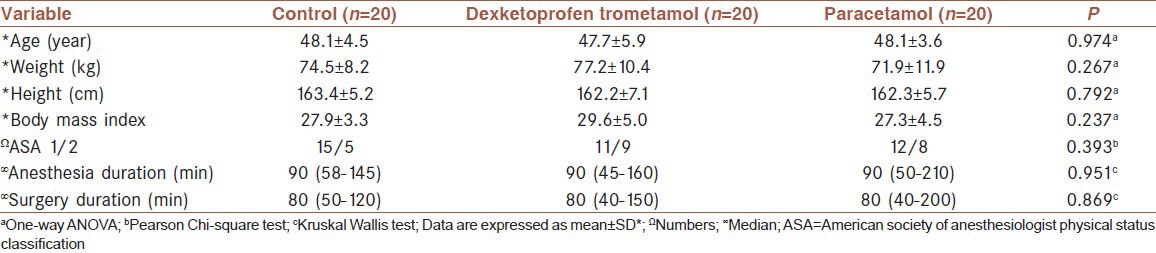
There were also no significant differences between groups for VAS scores at all follow-up times (P > 0.006) (as the significance of the difference between VAS scores were evaluated from 0th h to 24th h at 9 different evaluation times Type I error was approximately 37%. Bonferroni correction was used for controlling Type I error. When 0.05 was divided to subanalysis number the result was 0.006 (0.05/9 = 0.0056 ≈ 0.006). So the result P < 0.006 was accepted statistically significant according to Bonferroni correction. When P < 0.006 is accepted as significant Type I error was approximately 5%) [Figure 1]. Changes in VAS levels were statistically significant in each group itself (P < 0.001). Reductions in pain score at 12th, 20th, and 24th h compared with the baseline in control group, 12th, 16th, 20th, and 24th h in dexketoprofen trometamol group, and 16th, 20th, and 24th h in paracetamol group were found to be statistically significant (P < 0.0006) [Figure 1].
Figure 1.
Visual analog scale scores according to times and groups
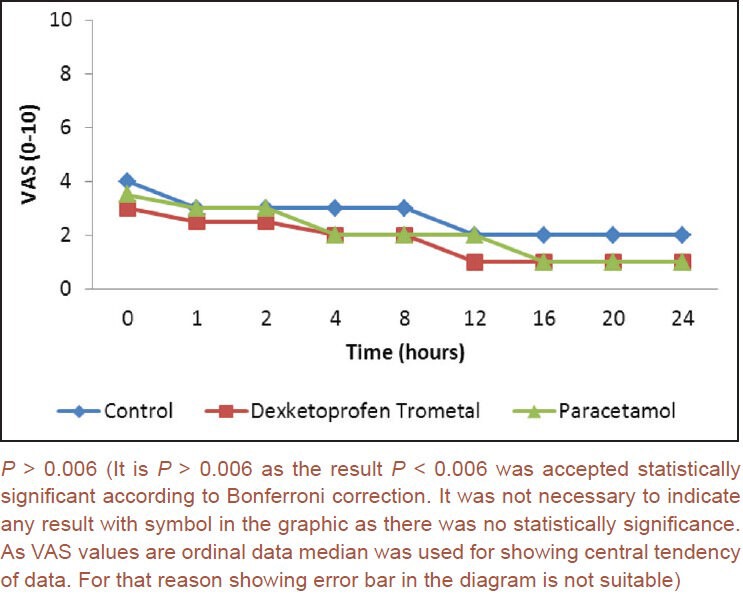
Changes in RSS levels according to time were found to be statistically significant in each group (P < 0.001). Reductions in RSS scores after the 2nd h in dexketoprofen trometamol group and 1st h in paracetamol group when compared to the baseline were found to be statistically significant (P < 0.0006). There were no statistically significant differences between groups for the RSS scores (P > 0.006) [Figure 2]. (Similar to VAS scores RSS scores were also analyzed from 0th h to 24th h at 9 different evaluation times Type I error was approximately 37%. Bonferroni correction was used for controlling Type I error. When 0.05 was divided to subanalysis number the result was 0.006 (0.05/9 = 0.0056 ≈ 0.006). So the result P < 0.006 was accepted statistically significant according to Bonferroni correction. When P < 0.006 accepted as significant Type I error was approximately 5%).
Figure 2.
Ramsay sedation scale scores according to times and groups
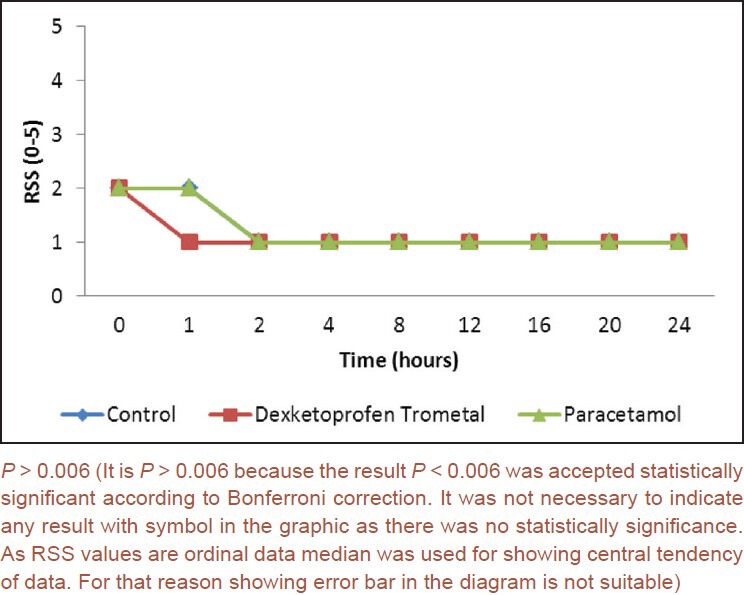
There were statistically significant differences between groups for the morphine use at 12th, 16th, 20th, and 24th h (P < 0.006). Morphine consumption in dexketoprofen trometamol group was significantly lower at 12th h when compared to control group (P < 0.006). Morphine usage was significantly lower in both dexketoprofen trometamol and paracetamol groups at 16th, 20th, and 24th h compared to control group (P < 0.006) [Figure 3].
Figure 3.
Morphine consumption according to times and groups (mL) (0.2 mg/mL)
Statistically significant differences were determined only in patient satisfaction scores at 0th and 1st h among all follow-up times (P < 0.006). Patient satisfaction scores of the control group at 0th h were significantly lower than both the dexketoprofen trometamol and the paracetamol groups (P < 0.006). However, 1st h scores of the control group were only significantly lower than the scores of the paracetamol group (P < 0.006). When the nausea and vomiting were assessed in the groups of our study, 13 patients in dexketoprofen trometamol group (65%), 10 patients in paracetamol group (50%), and 16 patients in control group (80%) had nausea complaints, and eight patients in dexketoprofen trometamol group (40%), 10 patients in paracetamol group (50%), and 13 patients in control group (65%) had vomiting complaints. There were no statistically significant differences between groups for the frequency of nausea and vomiting at all follow-up times (P > 0.006).
DISCUSSION
Pain after abdominal hysterectomy is composed of the pain in incision site, pain from visceral structures, and dynamic pain due to conditions such as coughing and mobilization, and some of these components may be felt more than others at different times of post-operative period.[7]
Combined use of opioids with paracetamol and Nonsteroidal Antiinflammatory Drugs is a known method in post-operative analgesia after total abdominal hysterectomy surgery, but intravenous use of dexketoprofen trometamol is a new method because of the lack of its parenteral form until recently and as a consequence the numbers of studies in this field are limited. Using the analgesics at the final periods of the surgery contributes to the pain control immediately after the operation. Analgesic effect of intravenous paracetamol emerges after 5-10 min of application and reaches to maximum levels in 1 h.[8,9]
In literature, various studies performed in different surgical groups notified that intravenous paracetamol usage provided lower pain scores in the post-operative period.[10,11,12]
Tuncer et al. applied oral and intravenous dexketoprofen to abdominal hysterectomy patients in their two different studies and they found that oral and iv dexketoprofen provided sufficient analgesia and decreased morphine consumption.[13,14]
We started the application of paracetamol and dexketoprofen trometamol when the closure of the incision started. Although, there were no significant differences between groups for the 0th min mean VAS values, also there weren’t any significant differences between groups for VAS scores at any follow-up times. Within groups VAS score comparisons revealed statistically significant differences for pain score reductions at 12th, 20th, and 24th h compared to baseline in control group, at 12th, 16th, 20th, and 24th h in dexketoprofen group, and at 16th, 20th, and 24th h in paracetamol group. Delbos and Boccard studied the effects of intravenous paracetemol on daily morphine use after orthopedic surgery, and found total morphine consumption significantly decreased in paracetamol group compared to placebo group.[15] Varrassi et al. found no significant difference for the morphine consumption between two groups in their study.[10] It was found in many studies that intravenous paracetamol decreased total morphine consumption compared to placebo in post-operative period.[11,16,17] It was also reported by authors that opioid consumption was decreased in dexketoprofen groups compared to placebos in their studies.[13,18,19] In our study, total consumed morphine amount decreased in 12th, 16th, 20th, and 24th h in dexketoprofen trometamol group, and in 16th, 20th, and 24th h in paracetamol group, compared to baseline during the 24 h of follow-up period. According to these, combined use of paracetamol or dexketoprofen trometamol with morphine is thought to achieve an effective analgesia by complementary impact, and will reduce the morphine consumption.
The HR and MAP were measured since post-operative pain and analgesics may also affect the hemodynamic parameters. In our study, it was found that, there were no differences between the groups regarding to MAP, HR, and respiratory rate; the inter-group differences were observed in parallel, and decreased and eventually returned to the normal levels. These reductions in HR and MAP may be due to both the reductions in VAS and anxiety, fluid resuscitation and the adverse effects of analgesic agents. In accordance with the literature data, we found that, none of our patients’ MAP and HR values decreased more than 20%; so the difference was not significant clinically.[11,19,20] We also think that, this result may be due to use of the optimum pain management.
Consort Diagram.
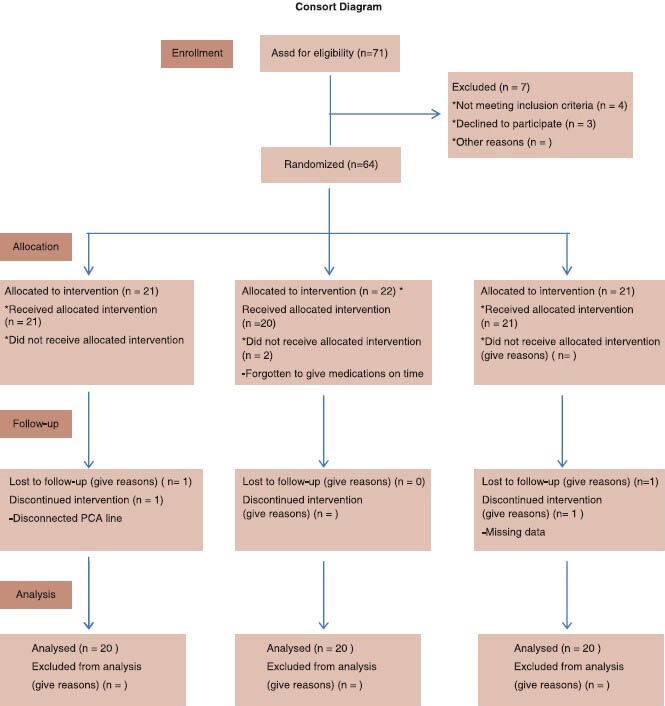
Patient randomization and disposition[29]
The main factors that are limiting the widespread use of the opioids are respiratory depression and sedation.[21,22] The deaths that are seen during the opioid usage depend on respiratory depression and sedation is a valuable parameter that is used for early recognition of respiratory depression.[23] Respiratory depression rate, which is the most feared side effect, is very low in the literature data. The respiratory rate did not decreased below 10/min during the opioid treatment in all groups in our study.
The Ramsay Sedation Score started to decrease significantly 2 h from the beginning in the control and the dexketoprofen trometamol groups whereas 1 h from the beginning in the paracetamol group. In their study, Hanna et al. found that there were higher sedation scores of dexketoprofen and ketoprofen compared to placebo at 2nd and 13th h interestingly; however, it was explained as the observers assessed sedation and sleep wrongly due to the quality of this analgesic.[18] In the study by Hernández-Palazón et al., it was found that the sedation was lower in i.v paracetamol group when compared with placebo and the difference was statistically significant.[17] In another study, efficacy of intravenous dexketoprofen trometamol was evaluated by adding i.v PCA tramadol for postdiscectomy pain, similar to the results of our study no significance was observed according to RSS.[24]
There are many factors in the etiology of the post-operative nausea and vomiting such as anesthetic gases and surgical procedures. Opioids are one of the most important factors that increase the incidence of the post-operative nausea and vomiting.[25] In our study, we did not determine any statistically significant differences between the groups in terms of nausea and vomiting. The amount of total morphine was lower in the dexketoprofen trometamol and paracetamol groups compared with control group; however, there were no differences in the incidence of nausea and vomiting between the groups. Beside opioid usage, it was because of the higher incidence of post-operative nausea and vomiting in gynecological illnesses.[26] In addition, this situation may be related to factors increasing the susceptibility of patients with nausea and vomiting and which we did not ask before (nausea due to vehicles, history of sensitivity to the drugs, which were used before etc.). The sample size of this study was calculated for evaluating the difference in 24 h total morphine consumption between groups so for evaluating nausea and vomiting a bigger sample size may be necessary. None of our patients had urinary retention, stomach ache, dizziness, and visual impairment.
The patient satisfaction is one of the most important criteria in assessing the efficacy of the analgesic and the anxiety of the patient. Since, the moods of the individuals and threshold of sensitivity to the painful stimuli can be different, it is expected that the satisfaction about the post-operative treatments may differ in between different people. In a study by Peduto et al., it was reported that global efficiency was defined as good or excellent in 87% of patients receiving i.v paracetamol, whereas 65% of patients in the control group.[16] Varrassi et al. reported that, global efficacy was assessed as good or excellent similarly in i.v paracetamol group and ketorolac group.[10] Tuncer et al. determined that they found similar patient satisfaction rates between the groups.[13] Cakan et al. found increased satisfaction scores in paracetamol management in their lumbar discectomy patients and they postulated that this may be the result of high serotonin levels caused by paracetamol.[27] Ekmekçi et al. added dexketoprofen trometamol to i.v PCA tramadol for post laparoscopic pain treatment and observed increased patient satisfaction in their dexketoprofen trometamol + tramadol group.[28] In our study, in all follow-up times, there was only significant difference between the 0th and 1st h satisfaction scores between the groups. Patient satisfaction score of the control group at 0th h was lower than both satisfaction scores of the dexketoprofen trometamol and the paracetamol groups. There was a significant difference in satisfaction scores compared to baseline scores only in the control group and satisfaction scores were increased at statistically significant level in all the follow-up time except the 1st and 4th h. In dexketoprofen trometamol group, only 6/20 patients (30%) stated patient satisfaction as good, whereas 14 (70%) as excellent. This rate is the same as the paracetamol group. However 10/20 patients (50%) indicated patient satisfaction as good, 8 (40%) as excellent and 2 (10%) as moderate.
As a result, paracetamol and dexketoprofen trometamol combined with PCA morphine provides only slight advantages on certain times such as higher patient satisfaction and lower sedation scores. According to results of the present study, routine addition of dexketoprofen trometamol and Paracetamol to PCA morphine after hysterectomies is not recommended.
ACKNOWLEDGMENTS
We thank all staff of Gynecology and Obstetrics Clinic and all the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kehlet H, Dahl JB. The value of “multimodal” or “balanced analgesia” in postoperative pain treatment. Anesth Analg. 1993;77:1048–56. doi: 10.1213/00000539-199311000-00030. [DOI] [PubMed] [Google Scholar]
- 2.Kehlet H. Controlling acute pain-role of preemptive analgesia, peripheral treatment and balanced analgesia and effects on outcome. In: Mitchell M, editor. Pain 1999-An Updated Review. Seattle: IASP Press; 1999. pp. 459–62. [Google Scholar]
- 3.Pinzur MS, Garla PG, Pluth T, Vrbos L. Continuous postoperative infusion of a regional anesthetic after an amputation of the lower extremity. A randomized clinical trial. J Bone Joint Surg Am. 1996;78:1501–5. doi: 10.2106/00004623-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bannwarth B, Péhourcq F. Pharmacologic basis for using paracetamol: Pharmacokinetic and pharmacodynamic issues. Drugs. 2003;63(Spec No 2):5–13. [PubMed] [Google Scholar]
- 5.Rodríguez MJ, Arbós RM, Amaro SR. Dexketoprofen trometamol: Clinical evidence supporting its role as a painkiller. Expert Rev Neurother. 2008;8:1625–40. doi: 10.1586/14737175.8.11.1625. [DOI] [PubMed] [Google Scholar]
- 6.Kesimci E, Gümüş T, Izdeş S, Sen P, Kanbak O. Comparison of efficacy of dexketoprofen versus paracetamol on postoperative pain and morphine consumption in laminectomy patients. Agri. 2011;23:153–9. doi: 10.5505/agri.2011.86548. [DOI] [PubMed] [Google Scholar]
- 7.Zohar E, Fredman B, Phillipov A, Jedeikin R, Shapiro A. The analgesic efficacy of patient-controlled bupivacaine wound instillation after total abdominal hysterectomy with bilateral salpingo-oophorectomy. Anesth Analg. 2001;93:482–7,. doi: 10.1097/00000539-200108000-00048. [DOI] [PubMed] [Google Scholar]
- 8.Collins VJ. Physiologic and Pharmacologic Bases of Anesthesiology. Pennsylvania, USA: Williams & Wilkins; 1996. Opiate and narcotic drugs; pp. 544–81. [Google Scholar]
- 9.Ferrante FM. Opioids. In: Ferrante FM, VadeBoncour TR, editors. Postoperative Pain Management. New York: Churchill-Livingstone Inc; 1993. pp. 367–90. [Google Scholar]
- 10.Varrassi G, Marinangeli F, Agrò F, Aloe L, De Cillis P, De Nicola A, et al. A double-blinded evaluation of propacetamol versus ketorolac in combination with patient-controlled analgesia morphine: Analgesic efficacy and tolerability after gynecologic surgery. Anesth Analg. 1999;88:611–6. doi: 10.1097/00000539-199903000-00028. [DOI] [PubMed] [Google Scholar]
- 11.Arici S, Gurbet A, Türker G, Yavaşcaoğlu B, Sahin S. Preemptive analgesic effects of intravenous paracetamol in total abdominal hysterectomy. Agri. 2009;21:54–61. [PubMed] [Google Scholar]
- 12.Hynes D, McCarroll M, Hiesse-Provost O. Analgesic efficacy of parenteral paracetamol (propacetamol) and diclofenac in post-operative orthopaedic pain. Acta Anaesthesiol Scand. 2006;50:374–81. doi: 10.1111/j.1399-6576.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 13.Tuncer S, Tavlan A, Köstekçi H, Reisli R, Otelcioğlu S. Postoperatif ağrida deksketoprofen kullanimi. Agri. 2006;18:30–5. [PubMed] [Google Scholar]
- 14.Tuncer S, Reisli R, Keçecioğlu M, Erol A. The effects of intravenous dexketoprofen on postoperative analgesia and morphine consumption in patients undergoing abdominal hysterectomy. Agri. 2010;22:98–102. [PubMed] [Google Scholar]
- 15.Delbos A, Boccard E. The morphine-sparing effect of propacetamol in orthopedic postoperative pain. J Pain Symptom Manage. 1995;10:279–86. doi: 10.1016/0885-3924(95)00004-I. [DOI] [PubMed] [Google Scholar]
- 16.Peduto VA, Ballabio M, Stefanini S. Efficacy of propacetamol in the treatment of postoperative pain. Morphine-sparing effect in orthopedic surgery. Italian Collaborative Group on Propacetamol. Acta Anaesthesiol Scand. 1998;42:293–8. doi: 10.1111/j.1399-6576.1998.tb04919.x. [DOI] [PubMed] [Google Scholar]
- 17.Hernández-Palazón J, Tortosa JA, Martínez-Lage JF, Pérez-Flores D. Intravenous administration of propacetamol reduces morphine consumption after spinal fusion surgery. Anesth Analg. 2001;92:1473–6. doi: 10.1097/00000539-200106000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Hanna MH, Elliott KM, Stuart-Taylor ME, Roberts DR, Buggy D, Arthurs GJ. Comparative study of analgesic efficacy and morphine-sparing effect of intramuscular dexketoprofen trometamol with ketoprofen or placebo after major orthopaedic surgery. Br J Clin Pharmacol. 2003;55:126–33. doi: 10.1046/j.1365-2125.2003.01727.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iohom G, Walsh M, Higgins G, Shorten G. Effect of perioperative administration of dexketoprofen on opioid requirements and inflammatory response following elective hip arthroplasty. Br J Anaesth. 2002;88:520–6. doi: 10.1093/bja/88.4.520. [DOI] [PubMed] [Google Scholar]
- 20.Avellaneda C, Gómez A, Martos F, Rubio M, Sarmiento J, de la Cuesta FS. The effect of a single intravenous dose of metamizol 2 g, ketorolac 30 mg and propacetamol 1 g on haemodynamic parameters and postoperative pain after heart surgery. Eur J Anaesthesiol. 2000;17:85–90. doi: 10.1046/j.1365-2346.2000.00607.x. [DOI] [PubMed] [Google Scholar]
- 21.Coetzee JF, van Loggerenberg H. Tramadol or morphine administered during operation: A study of immediate postoperative effects after abdominal hysterectomy. Br J Anaesth. 1998;81:737–41. doi: 10.1093/bja/81.5.737. [DOI] [PubMed] [Google Scholar]
- 22.Carlborg L, Lindoff C, Hellman A. Diclofenac versus pethidine in the treatment of pain after hysterectomy. Eur J Anaesthesiol. 1987;4:241–7. [PubMed] [Google Scholar]
- 23.Duante LT, Fernandes Mdo C, Costa VV, Saraiva RA. The Incidence of Postoperative Respiratory Depression in Patients undergoing Intravenous or Epidural Analgesia with Opioids. Rev Bras Anesthesiol. 2009;59:409–420. doi: 10.1590/s0034-70942009000400003. [DOI] [PubMed] [Google Scholar]
- 24.Yazar MA, Inan N, Ceyhan A, Sut E, Dikmen B. Postoperative analgesic efficacy of intravenous dexketoprofen in lumbar disc surgery. J Neurosurg Anesthesiol. 2011;23:193–7. doi: 10.1097/ANA.0b013e31820d1ebb. [DOI] [PubMed] [Google Scholar]
- 25.Stanley G, Appadu B, Mead M, Rowbotham DJ. Dose requirements, efficacy and side effects of morphine and pethidine delivered by patient-controlled analgesia after gynaecological surgery. Br J Anaesth. 1996;76:484–6. doi: 10.1093/bja/76.4.484. [DOI] [PubMed] [Google Scholar]
- 26.Jørgensen H, Fomsgaard JS, Dirks J, Wetterslev J, Andreasson B, Dahl JB. Effect of peri-and postoperative epidural anaesthesia on pain and gastrointestinal function after abdominal hysterectomy. Br J Anaesth. 2001;87:577–83. doi: 10.1093/bja/87.4.577. [DOI] [PubMed] [Google Scholar]
- 27.Cakan T, Inan N, Culhaoglu S, Bakkal K, Başar H. Intravenous paracetamol improves the quality of postoperative analgesia but does not decrease narcotic requirements. J Neurosurg Anesthesiol. 2008;20:169–73. doi: 10.1097/ANA.0b013e3181705cfb. [DOI] [PubMed] [Google Scholar]
- 28.Ekmekçi P, Kazak Bengisun Z, Kazbek BK, Öziş SE, Taştan H, Süer AH. The efficacy of adding dexketoprofen trometamol to tramadol with patient controlled analgesia technique in post-laparoscopic cholecystectomy pain treatment. Agri. 2012;24:63–8. doi: 10.5505/agri.2012.71501. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KF, Altman DG, Moher D for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. doi: 10.1136/bmj.c332. [DOI] [PMC free article] [PubMed] [Google Scholar]


