Abstract
Stroke scales can be classified as clinicometric scales and functional impairment, handicap scales. All studies describing stroke scales were reviewed by internet searching engines with the final search performed on January 1, 2013. The following string of keywords was entered into search engines; stroke, scale, score and disability. Despite advantages of modified National Institute of Health Stroke Scale and Scandinavian stroke scale comparing to the NIHSS, including their simplification and less inter-rater variability; most of the stroke neurologists around the world continue using the NIHSS. The modified Rankin scale (mRS) and Barthel index (BI) are widely used functional impairment and disability scales. Distinction between grades of mRS is poorly defined. The Asian stroke disability scale is a simplified functional impairment, handicap scale which is as valid as mRS and BI. At the present time, the NIHSS, mRS and BI are routine stroke scales because physicians have used to work with these scales for more than two decades, although it could not be an acceptable reason. On the other side, results of previous stroke trials, which are the basis of stroke management guidelines are driven using these scales.
Keywords: Disability, scale, score, stroke
INTRODUCTION
A reproducible and valid method for quantification of the neurological deficit that occurs after stroke is essential for monitoring patients; many stroke scales have been proposed for this purpose.[1] Stroke scales represent a useful tool for estimating the severity of stroke at onset and for assessing prognostic information in hospital. In general, a stroke scale consists of several variables for observing the signs and symptoms and each variable is categorized for scoring.[1] In developing an ideal stroke scale, issues of simplicity, reliability, validity and popularity of use must be pursued, especially if a scale is to be used by a broad array of practitioners.[2] Reliability of a stroke scales could be improved with a personal and videotape training.[3] Simplicity and time taking is important in any outcome measure, especially for use in stroke patients with cognitive problems and feelings of tiredness.[4] Stroke scales can be classified as parametric or clinicometric scales on the basis of physical deficit and functional impairment, handicap scales.[1,5] Evaluating the impact of new treatments requires the use of reliable and valid outcome measures.[6] Development of stroke outcome classification systems is necessary because neurological deficits often lead to permanent impairments, disabilities and compromised quality-of-life.[7]
METHODS
A retrospective review was performed about stroke scales. Medline, Ovid, PubMed, Google, Proquest, Scopus, Cochrane Library, Elsevier, Thompson, ISI, Index Medicus, Index Copernicus and Science Direct was used as search engines. The following string of keywords was selected (stroke) and (scale) and (score) and (disability) and (grade) with the final search performed on January, 1, 2013. At the other side, library archives of Mashhad University of Medical Sciences were searched for this purpose in paper journals published between 1970 and 2013.
Comparison of clinicometric stroke scales
The National Institute of Health Stroke Scale (NIHSS) is the most frequently used stroke deficit scale in routine clinical practice and clinical trials.[8] In spite of its great success, there are problems with the NIHSS. It contains items with poor reliability and has been criticized for its redundancy and complexity.[9,10] The NIHSS overall reliability is clear, however assessments have consistently shown specific items that yield low inter-rater reliability.[10,11] These items with poorer NIHSS reliability included facial palsy, ataxia, dysarthria and level of consciousness.[12] Among over 15,000 individuals who have taken online NIHSS certification, the NIHSS items with poorer inter-rater reliability included facial palsy (k = 0.25), ataxia (k = 0.15), level of consciousness (k = 0.43), dysarthria (k = 0.46) and gaze (k = 0.44).[13] These NIHSS items with poor inter-rater reliability have also been identified in Spanish, Italian and Chinese versions of NIHSS.[14,15,16] These elements may contribute to difficulties in practitioner communication, incorrect hospital care patterns that are based on the NIHSS; e.g., decisions to give thrombolytics, variable trial enrollments and even possible difficulties with assessing patient outcome in clinical trials.[13] Given the unreliability of some of the NIHSS items, patients may score high on the NIHSS when they actually have mild strokes but questionable other findings. Alternatively, patients may score as mild even if they have more sever deficits, because unreliability may result in certain items being unscored.[13,17] Patient with sever stroke may not be able to receive NIHSS scores for ataxia or dysarthria because their arousal state may preclude testing these items. Because these items are not scored abnormal unless patients produce testable behaviors, these patients may be too sick to score on these items.[13,18] Though the patients may clinically improve, their NIHSS scores may artificially worsen since now items such as ataxia and dysarthria can receive the scores that were previously unscored.[13,19] Since these items have been removed from the modified NIHSS, this difficulty can be avoided or at least lessened. The NIHSS was modified, which maintains similar internal structure.[11,13,18] Level of consciousness was redundant and dropped from the new scale. Ataxia showed poor reliability, so it was excluded. Facial palsy and dysarthria showed poor reliability and were redundant, so they were eliminated.[11,13,18] The sensory item was simplified due to poor reliability.[11] With fewer items and simpler grading, the modified NIHSS was intended to be simpler and easier to administer.[11,12,13] The resulting modified NIHSS has shown significantly higher reliability and validity than NIHSS.[13,18] In the NIHSS, 7 of 42 points are related to language function, while only 2 of 42 points are attributed to neglect functions.[20,21] Redundant items are noted in the NIHSS have been deleted from the modified NIHSS, resulting in a more balanced clinical scale. Therefore, lateralization bias may be minimized.[13,20,21] The author suggests scoring 0-3 to language function and including mute or global aphasia in score 3 as severe aphasia. This scoring strategy improves hemisphere balance between language and neglect items in modified NIHSS. Both NIHSS and modified NIHSS failed to accurately or reliably detect stroke severity in patients with posterior circulation findings.[13,22] With the removal of the ataxia item, there may be a concern that the modified NIHSS would be even less able to assess brainstem strokes. However, since ataxia is a poorly reliable NIHSS item, the benefit of using a scale that inconsistently assesses the posterior circulation, may not out weight the consistency of modified NIHSS.[12,13] Many clinical trials routinely include only anterior circulation strokes, so that there is less need to measure posterior circulation deficit for this purpose. However, stroke severity scale specialized for posterior circulation strokes has been developed and validated in Israel.[22] The Scandinavian stroke scale (SSS) is easier than NIHSS for clinical practice in acute stroke patients and has been used in many clinical trials. The NIHSS,[9,10,11,12,13,14,15,16,17,18,19,20,21] Canadian neurological scale,[23,24] European stroke scale (designed for patients with middle cerebral artery stroke),[25] SSS,[26] Japan stroke scale,[1] Orpington prognostic scale,[27] Orgogozo scale[28] and numerous other scales developed for clinicometric assessment of acute stroke patients.[13] The Orpington prognostic scale is easier than NIHSS in clinical practice and additionally evaluates the cognitive function.[27] Despite advantages of modified NIHSS and SSS comparing to the NIHSS (including their simplification and less inter-rater variability), most of the stroke Neurologists around the world continue using the NIHSS because they have used to work with it for more than two decades, although it could not be an acceptable reason. At the other side, results of previous stroke trials, which are the basis of stroke management guidelines are driven using the initial NIHSS. The stroke outcome classification of the American Heart Association is too comprehensive and time consuming to be used in the routine clinical practice and did not enjoy the widespread acceptance around the world.[7]
Comparison of functional impairment and handicap stroke scales
For quality-of-life and outcome measures after stroke, Duncan et al. in the US found that eight key areas (strength, hand function, activities of daily living, mobility, communication, memory, emotion and social participation) emerged as key areas from the patients perspective.[29] Similarly, Williams et al. reported that patients identified 12 key domains (mobility, energy, upper extremity function, work/productivity, mood, self-care, social roles, family roles, vision, language, thinking and personality).[30] The basic self-care tasks are feeding, grooming, dressing, bathing, toileting, including sphincter control and mobility, including transferring from place to place.[7] These are called basic activities of daily living. Independence in these activities could enable the stroke patient to live at home with the help from family or community providers for meals and other household tasks as needed.[7,29,30] More complex activities of daily living are called instrumental activities of daily living. These tasks are performed to maintain independence in the home and community and include shopping, using transportation, telephoning, preparing meals, handling finances and maintaining a household.[7,29,30] Other instrumental activities of daily living that affect quality-of-life are work skills, religious activities and leisure time and recreational activities.[7,29,30] Leisure activities are demonstrated as the strongest association to subject well-being.[31] The modified Rankin scale (mRS) and Barthel index (BI) are widely used functional impairment, disability scales, which have been proven to be a valid and reliable for defining outcome in stroke patients.[32] Despite BI, distinction between grades of mRS are poorly defined.[33] Inter-rater variability introduces noise into trial outcome assessments and reduces the power of clinical trials to detect treatment outcome.[34] A variety of approaches to minimize inter-rater variation of mRS have been described or proposed, including: (1) Use of a formal structured interview, (2) training and certification programs using written and video case vignettes and (3) central panel adjudication of local site-recorded video assessments.[34] However, the instruments and approaches developed to date have not consistently been shown to reduce inter-rater variability for mRS.[33,34] However, there is little consensus on the optimal implementation of the BI and mRS as an outcome measure in acute stroke trials[32] and it is unclear which outcome scale is preferable.[32] The Japan stroke scale[1] and Kurashiki pre-hospital stroke scale are clinicometric stroke scales which are designed in Asia.[1,35] Chinese stroke scale is a comprehensive functional impairment scale designed in Asian continent.[36] The Asian stroke disability scale (ASDS) was provided as a simplified functional impairment, handicap scale and inter-rater reliability of ASDS compared with mRS and BI.[37,38] Development procedure for the ASDS is similar to method of making Japan stroke scale.[1,37] The procedure is summarized as following steps: (1) Select the variables, (2) categorize the variables, (3) evaluate the categorization for their distribution and sensitivity, (4) modify and re-evaluate the categorization, (5) repeat procedures 1 through 4 until the appropriate categorizations are obtained.[1,37] Three items including; self-care, mobility and daily activities were selected as variables for development of the ASDS based on the contribution of each item to the prognosis and a review of currently available stroke scales.[1,37] The variables were provisionally graded on a 2- to 4-point scale based on the importance of each item. Each of the variables was categorized into three categories.[37] The total score for a patient could be calculated from the sum of the scores for each of the variables ranging from 0 to 8.[37] Table 1 shows details of the ASDS. The ASDS is simple, requires less than 1 min to perform the test and is as valid as mRS and BI in assessment of functional impairment of stroke patients.[37,38] The quantitative and qualitative inter-rater variability of ASDS is similar to the mRS and BI.[36,37] The paired inter-rater variability of mRS, BI and ASDS scores based on qualitative categorization was not significant for the three methods, P > 0.05.[37,38] Inter-rater reliability of mRS was poor (k = 0.16) in the study conducted by Quinn et al.[39] Comparing estimated scores between the paired assessors, there was again poor agreement in 30% and significant variability (k = 0.38) of mRS score.[39] In the evaluation of Rankin focused assessment tool, rater's scores concurred fully in 47 of 50 patients and in the remaining three patients, scores differed by one level.[34] A review of literature about inter-rate reliability of mRS revealed moderate inter-rater reliability, which improved with structured interviews.[40] The difference of disability scores based on the mRS, BI and SSS are small and these scores have excellent agreement with each other, whereas modified NIHSS has substantial agreement with mRS and BI in a UK study.[26] Another comparison study in UK was performed on 1400 patients.[19] When the mRS and BI scores were dichotomized at 95 and 1 respectively, the NIHSS appeared more sensitive than the BI or mRS.[41] Diagnostic accuracy of BI in serial assessments of ischemic stroke patients was performed in the Netherland.[42] Assessment of the BI in acute stroke showed good discriminative properties for the final outcome of BI at 6 months.[42] Another study in the Netherland compared with five stroke scales; the Orgogozo scale, the NIHSS, the Canadian neurological scale and the SSS with measures of disability and handicap and quality-of-life according to the mRS and BI.[28] The five stroke scales were highly related to one another but the correlation between stroke scales and functional scales was less than 0.70 and decreased from BI (47.5%) to mRS (36.5%).[28] Therefore, clinicometric stroke scales only partly explain functional health and impact of impairments on functional outcomes seems to be under estimated by the stroke scale weights.[28] The Frenchay stroke scale,[43,44] Canadian occupational performance measure,[45] stroke impact scale[46] and numerous other functional impairment scale have been developed for use in stroke patients by stroke specialists and occupational therapists.[1,5,6,47,48,49] Despite the development of better functional impairment scales, stroke neurologists around the world continue using the mRS and BI,[50] because they have used to work with these scales for decades, although it could not be an acceptable reason. At the other side, results of the previous stroke trials, which are the basis of stroke management guidelines are driven using the initial mRS and BI.
Table 1.
The Asian stroke disability scale*
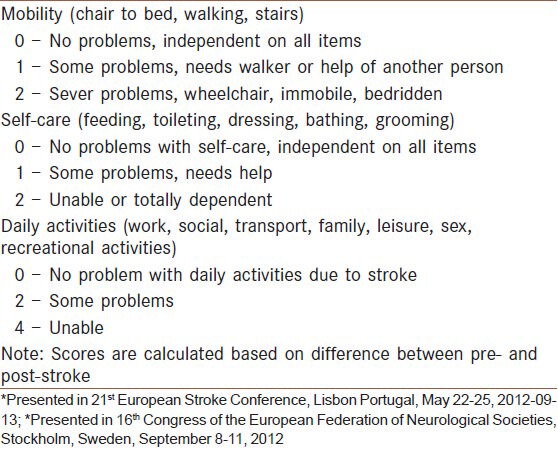
CONCLUSION
Despite advantages of modified NIHSS and SSS comparing to the NIHSS, most of the stroke neurologists around the world continue using the NIHSS. The mRS and BI are widely used functional impairment, disability scales and it is unclear, which outcome scale is preferable. The ASDS is a simplified functional impairment and disability scale, which is as valid as mRS and BI.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gotoh F, Terayama Y, Amano T. Stroke Scale Committee of the Japan Stroke Society. Development of a novel, weighted, quantifiable stroke scale: Japan stroke scale. Stroke. 2001;32:1800–7. doi: 10.1161/01.str.32.8.1800. [DOI] [PubMed] [Google Scholar]
- 2.Asplund K. Clinimetrics in stroke research. Stroke. 1987;18:528–30. doi: 10.1161/01.str.18.2.528. [DOI] [PubMed] [Google Scholar]
- 3.Lyden P, Brott T, Tilley B, Welch KM, Mascha EJ, Levine S, et al. Improved reliability of the NIH stroke scale using video training. NINDS TPA stroke study group. Stroke. 1994;25:2220–6. doi: 10.1161/01.str.25.11.2220. [DOI] [PubMed] [Google Scholar]
- 4.Buck D, Jacoby A, Massey A, Ford G. Evaluation of measures used to assess quality of life after stroke. Stroke. 2000;31:2004–10. doi: 10.1161/01.str.31.8.2004. [DOI] [PubMed] [Google Scholar]
- 5.Sturm JW, Osborne RH, Dewey HM, Donnan GA, Macdonell RA, Thrift AG. Brief comprehensive quality of life assessment after stroke: The assessment of quality of life instrument in the north East Melbourne stroke incidence study (NEMESIS) Stroke. 2002;33:2888–94. doi: 10.1161/01.str.0000040407.44712.c7. [DOI] [PubMed] [Google Scholar]
- 6.Baker K, Cano SJ, Playford ED. Outcome measurement in stroke: A scale selection strategy. Stroke. 2011;42:1787–94. doi: 10.1161/STROKEAHA.110.608505. [DOI] [PubMed] [Google Scholar]
- 7.Kelly-Hayes M, Robertson JT, Broderick JP, Duncan PW, Hershey LA, Roth EJ, et al. The American Heart Association stroke outcome classification: Executive summary. Stroke. 1998;97:2474–8. doi: 10.1161/01.cir.97.24.2474. [DOI] [PubMed] [Google Scholar]
- 8.Goldstein LB, Samsa GP. Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke. 1997;28:307–10. doi: 10.1161/01.str.28.2.307. [DOI] [PubMed] [Google Scholar]
- 9.Dewey HM, Donnan GA, Freeman EJ, Sharples CM, Macdonell RA, McNeil JJ, et al. Interrater reliability of the National Institutes of Health Stroke Scale: Rating by neurologists and nurses in a community-based stroke incidence study. Cerebrovasc Dis. 1999;9:323–7. doi: 10.1159/000016006. [DOI] [PubMed] [Google Scholar]
- 10.Kasner SE, Chalela JA, Luciano JM, Cucchiara BL, Raps EC, McGarvey ML, et al. Reliability and validity of estimating the NIH stroke scale score from medical records. Stroke. 1999;30:1534–7. doi: 10.1161/01.str.30.8.1534. [DOI] [PubMed] [Google Scholar]
- 11.Berthier E, Decavel P, Vuiller F, Verlut C, Moulin T, Medeiros de Bustos E. Review: Reliability of NIHSS by telemedicine. Eur Res Telemed. 2012;1:111–4. [Google Scholar]
- 12.Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, et al. Underlying structure of the National Institutes of Health Stroke Scale: Results of a factor analysis. NINDS TPA stroke trial investigators. Stroke. 1999;30:2347–54. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- 13.Meyer BC, Lyden PD. The modified National Institutes of Health Stroke Scale: Its time has come. Int J Stroke. 2009;4:267–73. doi: 10.1111/j.1747-4949.2009.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domínguez R, Vila JF, Augustovski F, Irazola V, Castillo PR, Rotta Escalante R, et al. Spanish cross-cultural adaptation and validation of the National Institutes of Health Stroke Scale. Mayo Clin Proc. 2006;81:476–80. doi: 10.4065/81.4.476. [DOI] [PubMed] [Google Scholar]
- 15.Pezzella FR, Picconi O, De Luca A, Lyden PD, Fiorelli M. Development of the Italian version of the National Institutes of Health Stroke Scale: It-NIHSS. Stroke. 2009;40:2557–9. doi: 10.1161/STROKEAHA.108.534495. [DOI] [PubMed] [Google Scholar]
- 16.Sun TK, Chiu SC, Yeh SH, Chang KC. Assessing reliability and validity of the Chinese version of the stroke scale: Scale development. Int J Nurs Stud. 2006;43:457–63. doi: 10.1016/j.ijnurstu.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Josephson SA, Hills NK, Johnston SC. NIH stroke scale reliability in ratings from a large sample of clinicians. Cerebrovasc Dis. 2006;22:389–95. doi: 10.1159/000094857. [DOI] [PubMed] [Google Scholar]
- 18.Meyer BC, Hemmen TM, Jackson CM, Lyden PD. Modified National Institutes of Health Stroke Scale for use in stroke clinical trials: Prospective reliability and validity. Stroke. 2002;33:1261–6. doi: 10.1161/01.str.0000015625.87603.a7. [DOI] [PubMed] [Google Scholar]
- 19.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology. 2001;56:1015–20. doi: 10.1212/wnl.56.8.1015. [DOI] [PubMed] [Google Scholar]
- 20.Woo D, Broderick JP, Kothari RU, Lu M, Brott T, Lyden PD, et al. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? NINDS t-PA stroke study group. Stroke. 1999;30:2355–9. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- 21.Millis SR, Straube D, Iramaneerat C, Smith EV, Jr, Lyden P. Measurement properties of the National Institutes of Health Stroke Scale for people with right-and left-hemisphere lesions: Further analysis of the clomethiazole for acute stroke study-ischemic (class-I) trial. Arch Phys Med Rehabil. 2007;88:302–8. doi: 10.1016/j.apmr.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Gur AY, Lampl Y, Gross B, Royter V, Shopin L, Bornstein NM. A new scale for assessing patients with vertebrobasilar stroke-the Israeli vertebrobasilar stroke scale (IVBSS): Inter-rater reliability and concurrent validity. Clin Neurol Neurosurg. 2007;109:317–22. doi: 10.1016/j.clineuro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Côté R, Battista RN, Wolfson C, Boucher J, Adam J, Hachinski V. The Canadian neurological scale: Validation and reliability assessment. Neurology. 1989;39:638–43. doi: 10.1212/wnl.39.5.638. [DOI] [PubMed] [Google Scholar]
- 24.Bushnell CD, Johnston DC, Goldstein LB. Retrospective assessment of initial stroke severity: Comparison of the NIH stroke scale and the Canadian neurological scale. Stroke. 2001;32:656–60. doi: 10.1161/01.str.32.3.656. [DOI] [PubMed] [Google Scholar]
- 25.Hantson L, De Weerdt W, De Keyser J, Diener HC, Franke C, Palm R, et al. The European stroke scale. Stroke. 1994;25:2215–9. doi: 10.1161/01.str.25.11.2215. [DOI] [PubMed] [Google Scholar]
- 26.Govan L, Langhorne P, Weir CJ. Categorizing stroke prognosis using different stroke scales. Stroke. 2009;40:3396–9. doi: 10.1161/STROKEAHA.109.557645. [DOI] [PubMed] [Google Scholar]
- 27.Lai SM, Duncan PW, Keighley J. Prediction of functional outcome after stroke: Comparison of the Orpington prognostic scale and the NIH stroke scale. Stroke. 1998;29:1838–42. doi: 10.1161/01.str.29.9.1838. [DOI] [PubMed] [Google Scholar]
- 28.De Haan R, Horn J, Limburg M, Van Der Meulen J, Bossuyt P. A comparison of five stroke scales with measures of disability, handicap, and quality of life. Stroke. 1993;24:1178–81. doi: 10.1161/01.str.24.8.1178. [DOI] [PubMed] [Google Scholar]
- 29.Duncan PW, Wallace D, Lai SM, Johnson D, Embretson S, Laster LJ. The stroke impact scale version 2.0. Evaluation of reliability, validity, and sensitivity to change. Stroke. 1999;30:2131–40. doi: 10.1161/01.str.30.10.2131. [DOI] [PubMed] [Google Scholar]
- 30.Williams LS, Weinberger M, Harris LE, Clark DO, Biller J. Development of a stroke-specific quality of life scale. Stroke. 1999;30:1362–9. doi: 10.1161/01.str.30.7.1362. [DOI] [PubMed] [Google Scholar]
- 31.Sveen U, Thommessen B, Bautz-Holter E, Wyller TB, Laake K. Well-being and instrumental activities of daily living after stroke. Clin Rehabil. 2004;18:267–74. doi: 10.1191/0269215504cr719oa. [DOI] [PubMed] [Google Scholar]
- 32.Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 2005;36:1984–7. doi: 10.1161/01.STR.0000177872.87960.61. [DOI] [PubMed] [Google Scholar]
- 33.Quinn TJ, Dawson J, Walters MR, Lees KR. Variability in modified Rankin scoring across a large cohort of international observers. Stroke. 2008;39:2975–9. doi: 10.1161/STROKEAHA.108.515262. [DOI] [PubMed] [Google Scholar]
- 34.Saver JL, Filip B, Hamilton S, Yanes A, Craig S, Cho M, et al. Improving the reliability of stroke disability grading in clinical trials and clinical practice: The Rankin focused assessment (RFA) Stroke. 2010;41:992–5. doi: 10.1161/STROKEAHA.109.571364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iguchi Y, Kimura K, Watanabe M, Shibazaki K, Aoki J. Utility of the Kurashiki prehospital stroke scale for hyperacute stroke. Cerebrovasc Dis. 2011;31:51–6. doi: 10.1159/000320854. [DOI] [PubMed] [Google Scholar]
- 36.Kong TK, Lum CM, Mo KK. Development of a hierarchical activities of daily living scale for Chinese stroke patients in geriatric day hospitals. Aging (Milano) 1995;7:173–8. doi: 10.1007/BF03324309. [DOI] [PubMed] [Google Scholar]
- 37.Ghandehari K. Design of Asian stroke disability scale (ASDS) Eur J Neurol. 2012;19(Suppl 1):188. [Google Scholar]
- 38.Ghandehari K, Ghandehari K, Saffarian-Toosi G, Masoudinezhad S, Yazdani S, Nooraddin A, et al. Comparative interrater reliability of Asian stroke disability scale, modified Rankin scale and Barthel index in patients with brain infarction. ARYA Atheroscler. 2012;8:153–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Quinn TJ, Dawson J, Walters MR, Lees KR. Exploring the reliability of the modified rankin scale. Stroke. 2009;40:762–6. doi: 10.1161/STROKEAHA.108.522516. [DOI] [PubMed] [Google Scholar]
- 40.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–6. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 41.Young FB, Weir CJ, Lees KR GAIN International Trial Steering Committee and Investigators. Comparison of the National Institutes of Health Stroke Scale with disability outcome measures in acute stroke trials. Stroke. 2005;36:2187–92. doi: 10.1161/01.STR.0000181089.41324.70. [DOI] [PubMed] [Google Scholar]
- 42.Kwakkel G, Veerbeek JM, Harmeling-van der Wel BC, van Wegen E, Kollen BJ Early Prediction of functional Outcome after Stroke (EPOS) Investigators. Diagnostic accuracy of the barthel index for measuring activities of daily living outcome after ischemic hemispheric stroke: Does early poststroke timing of assessment matter? Stroke. 2011;42:342–6. doi: 10.1161/STROKEAHA.110.599035. [DOI] [PubMed] [Google Scholar]
- 43.Schuling J, de Haan R, Limburg M, Groenier KH. The Frenchay activities index. Assessment of functional status in stroke patients. Stroke. 1993;24:1173–7. doi: 10.1161/01.str.24.8.1173. [DOI] [PubMed] [Google Scholar]
- 44.Post MW, de Witte LP. Good inter-rater reliability of the Frenchay activities index in stroke patients. Clin Rehabil. 2003;17:548–52. doi: 10.1191/0269215503cr648oa. [DOI] [PubMed] [Google Scholar]
- 45.Cup EH, Scholte OP, Reimer WJ, Thijssen MC, van Kuyk-Minis MA. Reliability and validity of the Canadian occupational performance measure in stroke patients. Clin Rehabil. 2003;17:402–9. doi: 10.1191/0269215503cr635oa. [DOI] [PubMed] [Google Scholar]
- 46.Duncan PW, Lai SM, Bode RK, Perera S, DeRosa J. Stroke impact scale-16: A brief assessment of physical function. Neurology. 2003;60:291–6. doi: 10.1212/01.wnl.0000041493.65665.d6. [DOI] [PubMed] [Google Scholar]
- 47.Jensen MB, Lyden P. Stroke scales: An update. Stroke Clinical Updates. 2006;16:1–7. [Google Scholar]
- 48.Wu CY, Chuang LL, Lin KC, Horng YS. Responsiveness and validity of two outcome measures of instrumental activities of daily living in stroke survivors receiving rehabilitative therapies. Clin Rehabil. 2011;25:175–83. doi: 10.1177/0269215510385482. [DOI] [PubMed] [Google Scholar]
- 49.Küçükdeveci AA, Yavuzer G, Elhan AH, Sonel B, Tennant A. Adaptation of the functional independence measure for use in Turkey. Clin Rehabil. 2001;15:311–9. doi: 10.1191/026921501676877265. [DOI] [PubMed] [Google Scholar]
- 50.Quinn TJ, Dawson J, Walters MR, Lees KR. Reliability of the modified Rankin scale. Stroke. 2007;38:144. doi: 10.1161/STROKEAHA.107.490110. [DOI] [PubMed] [Google Scholar]


