Abstract
A 30-year-old African woman in Kenya succumbed to severe swollen regional lymph nodes, development of painful boils and ulcer formation and rashes at specific tick-biting sites together with an intermittent fever and headache following repeated tick bites of Rhipicephalus pulchellus. She later developed nuchal lymphadenopathy-like condition and an eschar with edematous margins at bitten sites. A sustained high fever and fatigue then followed. She became well after treatment with antibiotics and topical application of anti-histamine daily for a week. This pose dangers of emerging tick-borne pathogens such as this one as their epidemiology, biology, socio-economics and prognosis remain unknown.
Keywords: Human host, inflammation, Rhipicephalus pulchellus, tick bites, tick-borne pathogens
INTRODUCTION
Ticks are considered to be second worldwide to mosquitoes as vectors of human diseases, while in livestock industry, ticks and tick-borne diseases (TandTBDs) and emanating secondary infections adversely impede its development particularly in sub-Saharan Africa, Latin America and Asia.[1,2,3] Wildlife,[4] domestic animals[2] and humans[5] are equally affected. However, the extend of the severity of the effects of the emerging and re-emerging ticks and tick-borne pathogens to their respective hosts remains considerably unknown due to unavailable data (as a result of unreported, undisclosed and unevaluated existing and potential cases). Since ticks are associated with domestic animals and wildlife more than humans, the worst of this unknown situation is the effects of ticks and tick-borne pathogens on humanity.
Tick-borne lymphadenopathy is one of the most recently known health conditions in human history, whose epidemiology, biology, sociology, economics and prognosis are poorly understood in health industry. The condition is characterized by lymphadenopathy and a rash and may either be one of the tick-borne conditions that might have gone unnoticed in human population or may be an emerging condition in the drastically changing world of complex vector-pathogen-host interfaces.[6] Nevertheless, lymphadenopathy and a rash per se, are recognized as symptoms and signs of many tropical diseases, of which bites from infected and non-infected tick species are. This condition was first described in 1997 in a female patient in France and its causative agent, bacterium, Rickettsia slovaca Sekeyová et al., 1998, is transmitted by Dermacentor Koch, 1844 ticks[7] while Rickettsia raoultii Mediannikov et al., 2008, causing the same condition was confirmed recently in Dermacentor marginatus Sulzer, 1776 ticks in Tuscany, Italy.[8] Although Dermacentor spp. are found in Kenya, little is known about the full continuum of rickettsial infections, which are variously reported as Kenya fever, Kenya typhus, Kenya tick fever and/or Kenya tick typhus with symptoms ranging from headache and nausea to muscle, back and joint pain with no skin rash.[1]
In the present case presentation, we report and discuss a tick-borne lymphadenopathy-like condition in an African woman in Kenya following repeated tick bites that she suffered during a survey study of ticks in Mwea National Reserve in Mwea division, Mbeere district, Eastern province. This is a human condition that has not been reported before in Kenya, more particularly in association with Rhipicephalus pulchellus Gerstäcker 1873, as tick vectors [Figure 1]. However, ticks of the genus: Rhipicephalus Koch, 1844 are known to transmit a wide range of bacterial (Rickettsiaceae: Rickettsia da Rocha-Lima, 1916 and Anaplasmataceae: Ehrlichia Moshkovski 1945) and viral (Bunyaviridae: Nairovirus) pathogens to humans worldwide.[2,9]
Figure 1.
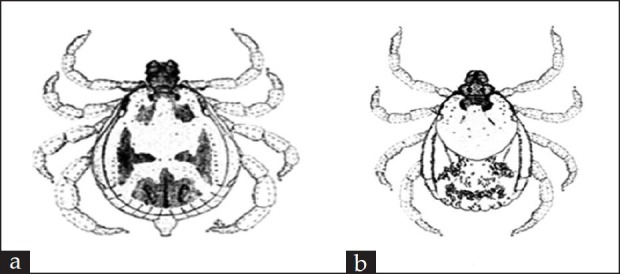
Rhipicephalus pulchellus Gerstäcker 1873 (Acari: Ixodidae) (also called Yellow backed tick and/or Zebra tick). The illustration represents both male (a) and female (unfed) (b) ticks, respectively. This tick species transmits Nairobi sheep disease virus and Theileria parva Theiler, 1904 and is also a vector of Theileria taurotragi Martin and Brocklesby, 1960, the cause of benign bovine theileriosis in Africa (as adopted from Walker et al., 2000[2])
CASE REPORT
While the International Centre of Insect Physiology and Ecology (ICIPE) was in the process of controlling and managing tsetse flies, the vectors for human and livestock trypanosomiasis between April, 2003 and December, 2006 in Mwea division, Eastern province of Kenya, it was noted that ticks had heavily infested several camping sites of Mwea National Reserve (covering an area of 68 square kilometers). The national reserve being one of the country's tourist attraction and wildlife management and conservation research centre, environmental friendly and sustainable strategies for prevention, control and management of tick infestation were sought by the Kenya Wildlife Service (KWS) to avert the anticipated negative effects on humanity, wildlife, livestock and hence, tourism industry at this location. It was on this basis that ICIPE was approached by KWS to help initiate these strategies, which started by launching a survey study program of ticks in Mwea National Reserve to evaluate the severity of tick infestation problem in the area and its impact on the livelihood of humanity, wildlife, livestock and tourism industry.
In the course of conducting the survey study, on 27th September, 2005, a 30-year-old African woman in Kenya reported back at the work station (at ICIPE in Nairobi) 2 weeks after suffering from repeated tick bites (of R. pulchellus) at the armpit of the right-hand side [Figures 2a and b] and the base of the neck [Figures 2c and d]. The patient had earlier visited a health center in the neighbourhood of her residence for medical advice and probably subsequent treatment following the recognition of itching and inflammatory reaction at specific body sites of tick bites and the emergence of painful swellings (boils) at the sites of tick bites accompanied with intermittent headache, persistent fever and malaise at the onset of these noticeable clinical symptoms. However, these efforts did not translate into any success as her swellings continued increasing in size [Figures 2a and b] and the associated clinical symptoms did not either subside as anticipated. At the health centre, she had been subjected to treatment with antibiotics and topical application of anti-histamine. These clinical symptoms started becoming noticeable 2 days after visiting and getting infested with ticks (of R. pulchellus) from Mwea National Reserve in Mbeere district, Eastern province where, together with others, had gone to survey, collect and identify ticks for use to evaluate the severity of tick infestation problem and its impact on the livelihoods of humanity, wildlife, livestock and tourism industry in the location.
Figure 2.
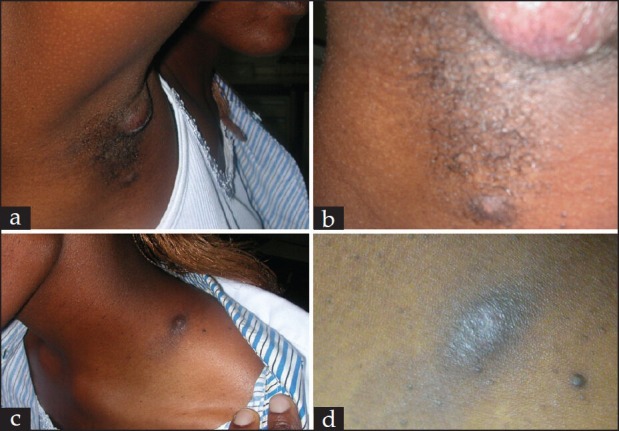
Boils were pale yellow, particularly at the site of tick bite and the entire surface became painful when they were about to drain greenish-yellowish pus (a) and (b). At the base of the neck, there was a tumor surrounded by a faded but noticeable rash around it (c). After healing, this mark (c) remained as a hard painless tumor. After discharging greenish-yellowish pus, the painful boil ulcerated and started healing gradually and became dry with an inoculation eschar including central necrosis, edematous margins and erythematous halo at the former site of the tick bite (d)
One day following a session of tick survey at Mwea National Reserve, mild but persistent itching and scratching ensued around the neck, on the back and in the armpit of the right hand side of a 30-year-old African woman. Little did it occur to her that this could be due to the ticks she had been surveying, collecting and storing a day before. She invited her friends to help with keen physical observation of her specific body sites and clothes so as to find out what the itching and scratching was all about. To her surprise, at the specific body sites of scratching, they recovered ticks, which were later identified to be R. pulchellus except on the back (probably had dropped off and probably at the time of biting, ticks had not been infected with the etiological agent(s)). Two days after the recovery of the ticks, she noticed redness followed by the emergence of severe swelling and ulcer formation at the specific biting sites along with swollen regional lymph nodes. This intensified the pain at the specific biting sites on her body, that later became systemic, thereby affecting some parts of the body that were not bitten by ticks. The patient succumbed to a rash accompanied with pain and constant but an intermittent fever and headache as though it was caused by Rocky Mountain spotted fever (RMSF).[10] She, however, continued to present high fever, malaise, general body weakness, headache, pain, nausea and nuchal lymphadenopathy [Figures 2a and b]. At the site of the tick bite an eschar with edematous margins formed [Figures 2c and d]. This followed with a sustained high fever associated with intermittent headache and fatigue. Physical examination of the patient's body was unexceptional. After 15 days of antimicrobial therapy of Doxycycline (taking100 mg twice a day after meals for 21 days) and bursting of the boils [Figure 2b] to release greenish-yellowish pus, these clinical symptoms started subsiding and healing ensued. Given that the patient had been subjected to antibiotic treatment and by the time she reported back to work station at ICIPE, healing had already started for some small swellings, which appeared dry at the base of the neck [Figure 2d], no clinical laboratory diagnoses were conducted at the ICIPE research station to confirm the identity of the pathogen apart from confirming the identity of the tick species, which were recovered from the patient's affected body sites and clothes.
DISCUSSION
Before the incident being described in this case report was encountered, previous tick survey studies in Kenya had indicated the emergence of tick-borne pathogens of R. pulchellus and other ticks transmissible to humans. For instance, from ticks infesting market livestock in Nairobi, Kenya, 26 isolates of Dugbe virus (DUGV) were identified and additionally obtained several other isolates of a virus that was identified as a Nairovirus related most closely to DUGV.[11] Six isolates of the virus were obtained from pools of Amblyomma gemma Dönitz, 1909 and R. pulchellus ticks collected from hides of cattle in Nairobi, Kenya, in October 1999[12] and also in Ethiopia.[13] In the 1999 Kenya abattoir survey, DUGV was isolated from four species of ticks, A. variegatum Fabricius, 1794, A. gemma, A. lepidum Dönitz, 1909, and R. pulchellus.[11] The DUGV is transmitted by ticks to vertebrates, including humans, and causes a mild febrile illness and thrombocytopenia[14] with complains of malaise, fatigue and general body weakness also experienced by the patient reported in this case, whereas Creamin-Congo Hemorrhagic fever (CCHF) virus of humans was first isolated from R. pulchellus ticks recovered from a dead sheep in Kenya (quoted by Morel, 1980[15]), the clinical symptoms experienced by the patient in this case were not in anyways closer to those of CCHF. By comparing and contrasting the pictures of patients, the health condition described in this case report was similar to the tick-borne lymphadenopathy that was first described in 1997 in a female patient in France and whose causative agent, Rickettsia slovaca Sekeyová et al., 1998, is transmitted by Dermacentor Koch, 1844 ticks.[7] The form of tick biting described in this case report presented a characteristic clinical picture, which resembled that of a 67-year-old woman who had also suffered from a tick bite (of Dermacentor) at the dorsal scalp in southern Germany in the vicinity of Freiburg (Baden-Wuerttemberg)[7] in February 2009. Nevertheless, the symptoms of the patient in this case report were closer to those caused by rickettsial infections transmitted by Dermacentor spp. with the exception of characteristic nature and picture of the skin rash of the affected person. Because the early symptoms experienced by the patient including: - the incubation period (2-15 days), pain, nausea, a sudden onset of a rash with constant but an intermittent fever and headache were non-specific, this particular health condition more or less paralleled that due to RMSF, which is the most lethal and most frequently reported rickettsial illness (caused by Rickettsia rickettsii Brumpt, 1922, a species of bacterium) in the United States whose vectors include: - Dermacentor variabilis Say, 1821, Dermacentor andersoni Stiles, 1908, Rhipicephalus sanguineus Latreille, 1806 and Amblyomma cajennense Fabricius, 1787.[10]
Other tick-borne pathogens such as Kupe virus have been recently isolated from Kenyan livestock ticks (A. variegatum, A. gemma, A. lepidum, and R. pulchellus) with unknown pathogenecity to humans as well as livestock and wildlife.[12] In another study conducted on the Kano Plain in Kenya, the identity of some viruses isolated from Rhipicephalus spp. and A. variegatum could not be confirmed.[16] And more recently, a new bacterial strain of the genus: Ehrlichia was isolated from deer ticks in Minnesota and Wisconsin, USA[17] where it had caused flu-like symptoms in at least 25 people. The foregoing, however, demonstrates both vector competence and vectorial capacity of R. pulchellus and the genus: Rhipicephalus in both pathogen and tick-borne disease transmission, respectively, and hence the need for developing effective integrated operations and strategies to prevent, control and manage both the vector and pathogen to avoid transmission of the disease within a given population and/or geographical location.[18]
The results of this report case provide a basis for the likelihood of emergence of unknown tick-borne diseases[19] in the locations where there is continued habitat-human-wildlife-livestock interface interactions without awareness campaigns, education and sustainable control and management of ticks. The situation may be worse particularly in an unfamiliar environments.[20] This, therefore, functions as very useful precautionary information to policy makers in the ministries of wildlife and tourism, public health and medical services, livestock and development, agriculture and education.
ACKNOWLEDGMENTS
We acknowledge the management and workers from the Mwea National Reserve of Kenya Wildlife Services for their cooperation and support during specimen collection. The first author wish to acknowledge the financial and material support received from the International Centre of Insect Physiology and Ecology (ICIPE), Nairobi, Kenya under the African Regional Postgraduate Programme in Insect Science (ARPPIS) and Wageningen University and Research Centre, Laboratory of Entomology under PhD Sandwich Fellowship. Ticks were collected by Dr. Jean Nguya Kalemba Maniania and Dr. Sopher Natuluku Ondiaka and to them all, we are very grateful.
Footnotes
Source of Support: The ICIPE-based Biovision Foundation of Switzerland provided funding that supported the survey study program of ticks. Part of facilities used for producing this manuscript was sponsored by the International Foundation for Sciences, Stockholm, Sweden and the Organization for the Prohibition of Chemical Weapons, The Hague, The Netherlands through a grant AB/12782-2.
Conflict of Interest: Nil.
REFERENCES
- 1.Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter RS, et al. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther. 2010;8:33–50. doi: 10.1586/eri.09.118. [DOI] [PubMed] [Google Scholar]
- 2.Walker JB, Keirans JE, Horak IG. Cambridge: Cambridge University Press; 2000. The genus Rhipicephalus (Acardi, Ixodidae): A guide to the brown ticks of the world; p. 643. [Google Scholar]
- 3.Jongean F, Uilenberg G. The global importance of ticks. Parasitol. 2004;129:S3–14. doi: 10.1017/s0031182004005967. [DOI] [PubMed] [Google Scholar]
- 4.Hasle G. PhD thesis. Norway: Faculty of Medicine, University of Oslo; 2011. Dispersal of ticks and tick-borne pathogens by birds. Dynamics of birds’ transport of ticks to Norway; p. 128. [Google Scholar]
- 5.Randolph SE. Tick-borne encephalitis virus, ticks and humans: Short-term and long-term dynamics. Curr Opin Infect Dis. 2008;21:462–7. doi: 10.1097/QCO.0b013e32830ce74b. [DOI] [PubMed] [Google Scholar]
- 6.Munderloh GU, Kurtti JT. Washington, DC: The State of the Science Workshop; 2010. Critical needs and gaps in understanding prevention, amelioration, and resolution of lyme and other tick-borne diseases: The short-term and long-term outcomes. Institute of Medicine Committee on Lyme Disease and Other Tick-borne Diseases; pp. 15–176. [Google Scholar]
- 7.Rieg S, Schmoldt S, Theilacker C, de With K, Wölfel S, Kern VW, et al. Tick-borne lymphadenopathy (TIBOLA) acquired in Southwestern Germany. BMC Infect Dis. 2011;11:167. doi: 10.1186/1471-2334-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Selmi M, Martello E, Bertolotti L, Bisanzio D, Tomassone L. Rickettsia slovaca and Rickettsia raoultii in Dermacentor marginatus ticks collected on wild boars in Tuscany, Italy. J Med Entomol. 2009;46:1490–3. doi: 10.1603/033.046.0636. [DOI] [PubMed] [Google Scholar]
- 9.de la Fuente J, Estrada-Pena A, Venzal MJ, Kocan MK, Sonenshine ED. Overview: Ticks as vectors of pathogens that cause disease in humans and animals. Front Biosci. 2008;13:6938–46. doi: 10.2741/3200. [DOI] [PubMed] [Google Scholar]
- 10.Masters EJ, Olson GS, Weiner SJ, Paddock CD. Rocky Mountain spotted fever: A clinician's dilemma. Arch Intern Med. 2003;163:769–74. doi: 10.1001/archinte.163.7.769. [DOI] [PubMed] [Google Scholar]
- 11.Sang R, Onyango C, Gachoya J, Mabinda E, Konongoi S, Ofula V, et al. Tickborue arbovirus surveillance in market livestock, Nairobi, Kenya. Emerg Infect Dis. 2006;12:1074–80. doi: 10.3201/eid1207.060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabtree MB, Sang R, Miller BR. Kupe virus, a new virus in the family Bunyaviridae, genus Nairovirus, Kenya. Emerg Infect Dis. 2009;15:147–54. doi: 10.3201/eid1502.080851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood OL, Lee VH, Ash JS, Casals J. Crimean-Congo Hemorrhagic Fever, Thogoto, Dugbe and Jos viruses isolated from ixodid ticks in Ethiopia. Am J Trop Med Hyg. 1978;27:600–4. doi: 10.4269/ajtmh.1978.27.600. [DOI] [PubMed] [Google Scholar]
- 14.Burt FJ, Spencer DC, Leman PA, Patterson B, Swanepoel R. Investigation of tick-borue viruses as pathogens of humans in South Africa and evidence of Dugbe virus infection in a patient with prolonged thrombocytopenia. Epidemiol Infect. 1996;116:353–61. doi: 10.1017/s0950268800052687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morels PC. Maisons-Alfort [France]: Institut d’elevage et de médecine vétérinaire des pays tropicaux; Addis Ababa [Ethiopia]: Ministry of Foreign Affairs, French Veterinary Mission in Ethiopia; 1980. A study on Ethiopian Ticks (Acarina, Ixodidae) p. 332. [Google Scholar]
- 16.Johnson BK, Chanas AC, Squires EJ, Shockley P, Simpson DI, Parsons J, et al. Arbovirus isolations from ixodid ticks infesting livestock, Kano Plain, Kenya. Trans R Soc Trop Med Hyg. 1980;74:732–7. doi: 10.1016/0035-9203(80)90188-1. [DOI] [PubMed] [Google Scholar]
- 17.Pritt SB, Sloan ML, Johnson DK, Munderloh GU, Paskewitz MS, McElroy MK, et al. Emergence of a new pathogenic Ehrlichia species, Wisconsin and Minnesota, 2009. N Engl J Med. 2011;365:422–9. doi: 10.1056/NEJMoa1010493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCluskey BJ. Biosecurity for arthropod-borne diseases. Vet Clin North Am Food Anim Pract. 2002;18:99–114. doi: 10.1016/s0749-0720(02)00010-5. [DOI] [PubMed] [Google Scholar]
- 19.Roberts CN, King JP. Policy entrepreneurs: Their activity, structure and function in the policy process. J Public Adm Res Theory. 1991;1:147–75. [Google Scholar]
- 20.Richards AL, Jiang J, Omulo S, Dare R, Abdirahman K, Ali A, et al. Human infection with Rickettsia felis, Kenya. Emerg Infect Dis. 2010;16:1081–6. doi: 10.3201/eid1607.091885. [DOI] [PMC free article] [PubMed] [Google Scholar]


