Abstract
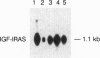
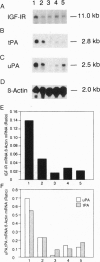
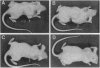
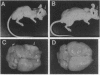
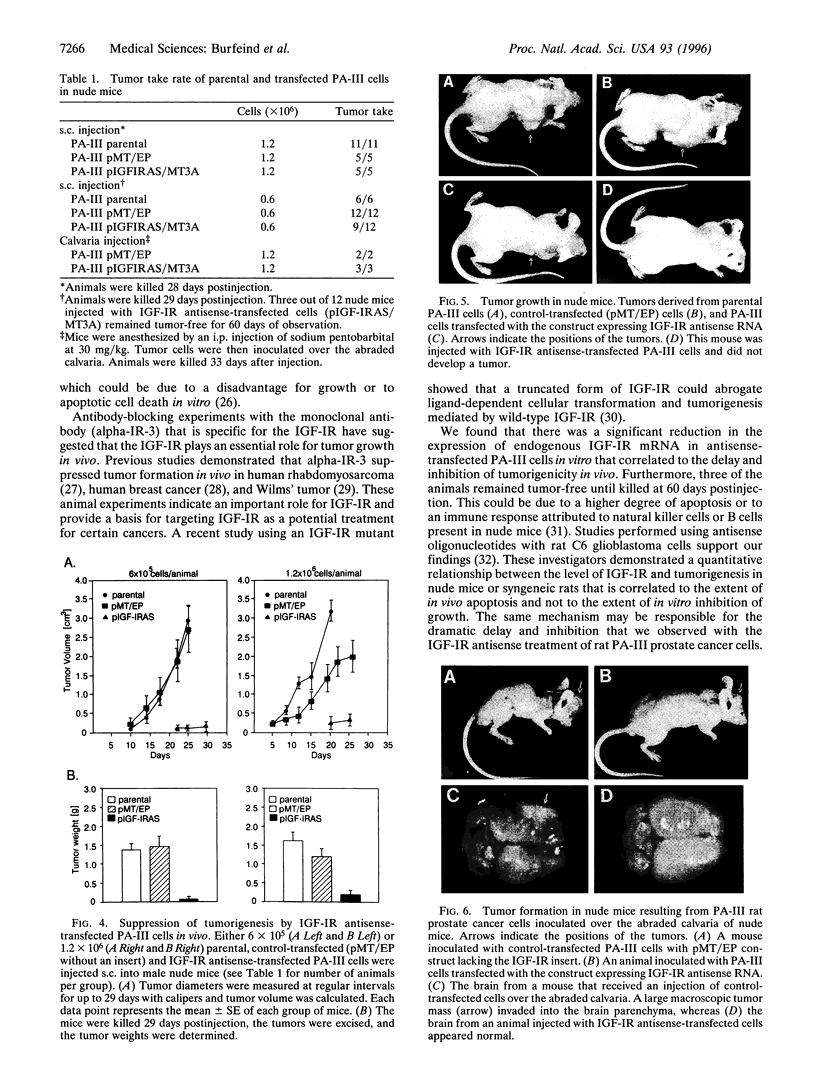
Prostate carcinoma is the second leading cause of death from malignancy in men in the United States. Prostate cancer cells express type I insulin-like growth factor receptor (IGF-IR) and prostate cancer selectively metastazises to bone, which is an environment rich in insulin-like growth factors (IGFs), thereby supporting a paracrine action for cancer cell proliferation. We asked whether the IGF-IR is coupled to tumorigenicity and invasion of prostate cancer. When rat prostate adenocarcinoma cells (PA-III) were stably transfected with an antisense IGF-IR expression construct containing the ZnSO4-inducible metallothionein-1 transcriptional promoter, the transfectants expressed high levels of IGF-IR antisense RNA after induction with ZnSO4, which resulted in dramatically reduced levels of endogenous IGF-IR mRNA. A significant reduction in expression both of tissue-type plasminogen activator and of urokinase-type plasminogen activator occurred in PA-III cells accompanying inhibition of IGF-IR. Subcutaneous injection of either nontransfected PA-III or PA-III cells transfected with vector minus the IGF-IR insert into nude mice resulted in large tumors after 4 weeks. However, mice injected with IGF-IR antisense-transfected PA-III cells either developed tumors 90% smaller than controls or remained tumor-free after 60 days of observation. When control-transfected PA-III cells were inoculated over the abraded calvaria of nude mice, large tumors formed with invasion of tumor cells into the brain parenchyma. In contrast, IGF-IR antisense transfectants formed significantly smaller tumors with no infiltration into brain. These results indicate an important role for the IGF/IGF-IR pathway in metastasis and provide a basis for targeting IGF-IR as a potential treatment for prostate cancer.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achbarou A., Kaiser S., Tremblay G., Ste-Marie L. G., Brodt P., Goltzman D., Rabbani S. A. Urokinase overproduction results in increased skeletal metastasis by prostate cancer cells in vivo. Cancer Res. 1994 May 1;54(9):2372–2377. [PubMed] [Google Scholar]
- Arteaga C. L., Kitten L. J., Coronado E. B., Jacobs S., Kull F. C., Jr, Allred D. C., Osborne C. K. Blockade of the type I somatomedin receptor inhibits growth of human breast cancer cells in athymic mice. J Clin Invest. 1989 Nov;84(5):1418–1423. doi: 10.1172/JCI114315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R. Oncogenes and the strategy of growth factors. Cell. 1994 Dec 16;79(6):927–930. doi: 10.1016/0092-8674(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Baserga R. The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res. 1995 Jan 15;55(2):249–252. [PubMed] [Google Scholar]
- Bautista C. M., Mohan S., Baylink D. J. Insulin-like growth factors I and II are present in the skeletal tissues of ten vertebrates. Metabolism. 1990 Jan;39(1):96–100. doi: 10.1016/0026-0495(90)90154-5. [DOI] [PubMed] [Google Scholar]
- Colombi M., Bellotti D., De Petro G., Barlati S. Plasminogen activators in nude mice xenotransplanted with human tumorigenic cells. Invasion Metastasis. 1995;15(1-2):22–33. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gansler T., Furlanetto R., Gramling T. S., Robinson K. A., Blocker N., Buse M. G., Sens D. A., Garvin A. J. Antibody to type I insulinlike growth factor receptor inhibits growth of Wilms' tumor in culture and in athymic mice. Am J Pathol. 1989 Dec;135(6):961–966. [PMC free article] [PubMed] [Google Scholar]
- Gleave M., Hsieh J. T., Gao C. A., von Eschenbach A. C., Chung L. W. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991 Jul 15;51(14):3753–3761. [PubMed] [Google Scholar]
- Hsieh W. S., Simons J. W. Systemic therapy of prostate cancer. New concepts from prostate cancer tumor biology. Cancer Treat Rev. 1993 Jul;19(3):229–260. doi: 10.1016/0305-7372(93)90037-r. [DOI] [PubMed] [Google Scholar]
- Iwamura M., Sluss P. M., Casamento J. B., Cockett A. T. Insulin-like growth factor I: action and receptor characterization in human prostate cancer cell lines. Prostate. 1993;22(3):243–252. doi: 10.1002/pros.2990220307. [DOI] [PubMed] [Google Scholar]
- Jansen M., van Schaik F. M., Ricker A. T., Bullock B., Woods D. E., Gabbay K. H., Nussbaum A. L., Sussenbach J. S., Van den Brande J. L. Sequence of cDNA encoding human insulin-like growth factor I precursor. Nature. 1983 Dec 8;306(5943):609–611. doi: 10.1038/306609a0. [DOI] [PubMed] [Google Scholar]
- Kalebic T., Tsokos M., Helman L. J. In vivo treatment with antibody against IGF-1 receptor suppresses growth of human rhabdomyosarcoma and down-regulates p34cdc2. Cancer Res. 1994 Nov 1;54(21):5531–5534. [PubMed] [Google Scholar]
- Koutsilieris M., Frenette G., Lazure C., Lehoux J. G., Govindan M. V., Polychronakos C. Urokinase-type plasminogen activator: a paracrine factor regulating the bioavailability of IGFs in PA-III cell-induced osteoblastic metastases. Anticancer Res. 1993 Mar-Apr;13(2):481–486. [PubMed] [Google Scholar]
- Koutsilieris M. Osteoblastic metastasis in advanced prostate cancer. Anticancer Res. 1993 Mar-Apr;13(2):443–449. [PubMed] [Google Scholar]
- Long L., Rubin R., Baserga R., Brodt P. Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res. 1995 Mar 1;55(5):1006–1009. [PubMed] [Google Scholar]
- Mignatti P., Rifkin D. B. Biology and biochemistry of proteinases in tumor invasion. Physiol Rev. 1993 Jan;73(1):161–195. doi: 10.1152/physrev.1993.73.1.161. [DOI] [PubMed] [Google Scholar]
- Pietrzkowski Z., Mulholland G., Gomella L., Jameson B. A., Wernicke D., Baserga R. Inhibition of growth of prostatic cancer cell lines by peptide analogues of insulin-like growth factor 1. Cancer Res. 1993 Mar 1;53(5):1102–1106. [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Effects of dichloromethylene diphosphonate on the osteolytic and osteoplastic effects of rat prostate adenocarcinoma cells. J Natl Cancer Inst. 1985 Nov;75(5):949–954. doi: 10.1093/jnci/75.5.949. [DOI] [PubMed] [Google Scholar]
- Pollard M. Spontaneous prostate adenocarcinomas in aged germfree Wistar rats. J Natl Cancer Inst. 1973 Oct;51(4):1235–1241. doi: 10.1093/jnci/51.4.1235. [DOI] [PubMed] [Google Scholar]
- Polychronakos C., Janthly U., Lehoux J. G., Koutsilieris M. Mitogenic effects of insulin and insulin-like growth factors on PA-III rat prostate adenocarcinoma cells: characterization of the receptors involved. Prostate. 1991;19(4):313–321. doi: 10.1002/pros.2990190405. [DOI] [PubMed] [Google Scholar]
- Prager D., Li H. L., Asa S., Melmed S. Dominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor I receptor mutant. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2181–2185. doi: 10.1073/pnas.91.6.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh T. D., Chang C., Uemura H., Weindruch R. Prostatic localization of spontaneous early invasive carcinoma in Lobund-Wistar rats. Cancer Res. 1994 Nov 15;54(22):5766–5770. [PubMed] [Google Scholar]
- Resnicoff M., Abraham D., Yutanawiboonchai W., Rotman H. L., Kajstura J., Rubin R., Zoltick P., Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995 Jun 1;55(11):2463–2469. [PubMed] [Google Scholar]
- Resnicoff M., Ambrose D., Coppola D., Rubin R. Insulin-like growth factor-1 and its receptor mediate the autocrine proliferation of human ovarian carcinoma cell lines. Lab Invest. 1993 Dec;69(6):756–760. [PubMed] [Google Scholar]
- Resnicoff M., Coppola D., Sell C., Rubin R., Ferrone S., Baserga R. Growth inhibition of human melanoma cells in nude mice by antisense strategies to the type 1 insulin-like growth factor receptor. Cancer Res. 1994 Sep 15;54(18):4848–4850. [PubMed] [Google Scholar]
- Resnicoff M., Sell C., Rubini M., Coppola D., Ambrose D., Baserga R., Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994 Apr 15;54(8):2218–2222. [PubMed] [Google Scholar]
- Rickles R. J., Darrow A. L., Strickland S. Molecular cloning of complementary DNA to mouse tissue plasminogen activator mRNA and its expression during F9 teratocarcinoma cell differentiation. J Biol Chem. 1988 Jan 25;263(3):1563–1569. [PubMed] [Google Scholar]
- Rinker-Schaeffer C. W., Partin A. W., Isaacs W. B., Coffey D. S., Isaacs J. T. Molecular and cellular changes associated with the acquisition of metastatic ability by prostatic cancer cells. Prostate. 1994 Nov;25(5):249–265. doi: 10.1002/pros.2990250505. [DOI] [PubMed] [Google Scholar]
- Schloesser M., Slomski R., Wagner M., Reiss J., Berg L. P., Kakkar V. V., Cooper D. N. Characterization of pathological dystrophin transcripts from the lymphocytes of a muscular dystrophy carrier. Mol Biol Med. 1990 Dec;7(6):519–523. [PubMed] [Google Scholar]
- Shevelev A. Ia, Kondratov R. V., Rybalkin I. N., Prasolov V. S. Ekspressiia urokinazy cheloveka v perevivaemykh kletkakh gryzunov. Utrata intronov v khode perenosa gena s pomoshch'iu retrovirusnogo vektora. Mol Biol (Mosk) 1992 Jan-Feb;26(1):208–217. [PubMed] [Google Scholar]
- Stempien M. M., Fong N. M., Rall L. B., Bell G. I. Sequence of a placental cDNA encoding the mouse insulin-like growth factor II precursor. DNA. 1986 Oct;5(5):357–361. doi: 10.1089/dna.1986.5.357. [DOI] [PubMed] [Google Scholar]
- Taghian A., Budach W., Zietman A., Freeman J., Gioioso D., Suit H. D. Quantitative comparison between the transplantability of human and murine tumors into the brain of NCr/Sed-nu/nu nude and severe combined immunodeficient mice. Cancer Res. 1993 Oct 15;53(20):5018–5021. [PubMed] [Google Scholar]
- Tranque P., Naftolin F., Robbins R. Differential regulation of astrocyte plasminogen activators by insulin-like growth factor-I and epidermal growth factor. Endocrinology. 1994 Jun;134(6):2606–2613. doi: 10.1210/endo.134.6.8194486. [DOI] [PubMed] [Google Scholar]
- Trojan J., Blossey B. K., Johnson T. R., Rudin S. D., Tykocinski M., Ilan J., Ilan J. Loss of tumorigenicity of rat glioblastoma directed by episome-based antisense cDNA transcription of insulin-like growth factor I. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):4874–4878. doi: 10.1073/pnas.89.11.4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Blossey B. K., Kelley K. M., Shevelev A., Abdul-Karim F. W., Anthony D. D., Tykocinski M. L., Ilan J. Gene therapy of murine teratocarcinoma: separate functions for insulin-like growth factors I and II in immunogenicity and differentiation. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6088–6092. doi: 10.1073/pnas.91.13.6088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Gray A., Tam A. W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986 Oct;5(10):2503–2512. doi: 10.1002/j.1460-2075.1986.tb04528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware J. L. Prostate tumor progression and metastasis. Biochim Biophys Acta. 1987 Nov 25;907(3):279–298. doi: 10.1016/0304-419x(87)90010-2. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Sasaki A., Mundy G. R. Osteolytic bone metastasis in breast cancer. Breast Cancer Res Treat. 1994;32(1):73–84. doi: 10.1007/BF00666208. [DOI] [PubMed] [Google Scholar]