Abstract
Background:
The facial asymmetry correction in complex craniofacial malformations presents a challenging problem for reconstructive surgeons. Progressive hemifacial atrophy (HFA) and hemifacial microsomia (HFM) can manifest in different grades of severity. Most patients require only soft-tissue augmentation. Free flaps are the best option for correction of severe facial soft-tissue deficiency.
Materials and Methods:
Twenty-two patients of HFM and HFA were included in this study from January 2006 to March 2009 in the Department of Plastic and Reconstructive Surgery, SMS Medical College and Hospital. In all cases, atrophy correction was done using de-epithelialised parascapular free flap with the de-epithelialised surface was placed under the skin. A small skin paddle was taken for monitoring.
Results:
All cases were reconstructed with de-epithelialised parascapular free flap. There was no flap loss in this series. Hematoma was noted in five cases. Debulking and removal of skin paddle were done in all cases after 6 months. Atrophy recurrence was not observed in any of the cases on follow-up.
Conclusion:
Contouring of face in cases of HMF and HFA is satisfactorily done with the parascapular free flap. It gives better cosmetic results with minimal donor site morbidity. Facial vessels are better recipient vessels for anastomosis. Keeping de-epithelialised surface of flap under the skin helped in preventing sagging.
KEY WORDS: Hemifacial atrophy, hemifacial microsomia, parascapular flap
INTRODUCTION
The facial asymmetry correction in complex craniofacial malformations presents a challenging problem for reconstructive surgeons. To achieve the optimal reconstructive results, deficiencies of both the facial skeleton and the overlying soft tissue must be addressed. Facial asymmetry may be seen in hemifacial microsomia (HFM), hemifacial atropy, post radiation sequelae, burn, trauma and some other congenital conditions.
Progressive hemifacial atrophy (HFA) and HFM can manifest in different grades of severity. While most patients require only soft-tissue augmentation, some cases do require restoration of deficiencies of both the facial skeleton and the overlying soft tissue for achieving an optimal three-dimensional reconstruction result.
Severe soft-tissue asymmetries are corrected with different technique such as silicon injection[1], fat graft,[2] dermal graft,[3] pedicled flap,[4] free flaps,[5,17,18] etc. Of these techniques, free flaps are the best for correction of severe facial soft-tissue deficiency. Currently, thanks to a rapid development in functional refinements in microsurgical reconstructions, many free flaps have become popular in facial contouring, such as the omental flap,[6,7] the scapular and parascapular flap,[8] the groin flap[9] and the deep inferior epigastric artery perforator flap.[10] A consensus on the best method has not yet been reached and efforts to identify the ideal surgical procedure continue.
Versatility of parascapular flap offers the surgeon to use this flap in reconstructing three-dimensional defects. The parascapular microvascular flap has become a workhorse in the correction of facial asymmetry. This further enhanced the possibility of including any combination of skin, subcutaneous tissue, fascia, muscle and bone, if required, in the composite flap.
The parascapular flap and its variations have been described extensively. Although the flap was not published in the literature until 1984, dos Santos[11] is credited with the first important anatomical study of the scapular system for the purpose of free tissue transfer. Kim et al.[12] reported the use of the dorsal thoracic fascia based on the circumflex scapular artery for microvascular transfer as a result of their anatomical studies and those of Cormack and Lamberty. Encouraged by our experience with the fleur-de-lis flap with variable fat contributions for breast reconstruction, as well as dorsal thoracic fascial flaps used to cover hand wounds, we have been able to customise soft-tissue flaps from the back to correct soft-tissue deficiency and camouflage skeletal deformity of the face. Upton et al.[8] have reported their experience with parascapular free flaps for facial deformity.
We present our experience with 22 patients of HFM and HFA treated with microvascular transfer of free parascapular fasciocutaneous flap with some modification of the surgical approach.
MATERIALS AND METHODS
Medical records of 50 patients with diagnosis of HFM and HFA from January 2006 to March 2009, seen at the Department of Plastic and Reconstructive Surgery of SMS Hospital, Jaipur, were reviewed. For this study, only those cases were included which fulfilled the diagnostic criteria and had complete medical records. Twenty-two patients were eligible for this study.
Medical record data were reviewed and analysed for demographic pattern, distribution and extent of deformity of orbit,mandible,ear, facial nerve, and soft tissue (OMENS classification used for HFM), surgical technique, clinical outcome in terms of patients’ satisfaction and symmetry, any secondary procedure and complications. Out of 22 patients, 6 patients were of HFM. Out of six patients of HFM, three patients had mandibular hypoplasia of body and ramus and two patients had severe ear deformity, and all the six had severe soft-tissue deficiency.
All patients were operated by the senior author. The follow-up evaluation was from 2 to 5 years, with an average of 3 years. The facial asymmetry was marked clinically on the face to accurately determine the tissue requirements in surface area and volume. Fabrication of facial moulage [Figure 1b] was part of preoperative planning for each patient.
Figure 1.

(a) Preoperative photo; (b) photo with template; (c) donor site marking; (d) postoperative photo
All parascapular flaps were composite flaps including skin, de-epithelialised skin fat and fascia. Intact skin paddle was used for flap monitoring postoperatively.
Operative procedure
The ipsilateral donor site was generally selected so that positioning on the operative table was favorable. Facial contour irregularities were marked preoperatively with the patient in the upright position. It is important to mark the asymmetry in three dimensions. In order to construct the two-dimensional template, the areas requiring most bulk and the areas requiring less depth were marked separately. The template was placed over the donor site and marking was done according to the required thickness. Consideration for recipient vessels was kept in mind while marking the flap over the donor site [Figure 1c].
The recipient site on the face was dissected through a standard preauricular face lift incision or submandibular incision, depending on which recipient vessels were used for anastomosis. When the superficial temporal artery and vein were adequate in diameter for microvascular free tissue transfer, the preauricular incision extended from the temporal scalp to the earlobe. When facial vessels were used as a recipient vessel, then submandibular incision was employed.
The dissection was carried out in the subcutaneous face lift plane approximately 1 cm beyond the limits of tissue deficiency, determined by preoperative marking. Although depth requirements in portions of the hemiface vary, deficiency frequently requires dissection just at the midline of the chin, especially in HFM, where the soft-tissue flap camouflages irregularities and asymmetry of the chin after mandibular surgery and genioplasty. Dissection may be required deep to the alar base at the piriform aperture to allow repositioning of the alar–facial junction and reconstruction of the deficient alar support base in an anterioposterior dimension.
Dissection was frequently required across the upper lip and adjacent to the oral commissure to lengthen the ipsilateral deficient upper lip. Malposition and deformity of the ear were addressed similarly by release of a tethering deficiency of tissue at the base of the ear, followed by placement of vascularised flap tissue as required to build the base support of the ear.
De-epithelialisation of flap was done before starting the elevation of flap [Figure 2a]. The skin and subcutaneous tissue were then incised and the flap elevated in a subfascial plane by the retrograde technique [Figure 2b]. The width of the skin island taken was determined by the width of the facial deformity requiring the most tissue augmentation. Where thin tissue was required, extensions of dorsal thoracic fascia between the subcutaneous and muscle planes were incorporated into the flap. These fascial extensions comprised the latissimus dorsi, trapezius and other regional muscle fascia.
Figure 2.

(a) De-epithelialisation at donor site; (b) fascial dissection all around the flap
End-to-end microvascular anastomosis was performed, venous followed by arterial, to prevent engorgement and flap layout across the face. Depth requirement and size of fascial extensions were verified. The transition between the edge of de-epithelialised skin and fascial extension corresponded to the junction on the face between areas of severe and moderate or minimal tissue requirements. The transitions were tapered to yield a natural facial contour.
The operative table was positioned for final contour insetting with patient sitting. The flap was secured in place near the anastomosis with several buried absorbable sutures. The flap was sutured to the periosteum of the infra-orbital rim and zygoma with non-absorbable suture by means of drill holes, if required, in an attempt to prevent downward shift. Fixation to the piriform aperture medially, though seldom necessary, was performed by means of an intraoral incision. Facial extensions were secured in place medially with nylon suture brought out through the skin and tied over small bolster of petroleum gauze [Figure 1d]. Similarly, the junction of the de-epithelialised, trimmed dermis and fascial extension was secured with bolsters at the boundaries of the major soft-tissue deficiency. The flap was always placed with the dermis side of the flap superficial.
The tie over petroleum gauze bolsters were removed prior to discharge on the fifth or sixth postoperative day.
RESULTS
From 2006 to 2009, 22 patients underwent microvascular reconstruction with average 3 years follow-up period. Patient related data are summarised in Table 1. In this study, there were 9 males and 13 females. All patients were having unilateral involvement. Age at operation ranged from 15 to 26 years. Average age at operation was 19.27 years. No patients were having positive family history.
Table 1.
Clinical details of the patients
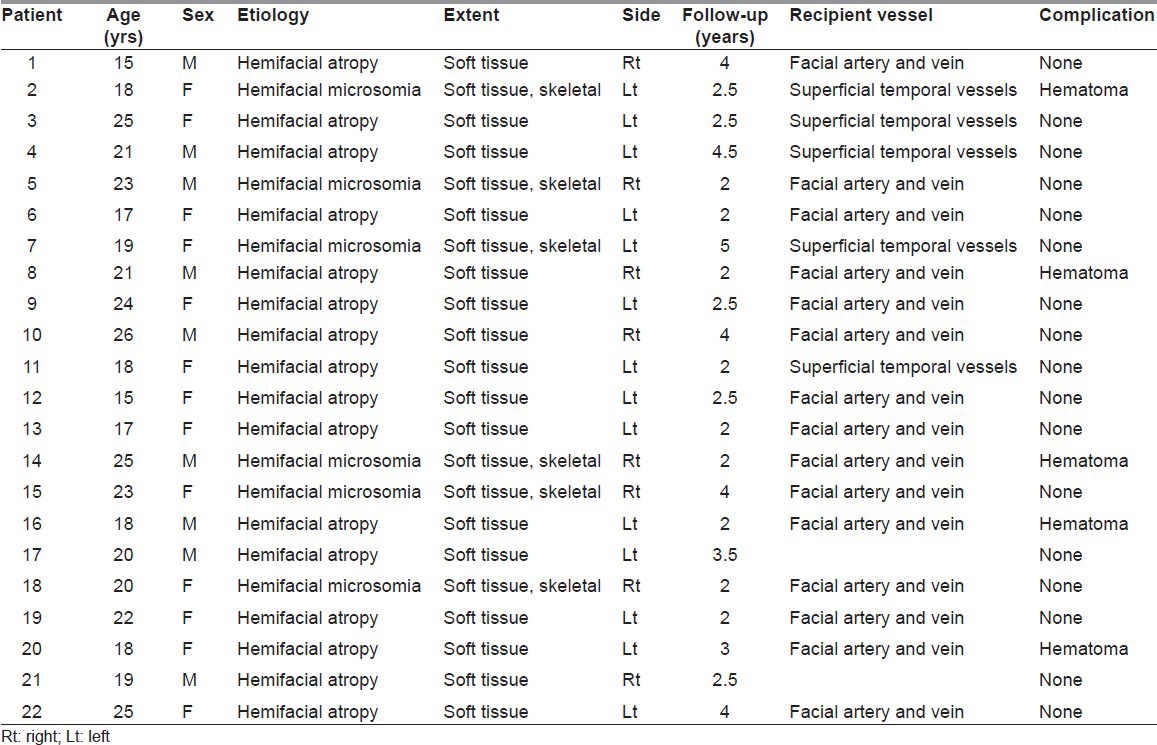
On the basis of definitive diagnostic features, 16 patients were of HFA and 6 patients were of HFM. In HFA patients, there was only soft-tissue involvement (skin, subcutaneous tissue and muscle). In HFM patients, along with soft-tissue atrophy, bone was also involved. The skeletal deformities in HFM were corrected with distractor osteogenesis before soft augmentation. Of the total six patients with HFM, three patients required skeletal correction by distraction osteogenesis.
In all 22 patients, fasciocutaneous parascapular flap, with skin paddle for monitoring, was used for soft-tissue augmentation.
Initially, in five cases the superficial temporal vessel was used as recipient vessel, but because of some technical difficulties, facial vessels were used in remaining 17 cases. Average operating time was 3.5 h, ranging from 3 to 5 h. There was no flap failure in this series. Recurrence of atrophy was not noted in any case during the follow-up period. Hematoma was noted in five patients, which was evacuated in the operation theatre, and the flap was inspected for active bleeding and anastomosis.
No significant donor site morbidity was found in immediate, postoperative and follow-up period.
Debulking or recontouring was done in all cases after 6 months, again in sitting position, for correction of minor asymmetry and the monitor skin paddle was excised too.
The secondary procedures were performed in the form of excision of monitor skin paddle (n = 22), debulking (n = 20) or flap resuspension (n = 1) to the malar region. In some patients, ancillary procedures were required and performed in the form of fat injection in five and resuspension with polypropylene mesh in one. Final results are shown in Figures 3a–d, 4a–d, and 5a–d. Regarding the satisfaction level of our 22 patients, subjective assessment by the patients revealed that in 8 patients it was very good, in 10 patients it was good, and the remaining 4 patients were not fully satisfied with the results.
Figure 3.

(a, b) Preoperative and postoperative (front view) after 4 years and (c, d) preoperative and postoperative (lateral view) after 5 years
Figure 4.

(a, b) Preoperative and postoperative (front view) after 4 years and (c, d) preoperative and postoperative (lateral view) after 4 years
Figure 5.
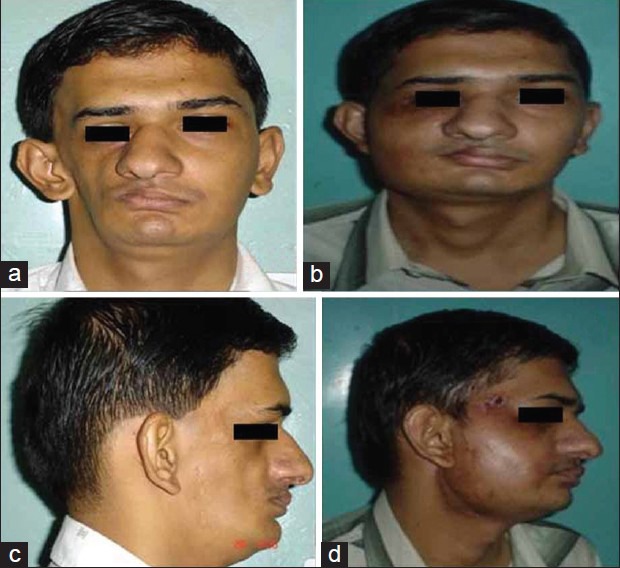
(a, b) Preoperative and postoperative (front view) after 4 years and (c, d) preoperative and postoperative (lateral view) after 4 years
DISCUSSION
HFM and HFA are the two most important striking and tragic deformities. Their etiology and pathophysiology are little understood. Facial asymmetry in these two conditions demands team approach comprising microvascular surgeons, craniofacial surgeon, orthodontist, prosthodontist and psychologist.
Because of severe cosmetic deformity, plastic surgeon is singled out to answer the challenge of these patients. In early days, both nonsurgical or surgical attempts were made to correct the deformity. In the nonsurgical method, injection of autologous and alloplastic materials was tried, and in the surgical method, autologous tissue graft and pedicled flap were tried by many authors, but none of the procedures have gained as much popularity as that of free flaps. The therapeutic approach for these rare disorders has changed dramatically with the introduction of microsurgical procedure. This innovative technique in facial contour reconstruction offers many advantages over the other techniques in terms of long-term functional and morphological results.
In this study of 22 cases, patients either had HFA or HFM. The demographic and presenting features of HFA patients in our study are quite similar to those described by Rogers.[13] Female preponderance was also found often with unilateral involvement. No patient in our study had bilateral involvement.
In this retrospective study, out of total six HFM patients, skeletal deformity was corrected with distraction osteogenesis in three patients. Soft-tissue contouring with parascapular flap was planned later as the second stage of treatment.[27]
Free tissue transplantation is the best option for correction of three-dimensional complex deformity because transplanted tissue bulk is maintained and there are no chances of recurrence.
There are different microvascular free tissue transfer options for correction of facial deformity, such as omentum,[6,7] groin,[9,14] rectus abdominis muscle, latissimus dorsi[15], serratus muscle,[16] deep inferior epigastric perforator,[17] scapular[8] and parascapular flap.[17,18]
Muscle flaps have the chances of recurrence due to muscle atrophy with time; therefore, fasciocutaneous flap is preferred.[17,18] We used parascapular flap in all cases because this flap has constant anatomy, long vascular pedicle, is compact in consistency and has minimal donor site morbidity.
Previously, surgeons preferred using superficial temporal vessel[17,18] for anastomosis. In this case series, the senior author had also used the same vessel initially in five cases, but some technical difficulties were encountered. Superficial temporal veins are very flimsy and small in diameter, as found by the senior author in the initial few cases, and this finding is similar to that reported by [Longaker and Siebert et al.[17,18] If superficial temporal vein was not found to be satisfactory, then either preauricular incision was extended into neck or postauricular extension was done. In the remaining cases, we used facial vessels as a recipient vessel. So, in our observation, selection of the recipient vessel for anastomosis is very important for success.
De-epithelialisation of parascapular flap is one of the most important steps in this surgery. Besides this, we also found that by doing the de-epithelialisation of skin before separation of flap, the donor site morbidity can be minimised. This finding is contrary to the findings in previous studies by Siebert et al.[18] and Longaker and Siebert[17] and similar to the report of Dunkley and Stevenson.[19]
There is little difference of opinion about insetting of flap as to which side is to be placed under the skin. Opinions vary as to whether or not it is beneficial to place the dermis side down (next to muscle) or up (lying immediately beneath the cheek skin). Tweed et al. suggested that a smoother cheek contour is achieved by placing the dermis outwards.[20] Harashina and Fujino[21] and Shintomi et al.,[22] however, argue that placing the dermis side down should reduce gravitational sagging. Luca vaienti et al. also mentioned that inserting this thick dermis layer turned towards the subcutis of the recipient site prevents subsequent downward shifting of the flap.[23] We also agree with their observation, as in this study, none of patients, except one, presented with gravitational sagging on follow-up.
Flap monitoring through skin paddle is still the most effective and reliable method,[24,25,26] particularly in developing countries where implantable laser Doppler methods for monitoring are relatively a costly affair. The small monitor skin paddle can be easily excised as a minor secondary procedure. Minor additional procedure was required in the form of thinning of margin and excision of skin paddle. Many studies have shown the need of ancillary procedure for aesthetic purpose, but in this study, ancillary procedure in form of fat injection was done in five patients and resuspension with polypropylene mesh was done in only one patient.[17,18,23] No partial or complete failure of flap was noted in our study, and also, no major complication was noted except five cases of hematoma. In this study, operative time was less as compared to previous studies. It depends largely on the surgeon's skill and experience with microvascular surgery. In this study, all patients were very much satisfied with the results except four patients. In these four patients, expectations were quite high and this was based on subjective assessment.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Rees TD, Ashley FL, Delgado JP. Silicone fluid injections for facial atrophy: A ten-year study. Plast Reconstr Surg. 1973;52:118–27. doi: 10.1097/00006534-197308000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Kanchwala SK, Bucky LP. Facial fat grafting: The search for predictable results. Facial Plast Surg. 2003;19:137–46. doi: 10.1055/s-2003-39140. [DOI] [PubMed] [Google Scholar]
- 3.Mordick TG, 2nd, Larossa D, Whitaker L. Whitaker soft tissue reconstruction of the face: A comparison of dermal fat grafting and vascularized tissue transfer. Ann Plast Surg. 1992;29:390–6. doi: 10.1097/00000637-199211000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Schmelzeisen R, Hausamen JE, Neukam FW, Schliephake H. Microsurgical reanastomosis of scapula transplants for maxillofacial bone reconstruction. Fortschr. Kiefer Gesichtschir. 1994;39:67–70. [PubMed] [Google Scholar]
- 5.Smith AA, Manktelow RT. The use of free tissue transfer to restore facial contour. Clin Plast Surg. 1990;17:655–61. [PubMed] [Google Scholar]
- 6.Losken A, Carlson GW, Culbertson JH, Scott Hultman C, Kumar AV, Jones GE, et al. Omental free flap reconstruction in complex head and neck deformities. Head Neck. 2002;24:326–31. doi: 10.1002/hed.10082. [DOI] [PubMed] [Google Scholar]
- 7.Walkinshaw M, Caffee HH, Wolfe SA. Vascularized omentum for facial contour restoration. Ann Plast Surg. 1983;10:292–300. doi: 10.1097/00000637-198304000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Upton J, Albin RE, Mulliken JB, Murray JE. The use of scapular and parascapular flaps for cheek reconstruction. Plast Reconstr Surg. 1992;90:959–71. doi: 10.1097/00006534-199212000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Harashina T, Fujino T. Reconstruction in Romberg's disease with free groin flap. Ann Plast Surg. 1981;7:289–94. doi: 10.1097/00000637-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Koshima I, Inagawa K, Urushibara K, Ohtsuki M, Moriguchi T. Deep inferior epigastric perforator dermal- fat or adiposal flap for correction of craniofacial contour deformities. Plast Reconstr Surg. 2000;106:10–5. doi: 10.1097/00006534-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 11.dos Santos LF. The vascular anatomy and dissection of the free scapular flap. Plast Reconstr Surg. 1984;73:599–604. doi: 10.1097/00006534-198404000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Kim PS, Gottlieb JR, Harris GD, Nagle DJ, Lewis VL. The dorsal thoracic fascia: Anatomic significance with clinical application in reconstructive microsurgery. Plast Reconstr Surg. 1987;79:72–80. [PubMed] [Google Scholar]
- 13.Rogers BO. Washington, D.C: Excerpta Medica; 1963. Progressive facial hemiatrophy (Romberg's disease): A review of 772 cases. In Transactions of the Third International Congress of Plastic Surgery; pp. 681–9. [Google Scholar]
- 14.Anderl H. Free vascularized groin fat flap in hypoplasia and hemiatrophy of the face (a 3 years observation) J Maxillofac Surg. 1979;7:327. doi: 10.1016/s0301-0503(79)80059-4. [DOI] [PubMed] [Google Scholar]
- 15.de la Fuente A, Jimenez A. Latissimus dorsi free flap for restoration of facial contour defects. Ann Plast Surg. 1989;22:1–8. doi: 10.1097/00000637-198901000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Angel MF, Bridges RM, Levine PA, Cantrell RW, Persing JA. The serratus anterior free tissue transfer for craniofacial reconstruction. J Craniofac Surg. 1992;3:207–12. doi: 10.1097/00001665-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Longaker MT, Siebert JW. Microvascular free flap correction of severe hemifacial atrophy. Plast Reconstr Surg. 1995;96:800–9. doi: 10.1097/00006534-199509001-00006. [DOI] [PubMed] [Google Scholar]
- 18.Siebert JW, Anson G, Longaker MT. Microsurgical correction of facial asymmetry in 60 consecutive cases. Plast Reconstr Surg. 1996;97:354–63. doi: 10.1097/00006534-199602000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Dunkley MP, Stevenson JH. Experience with the free _inverted_ groin flap in facial soft tissue contouring: A report on 6 flaps. Br J Plast Surg. 1990;43:154–8. doi: 10.1016/0007-1226(90)90154-r. [DOI] [PubMed] [Google Scholar]
- 20.Tweed AE, Manktelow RT, Zuker RM. Facial contour reconstruction with free flap. Ann Plast Surg. 1984;12:313–20. doi: 10.1097/00000637-198404000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Harashina T, Fujino T. Reconstruction in Romberg's disease with free groin flap. Ann Plast Surg. 1981;7:289–94. doi: 10.1097/00000637-198110000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Shintomi Y, Ohura T, Honda K, Iida K. The reconstruction of progressive facial hemi-atrophy by vascularized dermis-fat flaps. Br J Plast Surg. 1981;34:398–409. doi: 10.1016/0007-1226(81)90045-x. [DOI] [PubMed] [Google Scholar]
- 23.Vaienti L, Soresina M, Menozzi A. Parascapular free flap and fat graft: Combined surgical methods in morphological restoration of Hemifacial Progressive Atrophy. Plast Reconstr Surg. 2005;116:699–711. doi: 10.1097/01.prs.0000177449.12366.48. [DOI] [PubMed] [Google Scholar]
- 24.Jallali N, Ridha H, Butler PE. Postoperative monitoring of free flaps in UK plastic surgery units. Microsurgery. 2005;25:469–72. doi: 10.1002/micr.20148. [DOI] [PubMed] [Google Scholar]
- 25.Whitaker IS, Gulati V, Ross GL, Menon A, Ong TK. Variations in the post-operative management of free tissue transfers to the head and neck in the United Kingdom. Br J Oral Maxillofac Surg. 2007;45:16–8. doi: 10.1016/j.bjoms.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Whitaker IS, Oliver DW, Ganchi PA. Postoperative monitoring of microvascular tissue transfer: Current practice in the United Kingdom and Ireland. Plast Reconstruct Surg. 2003;111:2118–9. doi: 10.1097/01.PRS.0000057070.74385.AF. [DOI] [PubMed] [Google Scholar]
- 27.Kiichi I, Takahiko M, Isao K. Microsurgical correction of facial contour in hemifacial microsomia. Jpn J Plast Reconstruct Surg. 2005;48:881–90. [Google Scholar]


