Abstract
BACKGROUND
Low-dose tricyclic antidepressants have been used to treat chronic somatic and gastrointestinal pain disorders, including refractory functional dyspepsia. However, there are only limited data on the effects of these drugs on upper gastrointestinal function.
AIM
To compare the effects of two doses of amitriptyline (AMT) and placebo on gastric accommodation, emptying, satiation, and postprandial symptoms in healthy volunteers.
METHODS
Using a parallel-group, double-blind, placebo-controlled design, 41 healthy volunteers were randomized to AMT 25 mg, AMT 50 mg, or placebo for 2 wk. During the final 3 days of therapy, the following end points were assessed: fasting and postprandial gastric volumes, 2- and 4-h gastric emptying, time and volume to maximum satiation using a nutrient drink test, and postprandial symptoms 30 min later using 10-cm visual analog scales. AMT and metabolite levels were measured.
RESULTS
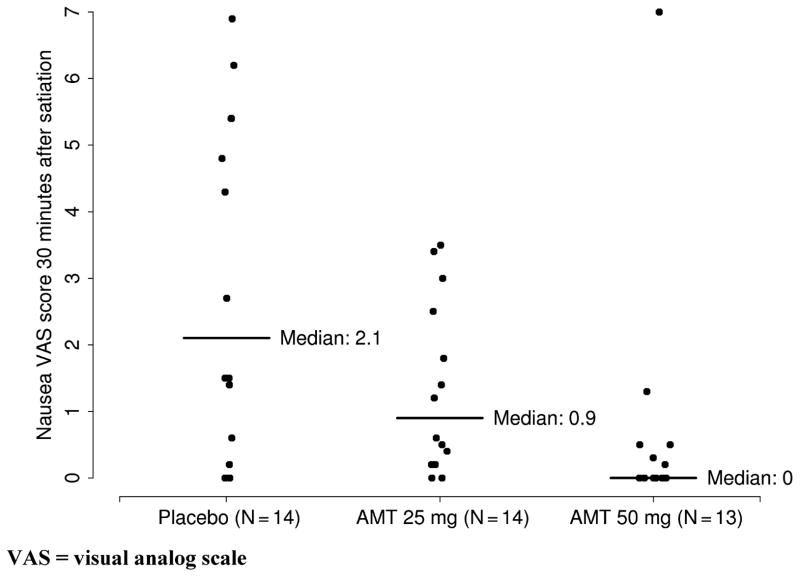
AMT slowed gastric emptying at 2 h (median 75% for placebo, 57% for AMT 25 mg, 67% for AMT 50 mg; P = 0.037) and 4 h (median 98% for placebo, 96% for AMT 25 mg, 92% for AMT 50 mg; P = 0.003). AMT did not affect gastric volumes or satiation volume, but it did reduce nausea scores at 30 min in a dose-dependent manner (median 2.1 for placebo, 0.9 for AMT 25 mg, and 0.0 for AMT 50 mg; P = 0.009).
CONCLUSION
In healthy volunteers, AMT slows gastric emptying of solids, but it does not significantly affect gastric volumes or satiation. AMT reduces nausea after challenge with a high calorie liquid load.
INTRODUCTION
Low-dose tricyclic antidepressants (TCAs) have been used to treat patients with chronic somatic and gastrointestinal pain disorders for many years (1–9). These agents are considered to work as neuromodulators, altering visceral sensation (10). However, the diverse pharmacological properties of TCAs, including their anticholinergics effects, suggest that they may have specific peripheral effects that cannot be extrapolated from their known central effects. One study that showed TCAs are effective in functional dyspepsia (FD) involved a small sample of patients, and the major symptom benefit was improved sleep (11). Despite extensive use in clinical practice, there are only limited data with regard to the effects of these agents directly on symptoms, or upper gastrointestinal motor or sensory function, either in health or disease states (11–14).
Postprandial symptoms, including early satiation, bloating, and nausea, are associated with impaired gastric accommodation in states such as FD, rumination syndrome, post vagotomy/gastric surgery, and diabetes mellitus when associated with vagal neuropathy (15–21). FD is a common, heterogeneous disorder of unclear etiology with limited treatment options (22–30). In addition to impaired gastric accommodation, a variety of motility and sensory disturbances have been implicated in the disorder (25, 27, 31–39). This suggests that measurement of gastrointestinal sensorimotor function may facilitate our understanding of upper gut symptoms and possibly enhance the choice of therapies in dyspepsia. However, research in the field of gastrointestinal sensorimotor function has been hindered by the invasiveness of investigations, such as the gastric barostat (15), which could alter physiology or symptoms generation (40).
With advances in the field of functional imaging and the development of novel technology that allows the noninvasive characterization of a variety of upper gut sensorimotor functions, we aimed to explore the gastric motor and sensory effects of amitriptyline (AMT), a drug commonly used in the management of dyspepsia. Low-dose AMT (50 mg) is also being tested in a National Institutes of Health-funded clinical trial of patients with FD in the United States (http://www2.niddk.nih.gov/). Our specific aim was to compare the effects of two doses of AMT and placebo on gastric emptying, gastric volumes, satiation, and postprandial symptoms in healthy volunteers.
MATERIALS AND METHODS
Study Overview
We performed a randomized, parallel group, two-dose, double-blind, placebo-controlled trial evaluating the effects of AMT on gastric motor and sensory function in healthy volunteers. Following an initial screening, 41 healthy volunteers were randomized to AMT 25 mg (n = 14), AMT 50 mg (n = 13), or placebo (n = 14) for 2 wk. During the final 3 days of therapy, we assessed the following end points: (a) gastric volumes using single photon emission computed tomography (SPECT), (b) gastric emptying using scintigraphy, (c) volume/time to maximum satiation using an Ensure (Ross Products, Division of Abbott Laboratories, Columbus, OH) drink satiation test, and (d) measurement of postprandial symptoms of bloating, fullness, nausea, and pain 30 min after reaching the maximum satiation using 10-cm visual analog scales (VAS).
Study Participants
Forty-one healthy participants were recruited by public advertisement. Each participant completed a validated bowel disease questionnaire (41) and was screened for any chronic gastrointestinal symptoms. Inclusion criteria were: 18- to 65-yr-old men and nonpregnant, nonbreastfeeding women. Women of childbearing potential were required to have a negative pregnancy test within 48 h of study.
Exclusion criteria included: abdominal surgery (other than appendectomy, caesarean section, or tubal ligation), positive symptoms on bowel disease questionnaire, use of medications that may alter gastrointestinal motility, current use of medications that could interact with AMT (per contraindications in the Physicians Desk Reference) (42), over-the-counter medication (except multivitamins) within 7 days of the study, present or previous chronic gastrointestinal illness or any systemic disease that could affect gastrointestinal motility, active cardiopulmonary disease requiring specialized monitoring, history of cardiac dysrhythmias (excluding sinus tachycardia, sinus arrhythmia, and premature atrial complexes), history of seizures, urinary retention, or angle-closure glaucoma, known intolerance to AMT, or symptoms of overt psychiatric disease.
Gastric Accommodation Test
We used a previously validated technique to noninvasively measure gastric accommodation; the method has been described in detail elsewhere (43). Following an overnight fast, participants were positioned supine on the imaging table of a SPECT system. A fasting SPECT scan was obtained 10 min after the intravenous injection of 10 m Ci 99mTc sodium pertechnetate. Upon the completion of the fasting scan, a 300 mL Ensure meal was ingested orally and followed by two subsequent postprandial SPECT scans and analyzed as described previously (44). Three-dimensional renderings of the stomach were then produced, and gastric volumes for fasting and postprandial scans were measured.
Measurement of Gastric Volumes
Following an overnight fast, participants ingested a standard egg-based 99mTc radiolabeled breakfast test meal. Scintigraphic gamma camera images were obtained immediately and 2 and 4 h after test meal ingestion as previously described (45).
Nutrient Drink Satiation Test
An adaptation of the method of Tack et al. was used (18). Briefly, patients were asked to ingest Ensure at a constant rate of 30 mL per minute (regulated by refilling the cup with Ensure using a constant-rate perfusion pump). The subjects were then instructed to maintain intake at the filling rate. At 5-min intervals, participants scored their satiation using a graphic rating scale that combines verbal descriptors on a scale graded 0–5: 0 = no symptoms, 1 = first sensation of satiation (threshold), 2 = mild, 3 = moderate, 4 = severe, 5 = maximum or unbearable satiation. Participants were told to stop meal intake when a score of 5 was obtained.
Thirty minutes after completing the test, participants scored their symptoms (bloating, fullness, nausea, pain) using a 10-cm VAS anchored with the words “unnoticeable” and “unbearable” at the left and right ends of the lines, respectively. The aggregate score was defined as the sum of VAS for each symptom (i.e., maximum 40). The timing of this symptom assessment was intended to be consistent with previous studies in the literature (46).
Amitriptyline/Metabolite Levels
As there is individual variability in drug metabolism, AMT and nortriptyline (NT) levels were obtained at a standardized time (8:00 am) the morning of the satiation test, within a 10–12 h window following the nightly ingestion of the drug (9:00–10:00 pm). This additional information was collected to improve understanding of the pharmacological effects of the drug and to allow some assessment of participant compliance.
Statistical Methods
PRIMARY AND SECONDARY END POINTS
The primary end points were the gastric volume ratio, gastric emptying at 2 h, and maximum tolerated volume of Ensure ingested. Secondary end points were gastric emptying at 4 h, time until a satiation score of 5 was reached and VAS scores for pain, bloating, nausea, and fullness were obtained 30 min after the time of maximal satiation.
Statistical Analysis
The effect of dose on the primary and various secondary end points was investigated using an intent-to-treat protocol by either a one-way analysis of variance, or a Kruskal-Wallis test for data that were not consistent with an assumption of normality (pain VAS score, nausea VAS score, fullness VAS score, and gastric emptying at 4 h). Sensitivity of results to possible confounding effects of age, sex, body mass index (BMI), or fasting gastric volume was investigated using linear regression models. Based on the relatively small sample size, no more than one variable in addition to dose was included in any one model. A further analysis compared primary end points according to raw measured combined AMT and NT levels (< 40 vs ≥ 40 ng/mL) with a two sample t-test. Statistical significance was determined at the 5% level. No adjustments for multiple testing were made.
RESULTS
Study Participants
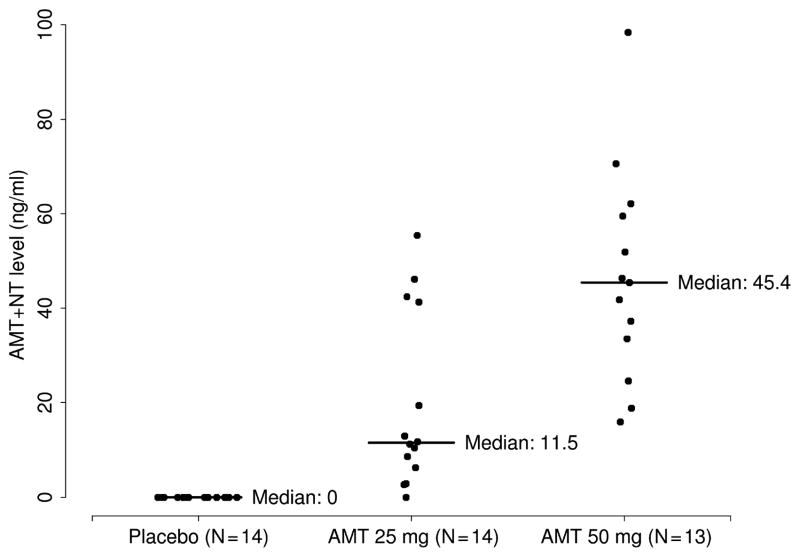
Table 1 shows the baseline characteristics and measured drug levels of the study population. Despite the randomization, there were some slight imbalances in age, sex, and BMI among the different treatment groups. Figure 1 shows the combined AMT and NT levels according to an assigned dose.
Table 1.
Characteristics of Treatment Groups
| Variable | Placebo (N = 14) | AMT 25 mg (N = 14) | AMT 50 mg (N = 13) |
|---|---|---|---|
| Age | 38 (31, 48) | 40 (37, 44) | 28 (24, 36) |
| Sex (Male) | 7 (50%) | 3 (21%) | 4 (31%) |
| BMI | 31.2 (26.5, 32.8) | 25.7 (22.7, 29.9) | 25.1 (23.6, 31.5) |
| NT level (ng/mL) | 0 (0, 0) | 5.5 (11.3, 20.9) | 14.3 (11.4, 27.2) |
| AMT level (ng/mL) | 0 (0, 0) | 8.5 (2.0, 12.4) | 23.8 (19.2, 30.2) |
Median (1st quartile, 3rd quartile) is shown for numerical variables.
AMT = amitriptyline; NT = nortriptyline; BMI = body mass index.
Figure 1.
Combined amitriptyline and nortriptyline drug levels according to assigned dose.
Gastric Volumes
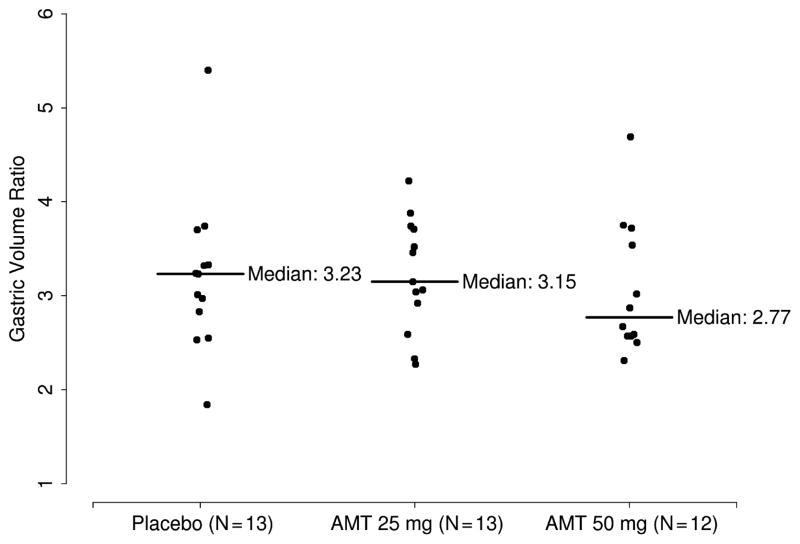
AMT dose had no effect on fasting volumes, fed volumes, the gastric volume ratio (postprandial volume/fasting volume, defined here as gastric accommodation, see Fig. 2), or the postprandial change in gastric volume (Table 2).
Figure 2.
Gastric volume ratio according to dose.
Table 2.
Effects of Amitriptyline on Gastric Volumes and Emptying
| Variable | Placebo (N = 14) | AMT 25 mg (N = 14) | AMT 50 mg (N = 13) | P Value |
|---|---|---|---|---|
| Fasting volume (mL) | 259 (191, 320) | 225 (198, 286) | 257 (244, 344) | 0.32 |
| Fed volume (mL) | 797 (749, 911) | 766 (674, 858) | 845 (798, 922) | 0.38 |
| Gastric volume ratio | 3.2 (2.8, 3.3) | 3.2 (2.9, 3.7) | 2.8 (2.6, 3.6) | 0.84 |
| Postprandial change in gastric volume (mL) | 539 (465, 578) | 523 (470, 578) | 572 (515, 593) | 0.79 |
| Gastric emptying at 2 h (%) | 75 (67, 86) | 57 (49, 72) | 67 (55, 75) | 0.037 |
| Gastric emptying at 4 h (%) | 98 (97, 99) | 96 (95, 97) | 92 (91, 96) | 0.003 |
Median (1st quartile, 3rd quartile) is shown.
AMT = amitriptyline; BMI = body mass index.
Kruskal-Wallis rank sum test used for gastric emptying at 4 h; one-way analysis of variance used for all other variables.
Gastric Emptying
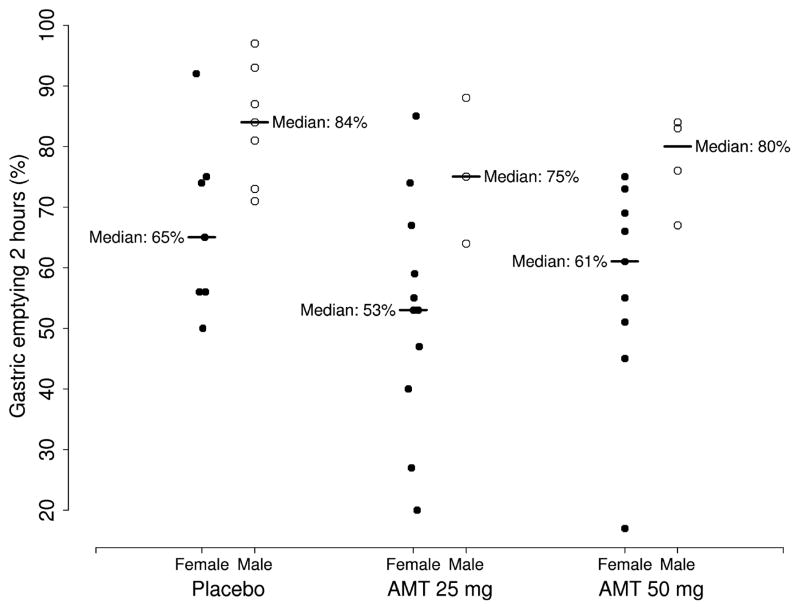
AMT slowed gastric emptying at 2 h (P = 0.037) and 4 h (P = 0.003) (Table 2). There was a significant sex effect on gastric emptying at 2 h (P < 0.001), with women tending to have slower gastric emptying than men. After controlling for sex, the observed difference in gastric emptying at 2 h between AMT dose groups was no longer statistically significant, although the trend remained (P = 0.14, Fig. 3). The greatest difference in gastric emptying at 4 h was observed between AMT 50 mg and placebo (median 92% vs 98%, respectively). No sex effect was observed at 4 h.
Figure 3.
Gastric emptying at 2 h according to dose and sex.
Nutrient Drink Satiation Test and Symptoms
AMT had no effect on volume of Ensure ingested or time to maximum satiation (Table 3). However, AMT did significantly reduce nausea at 30 min after reaching maximum satiation in an apparent dose-dependent manner (Table 3, Fig. 4). There was no evidence to suggest that there was any confounding with other patient variables.
Table 3.
Effects of Amitriptyline on Satiation Test End Points
| Variable | Placebo (N = 14) | AMT 25 mg (N = 14) | AMT 50 mg (N = 13) | P Value |
|---|---|---|---|---|
| VAS scores | ||||
| Pain | 0.9 (0.0, 4.7) | 0.8 (0.1, 1.6) | 0.8 (0.0, 1.8) | 0.82 |
| Bloating | 4.7 (3.1, 5.9) | 3.1 (2.4, 5.3) | 3.4 (2.4, 5.0) | 0.46 |
| Nausea | 2.1 (0.8, 5.3) | 0.9 (0.3, 2.3) | 0.0 (0.0, 0.5) | 0.009 |
| Fullness | 7.7 (6.9, 9.3) | 6.8 (6.4, 9.3) | 7.0 (5.0, 9.6) | 0.92 |
| Total | 16.7 (10.4, 23.5) | 13.2 (9.5, 15.4) | 12.0 (8.2, 14.8) | 0.23 |
| Volume ingested (mL) | 1,165 (1,024, 1,238) | 1,080 (919, 1,226) | 990 (900, 1,425) | 0.87 |
| Time to maximum satiation (min) | 35 (31, 38) | 34 (26, 37) | 32 (30, 42) | 0.57 |
Median (1st quartile, 3rd quartile) is shown.
AMT = amitriptyline; BMI = body mass index.
Kruskal-Wallis rank sum test used for pain, nausea and fullness VAS scores; one-way analysis of variance used for all other variables.
Figure 4.
Degree of nausea (VAS score) 30 min after reaching maximum satiation. VAS = visual analog scale.
Amitriptyline/Metabolite Levels
As noted in Figure 1, one participant in the 25 mg dose group had no detectable drug levels; study results did not change when this patient was re-assigned to the placebo group. Table 4 shows primary end points according to combined raw AMT and NT levels. Because the accuracy of any measurement lower than 20 ng/mL was uncertain, a cutoff point of 40 ng/mL was chosen to create a binary category for the combined raw drug level. None of the conclusions made in this report regarding primary end points changed when considering the raw drug levels directly.
Table 4.
Primary End Points According to Raw Measured Drug Level
| Variable | Combined Drug Level < 40 ng/mL (N = 29) | Combined Drug Level ≥ 40 ng/mL (N = 12) | P Value |
|---|---|---|---|
| Gastric volume ratio | 3.1 (2.6, 3.6) | 3.0 (2.6, 3.7) | 0.83 |
| Gastric emptying at 2 h (%) | 71 (56, 83) | 67 (53, 73) | 0.64 |
| Volume ingested (mL) | 1,080 (990, 1,260) | 1,058 (956, 1,429) | 0.83 |
Median (1st quartile, 3rd quartile) is shown.
P -Values from a two sample t-test.
DISCUSSION
TCAs have been used widely in gastroenterology practice, with several studies suggesting efficacy in the treatment of patients with a variety of functional gastrointestinal syndromes (1–4, 11, 47, 48) and somatic pain syndromes, including diabetic neuropathy, postherpetic neuralgia, arthritis, chronic back pain, headaches, and fibromyalgia (5, 6, 8). However, our understanding of the mechanism of action of these agents is limited. We found that AMT slowed gastric emptying of solids but had no significant effect on gastric volumes or maximum tolerated volume of a liquid nutrient load. While AMT reduced nausea, other meal-induced symptoms were not affected in this study.
The results here do not suggest that a TCA will not reduce pain- or meal-related symptoms in FD. In other studies, the therapeutic effect of TCAs does not appear to be related to the etiology of the pain (organic or functional), the presence or absence of depression (or an antidepressant effect of the drug), the dose of the TCA (low-dose or full antidepressant dose), or the sedating properties of TCAs (8, 11).
Because the peripheral effects of TCAs have not been well characterized, the overall effects of these medications are often attributed to their central nervous system activity. A commonly held hypothesis is that TCAs, which indirectly stimulate norepinephrine and serotonin receptors by blocking the reuptake of norepinephrine and serotonin, work as neuromodulators, affecting the brain-gut axis by altering neurotransmitter systems within the limbic system and other pain centers of the brain (e.g., anterior cingulate cortex) (11). Morgan and colleagues have shown that AMT is associated with reduced pain-related cerebral activation in the anterior cingulate cortex and left posterior parietal complex during stress (49), suggesting a central action of the drug. There is one small, randomized trial of AMT in FD showing improved symptoms with therapy that altered sleep patterns (11). One plausible explanation is that altering sleep patterns modulates the regulation of noradrenergic systems of the locus coeruleus (a brain center inhibited during sleep), which alters nociception (11).
Our study shows a significant effect of AMT on the sensation of nausea, with a dose-dependent decrease in nausea with escalating AMT dose. As seen in our previous study exploring the effects of desipramine on nutrient drink induced symptoms (50), AMT had no effect on other symptoms. Further, we demonstrated in the present study that there was no significant effect of AMT on gastric volumes, which has not previously been directly assessed. It should be noted that the participants in this trial were healthy volunteers, presumably with normal baseline physiology. It is possible that FD patients with abnormal gastric emptying, decreased accommodation, or visceral hyperalgesia may respond differently to AMT.
A possible concern with AMT, which has the highest antimuscarinic potency of the TCAs, is the side effect profile generated by those anticholinergic properties (in addition to interaction with adrenergic, serotonergic, and histaminergic receptors). However, the anticholinergic properties of AMT are relatively weak, less than atropine (51, 52). It is also possible that the usually mild symptoms may actually enhance or potentiate the medication effect and therapeutic efficacy. In addition, the sedative effects and their impact on sleep may be beneficial as outlined previously.
A theoretical downside of AMT is a slowing of gastric emptying. In this study, we did see slowing of gastric emptying. As gastric emptying tends to correlate poorly with symptoms in patients with FD and diabetes (31, 53–57), the clinical significance of this effect is uncertain. A retrospective study of TCAs in patients with diabetes and chronic vomiting did show clinical benefit, irrespective of duration of diabetes, presence of neuropathy, delayed gastric emptying, or psychiatric status 58. Based on these data and our findings, it would seem reasonable to try TCAs in symptomatic patients with delayed gastric emptying. Prospective trials in this patient population are warranted.
As the formation and expression of dyspeptic symptoms is likely complex, with both sensory and affective components, it is conceivable that AMT could be active at multiple sites. However, studies have revealed no effect of AMT on perception of rectal and esophageal distention (13), no effect of a single AMT dose on rectal compliance or visceral perception (14), and no alteration of perceptual responses to gastric distention (11). There does appear to be an effect on gastrointestinal motility, with slowed orocecal transit (12), inhibited muscular contraction in vitro (59), and inhibited ATP-sensitive K(+) channels in cultured murine interstitial cells of Cajal (60). What sensory or clinical impact these physiologic perturbations may have is difficult to say based on our current understanding of symptom generation.
The strengths of the current study include the use of novel techniques that allow the noninvasive measurement of gastric volumes, emptying, and sensation. We are unaware of a previous study of this kind to investigate the physiologic effects of this commonly used therapy. The study was powered to be able to detect what were considered to be clinically important effects of AMT on the primary end points. However, sample sizes were still relatively small, and, as such, power was limited to detect more subtle effects. It is unknown whether such minor effects may be relevant in symptom generation, such as the improvement in nausea seen in this study. Given the possibility that variability in body size and weight might affect many of the outcomes in this study, secondary analyses comparing end points across groups adjusting for BMI were performed; however, results were not qualitatively different.
The physiologic investigations used in this study are validated techniques that have been used to examine dyspeptic symptoms. However, physiologic correlates of gut-related symptoms are unclear, and the best way to investigate symptom generation is uncertain. It is possible that available testing lacks the sensitivity to capture the clinically relevant physiologic changes that impact gut-related symptoms, such as the effect on nausea seen in this study. Alternatively, we propose that the lack of significant physiologic correlates demonstrated in this healthy population highlights the primary role of the central nervous system in symptom generation in response to AMT, supporting the need for further research into visceral afferent function and subsequent central processing.
It is possible that the dose of AMT was too low to demonstrate more distinct physiologic effects. However, the doses chosen are those used in clinical practice and have been shown to be effective in both organic and functional pain syndromes (2–4, 7). The pharmacokinetics of the drug are such that steady state was achieved before the various physiologic tests, and drug levels were obtained and used in our analysis. In a study of TCA use in IBS, most patients achieved symptom improvement with a median dose of 50 mg (4). Furthermore, Halpert and colleagues found that the clinical response to TCAs in functional gastrointestinal disorders was not related to dosage, and there was no relationship between total dose or plasma level and the clinical response (61). Aside from the effect on nausea, our findings on dose were similar, and no therapeutic effect was seen based on plasma drug levels. As we typically wait for several weeks to assess TCA effect in clinical practice, it is possible that a longer trial could have revealed different effects on symptoms and the physiologic responses measured. In addition, drug effects may be different in a symptomatic versus healthy population.
We conclude that low-dose AMT slows gastric emptying of solids but does not significantly affect gastric volumes. AMT reduces nausea after challenge with a nutrient load, but does not impact other meal-induced symptoms at the doses and duration of therapy tested in this study of healthy volunteers. AMT and other TCAs may impact physiology and symptoms in various dyspeptic syndromes, and randomized controlled trials in patient groups are warranted.
STUDY HIGHLIGHTS.
What Is Current Knowledge
Tricyclic antidepressant agents (TCAs) are widely used in the management of various gastrointestinal disorders.
TCAs are considered to work as neuromodulators, affecting visceral sensation.
There is limited data on the specific effects of TCAs on upper gut motor or sensory function.
What Is New Here
Amitriptyline (AMT) affects gastric emptying of solids but has no significant effect on gastric accommodation or satiation.
AMT reduces nausea after a nutrient-rich liquid meal.
This study suggests that AMT and other TCAs may impact physiology and symptoms in various dyspeptic syndromes, warranting further investigation in patient populations.
Acknowledgments
The authors would like to thank Katherine Purcell for her assistance in the preparation of the manuscript.
Financial support: Ernest P. Bouras was supported by a clinical research grant from Mayo Clinic Jacksonville. Nicholas J. Talley is supported by NIH grant U01 065713-01. Michael Camilleri is supported by K24 DK 02638 and RO1 DK 54681.
Footnotes
Guarantor of the article: Ernest P. Bouras, M.D.
Specific author contributions: Concept, design and conduct of the study—Dr. Bouras, who served as the principal investigator, with input on design from all authors. Analysis of scintigraphic data—Burton. Statistical analysis—Crook and Heckman. Overall analysis and interpretation—Bouras, Talley, Camilleri, Richelson. Manuscript draft—Bouras. Critical revision of manuscript—Talley, Camilleri, Richelson.
Potential competing interests: None.
References
- 1.Cannon RO, 3rd, Quyyumi AA, Mincemoyer R, et al. Imipramine in patients with chest pain despite normal coronary angiograms. N Engl J Med. 1994;330:1411–7. doi: 10.1056/NEJM199405193302003. [DOI] [PubMed] [Google Scholar]
- 2.Clouse RE. Antidepressants for functional gastrointestinal syndromes. Dig Dis Sci. 1994;39:2352–63. doi: 10.1007/BF02087651. [DOI] [PubMed] [Google Scholar]
- 3.Clouse RE, Lustman PJ. Use of psychopharmacological agents for functional gastrointestinal disorders. Gut. 2005;54:1332–41. doi: 10.1136/gut.2004.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clouse RE, Lustman PJ, Geisman RA, et al. Antidepressant therapy in 138 patients with irriTable bowel syndrome: a five-year clinical experience. Aliment Pharmacol Ther. 1994;8:409–16. doi: 10.1111/j.1365-2036.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 5.Magni G. The use of antidepressants in the treatment of chronic pain. A review of the current evidence. Drugs. 1991;42:730–48. doi: 10.2165/00003495-199142050-00002. [DOI] [PubMed] [Google Scholar]
- 6.Max MB, Lynch SA, Muir J, et al. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 7.McQuay HJ, Carroll D, Glynn CJ. Low dose amitriptyline in the treatment of chronic pain. Anaesthesia. 1992;47:646–52. doi: 10.1111/j.1365-2044.1992.tb02383.x. [DOI] [PubMed] [Google Scholar]
- 8.Onghena P, Van Houdenhove B. Antidepressant-induced analgesia in chronic non-malignant pain: A meta-analysis of 39 placebo-controlled studies. Pain. 1992;49:205–19. doi: 10.1016/0304-3959(92)90144-Z. [DOI] [PubMed] [Google Scholar]
- 9.Richelson E. The use of tricyclic antidepressants in chronic gastrointestinal pain. J Clin Psychiatry. 1982;43:50–5. [PubMed] [Google Scholar]
- 10.Mayer EA, Tillisch K, Bradesi S. Review article: Modulation of the brain-gut axis as a therapeutic approach in gastrointestinal disease. Aliment Pharmacol Ther. 2006;24:919–33. doi: 10.1111/j.1365-2036.2006.03078.x. [DOI] [PubMed] [Google Scholar]
- 11.Mertz H, Fass R, Kodner A, et al. Effect of amitriptyline on symptoms, sleep, and visceral perception in patients with functional dyspepsia. Am J Gastroenterol. 1998;93:160–5. doi: 10.1111/j.1572-0241.1998.00160.x. [DOI] [PubMed] [Google Scholar]
- 12.Gorard DA, Libby GW, Farthing MJ. Influence of antide-pressants on whole gut and orocaecal transit times in health and irritable bowel syndrome. Aliment Pharmacol Ther. 1994;8:159–66. doi: 10.1111/j.1365-2036.1994.tb00273.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorelick AB, Koshy SS, Hooper FG, et al. Differential effects of amitriptyline on perception of somatic and visceral stimulation in healthy humans. Am J Physiol. 1998;275:G460–6. doi: 10.1152/ajpgi.1998.275.3.G460. [DOI] [PubMed] [Google Scholar]
- 14.Siproudhis L, Dinasquet M, Sebille V, et al. Differential effects of two types of antidepressants, amitriptyline and fluoxetine, on anorectal motility and visceral perception. Aliment Pharmacol Ther. 2004;20:689–95. doi: 10.1111/j.1365-2036.2004.02151.x. [DOI] [PubMed] [Google Scholar]
- 15.Azpiroz F, Malagelada JR. Gastric tone measured by an electronic barostat in health and postsurgical gastroparesis. Gastroenterology. 1987;92:934–43. doi: 10.1016/0016-5085(87)90967-x. [DOI] [PubMed] [Google Scholar]
- 16.Salet GA, Samsom M, Roelofs JM, et al. Responses to gastric distension in functional dyspepsia. Gut. 1998;42:823–9. doi: 10.1136/gut.42.6.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samsom M, Salet GA, Roelofs JM, et al. Compliance of the proximal stomach and dyspeptic symptoms in patients with type I diabetes mellitus. Dig Dis Sci. 1995;40:2037–42. doi: 10.1007/BF02208676. [DOI] [PubMed] [Google Scholar]
- 18.Tack J, Piessevaux H, Coulie B, et al. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–52. doi: 10.1016/s0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 19.Thumshirn M, Camilleri M, Hanson RB, et al. Gastric mechanosensory and lower esophageal sphincter function in rumination syndrome. Am J Physiol. 1998;275:G314–21. doi: 10.1152/ajpgi.1998.275.2.G314. [DOI] [PubMed] [Google Scholar]
- 20.Thumshirn M, Camilleri M, Saslow SB, et al. Gastric accommodation in non-ulcer dyspepsia and the roles of Helicobacter pylori infection and vagal function. Gut. 1999;44:55–64. doi: 10.1136/gut.44.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijnhoven BP, Salet GA, Roelofs JM, et al. Function of the proximal stomach after Nissen fundoplication. Br J Surg. 1998;85:267–71. doi: 10.1046/j.1365-2168.1998.00505.x. [DOI] [PubMed] [Google Scholar]
- 22.Camilleri CE, Carlson PJ, Camilleri M, et al. A study of candidate genotypes associated with dyspepsia in a U.S. community. Am J Gastroenterol. 2006;101:581–92. doi: 10.1111/j.1572-0241.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 23.Castillo EJ, Camilleri M, Locke GR, et al. A community-based, controlled study of the epidemiology and pathophysiology of dyspepsia. Clin Gastroenterol Hepatol. 2004;2:985–96. doi: 10.1016/s1542-3565(04)00454-9. [DOI] [PubMed] [Google Scholar]
- 24.Choung RS, Talley NJ. Novel mechanisms in functional dyspepsia. World J Gastroenterol. 2006;12:673–7. doi: 10.3748/wjg.v12.i5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delgado-Aros S, Camilleri M, Cremonini F, et al. Contributions of gastric volumes and gastric emptying to meal size and postmeal symptoms in functional dyspepsia. Gastroenterology. 2004;127:1685–94. doi: 10.1053/j.gastro.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 26.Fischler B, Tack J, De Gucht V, et al. Heterogeneity of symptom pattern, psychosocial factors, and pathophysiological mechanisms in severe functional dyspepsia. Gastroenterology. 2003;124:903–10. doi: 10.1053/gast.2003.50155. [DOI] [PubMed] [Google Scholar]
- 27.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239–55. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 28.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–79. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 29.Talley NJ. American Gastroenterological Association medical position statement: Evaluation of dyspepsia. Gastroenterology. 2005;129:1753–5. doi: 10.1053/j.gastro.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 30.Talley NJ, Locke GR, 3rd, Lahr BD, et al. Functional dyspepsia, delayed gastric emptying, and impaired quality of life. Gut. 2006;55:933–9. doi: 10.1136/gut.2005.078634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bredenoord AJ, Chial HJ, Camilleri M, et al. Gastric accommodation and emptying in evaluation of patients with upper gastrointestinal symptoms. Clin Gastroenterol Hepatol. 2003;1:264–72. [PubMed] [Google Scholar]
- 32.Chen JD, Ke MY, Lin XM, et al. Cisapride provides symptomatic relief in functional dyspepsia associated with gastric myoelectrical abnormality. Aliment Pharmacol Ther. 2000;14:1041–7. doi: 10.1046/j.1365-2036.2000.00801.x. [DOI] [PubMed] [Google Scholar]
- 33.David D, Mertz H, Fefer L, et al. Sleep and duodenal motor activity in patients with severe non-ulcer dyspepsia. Gut. 1994;35:916–25. doi: 10.1136/gut.35.7.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holtmann G, Gschossmann J, Neufang-Huber J, et al. Differences in gastric mechanosensory function after repeated ramp distensions in non-consulters with dyspepsia and healthy controls. Gut. 2000;47:332–6. doi: 10.1136/gut.47.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mertz H, Fullerton S, Naliboff B, et al. Symptoms and visceral perception in severe functional and organic dyspepsia. Gut. 1998;42:814–22. doi: 10.1136/gut.42.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piessevaux H, Tack J, Wilmer A, et al. Perception of changes in wall tension of the proximal stomach in humans. Gut. 2001;49:203–8. doi: 10.1136/gut.49.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rhee PL, Kim YH, Son HJ, et al. The etiologic role of gastric hypersensitivity in functional dyspepsia in Korea. J Clin Gastroenterol. 1999;29:332–5. doi: 10.1097/00004836-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Tack J, Caenepeel P, Fischler B, et al. Symptoms associated with hypersensitivity to gastric distention in functional dyspepsia. Gastroenterology. 2001;121:526–35. doi: 10.1053/gast.2001.27180. [DOI] [PubMed] [Google Scholar]
- 39.Troncon LE, Bennett RJ, Ahluwalia NK, et al. Abnormal intragastric distribution of food during gastric emptying in functional dyspepsia patients. Gut. 1994;35:327–32. doi: 10.1136/gut.35.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Zwart IM, Haans JJ, Verbeek P, et al. Gastric accommodation and motility are influenced by the barostat device: Assessment with magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol. 2007;292:G208–14. doi: 10.1152/ajpgi.00151.2006. [DOI] [PubMed] [Google Scholar]
- 41.Talley NJ, Phillips SF, Melton J, 3rd, et al. A patient questionnaire to identify bowel disease. Ann Intern Med. 1989;111:671–4. doi: 10.7326/0003-4819-111-8-671. [DOI] [PubMed] [Google Scholar]
- 42.PDR Staff. Physicians Desk Reference 2003. 57. Thomson Healthcare; 2002. [Google Scholar]
- 43.Bouras EP, Delgado-Aros S, Camilleri M, et al. SPECT imaging of the stomach: Comparison with barostat, and effects of sex, age, body mass index, and fundoplication. Single photon emission computed tomography. Gut. 2002;51:781–6. doi: 10.1136/gut.51.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuiken SD, Samsom M, Camilleri M, et al. Development of a test to measure gastric accommodation in humans. Am J Physiol. 1999;277:G1217–21. doi: 10.1152/ajpgi.1999.277.6.G1217. [DOI] [PubMed] [Google Scholar]
- 45.Camilleri M, Zinsmeister AR, Greydanus MP, et al. Towards a less costly but accurate test of gastric emptying and small bowel transit. Dig Dis Sci. 1991;36:609–15. doi: 10.1007/BF01297027. [DOI] [PubMed] [Google Scholar]
- 46.Tosetti C. Reproducibility of a water load test in healthy subjects and symptom profile compared to patients with functional dyspepsia. Gastroenterology. 1999;116:A336. [Google Scholar]
- 47.Greenbaum DS, Mayle JE, Vanegeren LE, et al. Effects of desipramine on irritable bowel syndrome compared with atropine and placebo. Dig Dis Sci. 1987;32:257–66. doi: 10.1007/BF01297051. [DOI] [PubMed] [Google Scholar]
- 48.Myren J, Lovland B, Larssen SE, et al. A double-blind study of the effect of trimipramine in patients with the irritable bowel syndrome. Scand J Gastroenterol. 1984;19:835–43. [PubMed] [Google Scholar]
- 49.Morgan V, Pickens D, Gautam S, et al. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005;54:601–7. doi: 10.1136/gut.2004.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Talley NJ, Camilleri M, Chitkara DK, et al. Effects of desipramine and escitalopram on postprandial symptoms induced by the nutrient drink test in healthy volunteers: A randomized, double-blind, placebo-controlled study. Digestion. 2005;72:97–103. doi: 10.1159/000088363. [DOI] [PubMed] [Google Scholar]
- 51.Richelson E, Nelson A. Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther. 1984;230:94–102. [PubMed] [Google Scholar]
- 52.Snyder SH, Yamamura HI. Antidepressants and the muscarinic acetylcholine receptor. Arch Gen Psychiatry. 1977;34:236–9. doi: 10.1001/archpsyc.1977.01770140126014. [DOI] [PubMed] [Google Scholar]
- 53.Bouras EP, Maleki D, Worra JB, et al. Prevalence of abnormal gastrointestinal transit in symptomatic patients with diabetes mellitus at a tertiary care center. Gastroenterology. 1997;112:A703. [Google Scholar]
- 54.Jones KL, Russo A, Stevens JE, et al. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 55.Loo FD, Palmer DW, Soergel KH, et al. Gastric emptying in patients with diabetes mellitus. Gastroenterology. 1984;86:485–94. [PubMed] [Google Scholar]
- 56.Talley NJ, Shuter B, McCrudden G, et al. Lack of association between gastric emptying of solids and symptoms in nonulcer dyspepsia. J Clin Gastroenterol. 1989;11:625–30. doi: 10.1097/00004836-198912000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Talley NJ, Verlinden M, Jones M. Can symptoms discriminate among those with delayed or normal gastric emptying in dysmotility-like dyspepsia? Am J Gastroenterol. 2001;96:1422–8. doi: 10.1111/j.1572-0241.2001.03683.x. [DOI] [PubMed] [Google Scholar]
- 58.Sawhney MS, Prakash C, Lustman PJ, et al. Tricyclic antidepressants for chronic vomiting in diabetic patients. Dig Dis Sci. 2007;52:418–24. doi: 10.1007/s10620-006-9378-8. [DOI] [PubMed] [Google Scholar]
- 59.James AN, Ryan JP, Parkman HP. Effects of clonidine and tricyclic antidepressants on gastric smooth muscle contractility. Neurogastroenterol Motil. 2004;16:143–53. doi: 10.1111/j.1365-2982.2004.00515.x. [DOI] [PubMed] [Google Scholar]
- 60.Choi S, Park CG, Kim MY, et al. Action of imipramine on activated ATP-sensitive K(+) channels in interstitial cells of Cajal from murine small intestine. Life Sci. 2006;78:2322–8. doi: 10.1016/j.lfs.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 61.Halpert A, Dalton CB, Diamant NE, et al. Clinical response to tricyclic antidepressants in functional bowel disorders is not related to dosage. Am J Gastroenterol. 2005;100:664–71. doi: 10.1111/j.1572-0241.2005.30375.x. [DOI] [PubMed] [Google Scholar]