Abstract
BACKGROUND:
Peroxisome proliferator activator receptor gamma (PPARγ) is a nuclear transcription factor regulating multiple genes involved in cell growth, differentiation, carbohydrate and lipid metabolism and energy production. Several genetic variations in the PPARγ gene have been identified to be associated with diabetes, obesity, dyslipidemia, insulin resistance, metabolic syndrome and coronary artery disease. The present study was designed to explore the distribution of two common single nucleotide polymorphisms of the PPARγ gene (C1431T and Pro12Ala) in an Iranian population.
MATERIALS AND METHODS:
Genotype frequencies for these two polymorphisms were compared for 160 healthy Iranian individuals with reports from other populations. The Genotyping was performed using real-time polymerase chain reaction.
RESULTS:
The genotype distribution of the C1431T PPARγ polymorphism was 0.869 for the CC genotype, 0.119 for the CT genotype and 0.013 for uncommon TT genotype. Allelic frequencies were 0.93 for C and 0.07 for T allele respectively. For the Pro12Ala polymorphism of PPARγ gene, genotypic distributions and allelic frequencies were, 0.813 for CC, 0.181 for CG and 0.06 for GG and 0.903 for C and 0.097 for G respectively. Allelic and genotypic frequencies for both polymorphisms of PPARγ gene were in Hardy-Weinberg equilibrium.
CONCLUSIONS:
Iran is a country with an ethnically diverse population and a comparison of allelic and genotypic frequencies of PPARγ C1431T and Pro12Ala polymorphisms between our population and others showed significant differences.
Keywords: Iranian population, PPARγ gene, single nucleotide polymorphism
Introduction
Peroxisome proliferator activator receptor gamma (PPARγ) is a member of the nuclear receptor superfamily and is a ligand-activated transcription factor that regulates genes responsible for some very important biological functions including: Cell growth, differentiation and metabolism and acts as dietary lipid sensor.[1]
The PPARγ gene is located on the human chromosome 3p25-24 and consists of nine exons and as a result of alternative splicing and promoter usage produces four isoforms; PPARγ1, PPARγ2, PPARγ3 and PPARγ4. PPARγ1 is expressed by most tissues.[2] PPARγ2 is predominantly expressed in adipose tissue and regulates adipocyte differentiation.[3] PPARγ3 expression appears to be confined to macrophages, adipose tissue and the colon.[4] Primer extension studies have confirmed that PPARγ4 mRNA is present in adipose tissue suggesting that the PPARγ4 isoform has a role in adipocyte biology.[5]
PPARγ plays critical roles in regulating lipid and carbohydrate metabolism (energy balance), adipocyte differentiation, proliferation and insulin sensitivity and create a relationship between environmental factors and metabolic processes of the organism.[6] PPARγ also plays roles in macrophages by enhancing foam cell formation and suppressing inflammatory cytokine production. It may also participate in controlling systemic glucose and lipid metabolism in the liver.[7,8] PPARγ may therefore affect processes involved in atherosclerosis.[9]
Since PPARγ is a nuclear transcription factor regulating multiple genes involved in energy production, glucose and lipid metabolism it may be a promising candidate gene for several major common diseases including cardiovascular disease, cancer, diabetes, inflammation and polymorphisms in this receptor may influence the pathology of these diseases. The PPARγ gene has been reported to be associated with various metabolic disorders including diabetes,[10] obesity,[11] dyslipidemia, insulin resistance, metabolic syndrome[12] and coronary artery disease (CAD).[13]
Several PPARγ2 gene single nucleotide polymorphisms (SNPs) have been reported to be associated with diabetes, obesity, dyslipidemia, insulin resistance, metabolic syndrome and CAD. Several genetic variations of the PPAR gene have been found in the activation function domain 1, deoxyribonucleic acid binding domain and ligand binding domain (LBD) of the receptor and confer some conformational changes in protein structure that may affect transcriptional activity.[14]
Among several genetic variants of the PPARγ gene, two polymorphisms Pro12Ala of the exon B (rs1801282) and the C1431T silent substitution (rs3856806) in the 6th exon are the most frequently occurring SNPs and have been associated with various diseases and extensive studies have been undertaken to assess the effects of these polymorphisms on many aspects of human physiology. The C1431T polymorphism is also known as the C161T or His477His was identified in 1998 by Meirhaeghe et al.,[15] and has been studied in relation to bone metabolism, metabolic syndrome, CAD, obesity and glucose intolerance. The Pro12Ala protein polymorphism is due to a CCA-to-GCA missense mutation and was identified by Yen et al.[16] It is associated with type-2 diabetes mellitus, insulin resistance, obesity and metabolic disorders. In addition, numerous studies have demonstrated rare alleles of these two common variants play a role in the complex pathogenetic mechanism of major diseases supposed to be useful markers to evaluate the connection between PPARγ and metabolic derived disorders.
Given the pivotal roles of PPARγ in regulating metabolism several studies in various ethnic populations including, Caucasians,[17] Mexican-Americans,[18] African-Americans,[19,13] Asian,[20] Eastern Asian,[12] European,[10] Hispanic and non-Hispanic[21] have been conducted to determine allelic frequencies and genotypic distribution. There have been no studies of the distribution of C1431T polymorphism in an Iranian population and there is some controversy about the frequency of Pro12Ala polymorphism. Therefore, the present study was designed to explore the distribution of these two common variants of PPAR gene in Iranian population and compare the finding with other populations. A number of 160 healthy individuals were analyzed to evaluate the frequency of PPARγ alleles and genotypes in a healthy Iranian population.
Materials and Methods
A total of 160 healthy volunteer subjects comprised of 70 males and 90 females, mainly people of Iranian descent (primarily Persian), were selected randomly from all parts of Mashhad as a second largest city in Iran and enrolled in this study. A health questionnaire, including questions such as name, ethnicity, family history, age, sex and dietary habits, was provided to each participant. Blood was taken in accordance with the World Health Organization protocol for blood donation and the healthy state of the all the participants was determined by medical history, physical examination and blood chemistry tests. Subjects were excluded from the study if they had a history of congestive heart disease, liver and/or renal disease, endocrinological abnormalities and alcohol consumption or were under medications that altered blood pressure, glucose or lipid metabolism. The clinical and biochemical characteristics of the all the individuals enrolled into the study were normal at baseline. The protocol was approved by the Ethics Committee of the Mashhad University of Medical Science and Informed consent was obtained from all participants.
Genomic deoxyribonucleic acid (DNA) was extracted from whole blood using the FlexiGene DNA isolation Kit (Qiagen). C1431T and Pro12Ala polymorphisms of the PPARγ gene were determined by a predesigned TaqMan SNP genotyping assay (Applied Biosystems). Oligonucleotides used for allelic discrimination assays for Pro12Ala and C1431T were as follows:
-
Context sequences for Pro/12Ala ([VIC/FAM]) (Applied Biosystems ID: C_1129864_10):
- AACTCTGGGAGATTCTCCTATTGAC[C/G] CAGAAAGCGATTCCTTCACTGATAC
- Pro12 Probe (Vic labeled): C_1129864_10-C
- Ala12 Probe (Fam labeled): C_1129864_10-G.
-
Context sequences for C1431T ([VIC/FAM]) (Applied Biosystems ID: C_11922961_30):
- ACCTCAGACAGATTGTCACGGAACA[C/T] GTGCAGCTACTGCAGGTGATCAAGA
- C1431 Probe (Vic labeled): C_11922961_30-C
- T1431 Probe (Fam labeled): C_11922961_30-T.
The reaction was performed in 25 μl final volume with real-time polymerase chain reaction (PCR) using 96-well plates on an ABI 7500 real time PCR system (Applied Biosystems). The PCR conditions were 95°C for 10 min and 40 cycles of 92°C for 15 s and 60°C for 1 min. Individual genotypes identification was analyzed by SDS software version 1.3 (Applied Biosystems). For genotyping quality control, duplicate samples and negative controls were included to ensure the accuracy.
Allele frequencies of two SNPs were computed using genotype data obtained from these healthy controls and were compared with those reported in other populations. Each SNP was tested for Hardy-Weinberg equilibrium (HWE) conformity. The statistical significance of the differences between genotypic distributions in populations was tested by the Chi-square (χ2) or Fisher's exact test using the SPSS 16.0 statistical package. (SPSS Inc., Chicago, IL) P < 0.05 was considered to be significant.
Results
The genotype characteristics of studied individuals are shown in Table 1. Allelic and genotypic frequencies for both polymorphisms of PPARγ gene were in HWE (P ≥ 0.05) [Table 1]. The genotypic distribution of the C1431T PPARγ polymorphism were 0.869 for CC, 0.119 for CT and 0.013 for TT and allelic frequencies were 0.93 for C and 0.07 for T respectively [Table 1]. For another Pro12Ala variant of PPARγ, genotypic distributions and allelic frequencies were, 0.813 for CC, 0.181 for CG and 0.06 for GG and 0.903 for C and 0.097 for G respectively [Table 1]. Differences in allelic frequencies and genotype distribution of the polymorphisms between Iranian population and those reported for other populations are shown in Tables 2 and 3 respectively.
Table 1.
Allelic and genotypes frequency of studied population
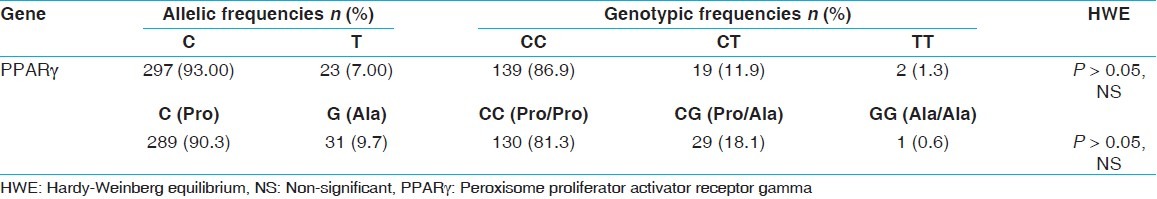
Table 2.
Comparison of allelic and genotypic frequencies of PPARγ C1431T variant between our population and others
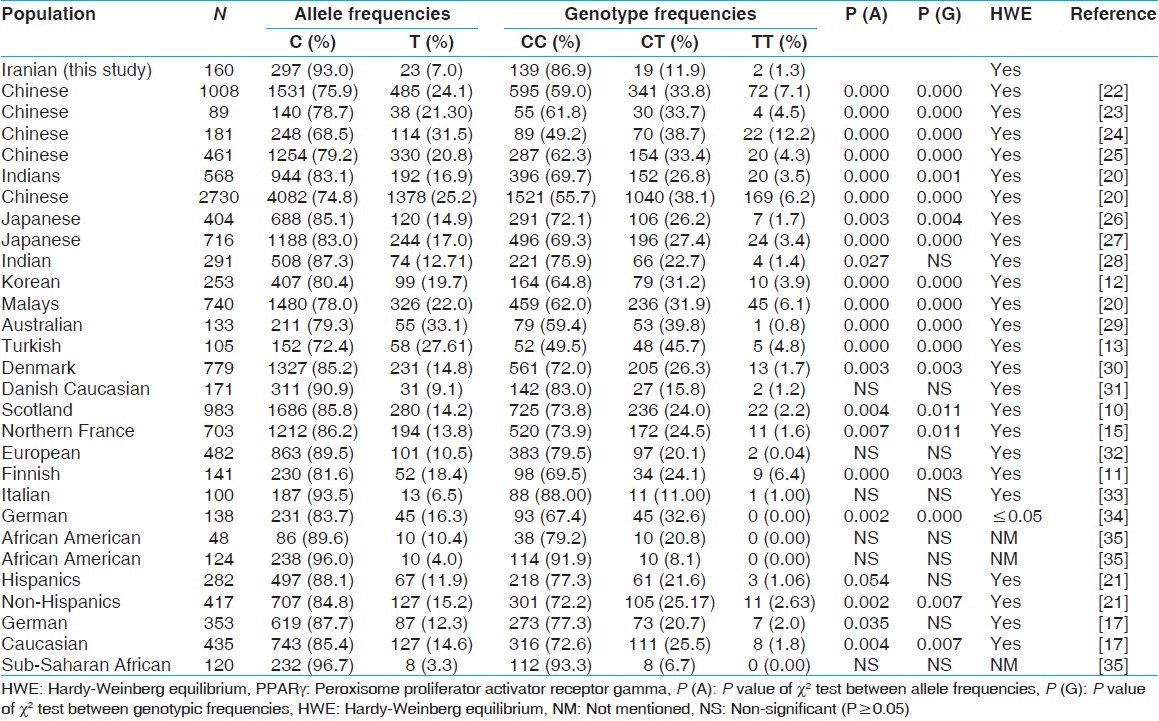
Table 3.
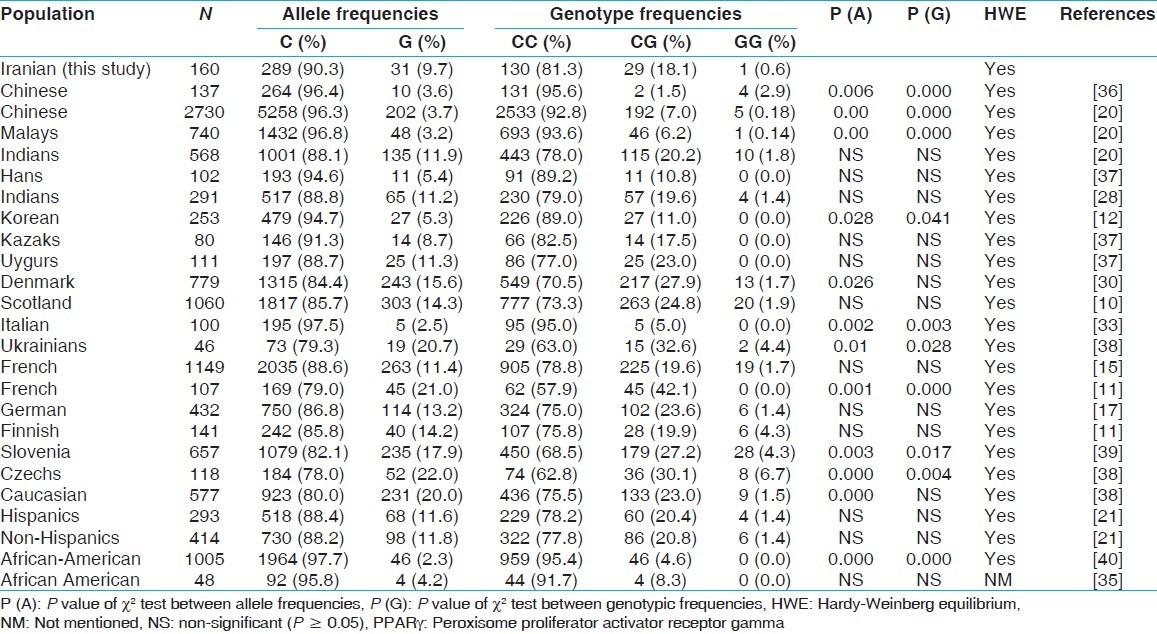
Comparison of allelic and genotypic frequencies of PPARγ Pro12Ala variant between our population and others
Discussion
The present study represents the examination of the PPARγ gene C1431T and Pro12Ala polymorphisms distribution in Iranian healthy population and comparison with other populations. Several studies have demonstrated these two common polymorphisms of PPARγ gene have a relationship with some major diseases. This current study is part of another study dealing with patients with metabolic syndrome and CADs (unpublished data); many studies have showed these two common variants of PPARγ are associated with decreased risk of CAD and MS in various populations. On the other hand, C1431T polymorphism has not been studied in our population before, so this is why we selected these two common variants to evaluate in our population.
Allelic and genotypic frequencies of PPARγ C1431T polymorphism in our population showed significant differences from other populations listed in Table 2 except for two reports from European,[31,32] one from Italy,[33] two in African-American,[35] and one from sub-Saharan African population.[35] Allelic but not genotypic frequencies in one report from European,[32] one from Caucasian,[17] one from Hispanic[21] and one from Indian[28] showed significant differences from what we found in our Iranian population. In all the populations the frequency of rare allele homozygote's (TT) was low (0-7%), but there was one exception for a Chinese population that showed more than 12% frequency that it might be due to small sample size.[24] In general, Chinese and other Asian populations showed a higher frequency than others. In this case, our data was different from those of Asian populations and was similar to those in Australian, European and Caucasian.
With respect to Pro12Ala polymorphism, comparison of allelic and genotypic frequencies between our data and other studies listed in Table 3 shows no significant differences with most of them. However there were some significant differences with two reports from China (P = 0.00),[20,36] one from Korea (P = 0.041),[12] one from Asia (P = 0.00),[20] and a few reports from Europe including, Italy (P = 0.003), Slovenia (P = 0.017),[39] The Czech Republic (P = 0.004),[38] France (P = 0.00)[11] and in an African-American population (P = 0.00)[40] as well. One report from Europe and one within a Caucasian population showed significance differences in allelic but not genotypic frequencies with our results.[38,30] As shown in Table 3, the CC ((Pro/Pro) genotype was the predominant form in all populations. Data are presented in Table 3 shows some inconsistency among different studies in the distribution of the G (12Ala) allele that might be due to the difference in racial and ethnic groups. The highest distribution of the G (12Ala) allele belongs to European and Caucasian populations and the least is relate to Chinese and African-American populations.
Most of the population in Iran is genetically close to Caucasian and their ancestors were the Aryans who had migrated from Central Asia to Iran. The main reason for the significant differences between our data and other populations results from Iran as a country with an ethnically diverse population including Pars, Turk, Kurd, Tajik, Turkmen, Baloch and special religious such as Muslims, Zoroastrians, Jews, Christians and Assyrians. However, Iran is located along the ancient Silk Road and connected Asia to Europe and during the course of history, Iranian population has encountered foreigners including Macedonians, Arabs, Turks and Mongols on various occasion. Therefore, the population living in this country might be mixed due to contacting with others and the immigration of some people from neighboring nations.[41]
Several studies have been undertaken on the association between the Pro12Ala and C1431T mutations with numerous diseases such as cardiovascular disease, cancer, diabetes, inflammation on various populations and the results showed controversy. The controversial findings related to these polymorphisms may be attributable to genetically differences in populations. Therefore, it is necessary to study in different populations to reach a general consensus in this field.
An important limitation in our study is the relatively small sample size. Further association study with a larger population base is needed to confirm our results and may be useful in understanding these SNPs roles in pathology of the main disease in the future.
Conclusion
Statistical differences in the distribution of two common polymorphisms of PPARγ gene between Iranian population and others showed the importance of studying these SNPs in relation to some major diseases. Regards to Iranian different genetic with some other populations in Asia and other continents, it seems that our study confirms this difference concerning the two common PPARγ polymorphisms.
Acknowledgments
This work was financially supported as a PhD thesis (no. 316) by Gastroenterology and Liver Disease Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Footnotes
Source of Support: This work was financially supported as a PhD thesis (no. 316) by Gastroenterology and Liver Disease Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Conflict of Interest: No.
References
- 1.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 2.Beamer BA, Negri C, Yen CJ, Gavrilova O, Rumberger JM, Durcan MJ, et al. Chromosomal localization and partial genomic structure of the human peroxisome proliferator activated receptor-gamma (hPPAR gamma) gene. Biochem Biophys Res Commun. 1997;233:756–9. doi: 10.1006/bbrc.1997.6540. [DOI] [PubMed] [Google Scholar]
- 3.Auwerx J. PPARgamma, the ultimate thrifty gene. Diabetologia. 1999;42:1033–49. doi: 10.1007/s001250051268. [DOI] [PubMed] [Google Scholar]
- 4.Fajas L, Fruchart JC, Auwerx J. PPARgamma3 mRNA: A distinct PPARgamma mRNA subtype transcribed from an independent promoter. FEBS Lett. 1998;438:55–60. doi: 10.1016/s0014-5793(98)01273-3. [DOI] [PubMed] [Google Scholar]
- 5.Al-Shali K, Cao H, Knoers N, Hermus AR, Tack CJ, Hegele RA. A single-base mutation in the peroxisome proliferator-activated receptor gamma4 promoter associated with altered in vitro expression and partial lipodystrophy. J Clin Endocrinol Metab. 2004;89:5655–60. doi: 10.1210/jc.2004-0280. [DOI] [PubMed] [Google Scholar]
- 6.González Sánchez JL, Serrano Ríos M, Fernández Perez C, Laakso M, Martínez Larrad MT. Effect of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor gamma-2 gene on adiposity, insulin sensitivity and lipid profile in the Spanish population. Eur J Endocrinol. 2002;147:495–501. doi: 10.1530/eje.0.1470495. [DOI] [PubMed] [Google Scholar]
- 7.Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–52. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- 8.Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, et al. Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem. 2003;278:34268–76. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- 9.Marx N, Sukhova G, Murphy C, Libby P, Plutzky J. Macrophages in human atheroma contain PPARgamma: Differentiation-dependent peroxisomal proliferator-activated receptor gamma (PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro. Am J Pathol. 1998;153:17–23. doi: 10.1016/s0002-9440(10)65540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doney AS, Fischer B, Cecil JE, Boylan K, McGuigan FE, Ralston SH, et al. Association of the Pro12Ala and C1431T variants of PPARG and their haplotypes with susceptibility to Type 2 diabetes. Diabetologia. 2004;47:555–8. doi: 10.1007/s00125-003-1323-1. [DOI] [PubMed] [Google Scholar]
- 11.Valve R, Sivenius K, Miettinen R, Pihlajamäki J, Rissanen A, Deeb SS, et al. Two polymorphisms in the peroxisome proliferator-activated receptor-gamma gene are associated with severe overweight among obese women. J Clin Endocrinol Metab. 1999;84:3708–12. doi: 10.1210/jcem.84.10.6061. [DOI] [PubMed] [Google Scholar]
- 12.Rhee E, Oh K, Lee W, Kim SY, Oh ES, Baek KH, et al. Effects of two common polymorphisms of peroxisome proliferator-activated receptor-γ gene on metabolic syndrome. Arch Med Res. 2006;37:86–94. doi: 10.1016/j.arcmed.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Wei Q, Jacobs DR, Jr, Schreiner PJ, Siscovick DS, Steffes MW, Fornage M. Patterns of association between PPARgamma genetic variation and indices of adiposity and insulin action in African-Americans and whites: The CARDIA Study. J Mol Med (Berl) 2006;84:955–65. doi: 10.1007/s00109-006-0088-7. [DOI] [PubMed] [Google Scholar]
- 14.Agostini M, Schoenmakers E, Mitchell C, Szatmari I, Savage D, Smith A, et al. Non-DNA binding, dominant-negative, human PPARgamma mutations cause lipodystrophic insulin resistance. Cell Metab. 2006;4:303–11. doi: 10.1016/j.cmet.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meirhaeghe A, Fajas L, Helbecque N, Cottel D, Lebel P, Dallongeville J, et al. A genetic polymorphism of the peroxisome proliferator-activated receptor gamma gene influences plasma leptin levels in obese humans. Hum Mol Genet. 1998;7:435–40. doi: 10.1093/hmg/7.3.435. [DOI] [PubMed] [Google Scholar]
- 16.Yen CJ, Beamer BA, Negri C, Silver K, Brown KA, Yarnall DP, et al. Molecular scanning of the human peroxisome proliferator activated receptor gamma (hPPAR gamma) gene in diabetic Caucasians: Identification of a Pro12Ala PPAR gamma 2 missense mutation. Biochem Biophys Res Commun. 1997;241:270–4. doi: 10.1006/bbrc.1997.7798. [DOI] [PubMed] [Google Scholar]
- 17.Mössner R, Meyer P, Jankowski F, König IR, Krüger U, Kammerer S, et al. Variations in the peroxisome proliferator-activated receptor-gamma gene and melanoma risk. Cancer Lett. 2007;246:218–23. doi: 10.1016/j.canlet.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Cole SA, Mitchell BD, Hsueh WC, Pineda P, Beamer BA, Shuldiner AR, et al. The Pro12Ala variant of peroxisome proliferator-activated receptor-gamma2 (PPAR-gamma2) is associated with measures of obesity in Mexican Americans. Int J Obes Relat Metab Disord. 2000;24:522–4. doi: 10.1038/sj.ijo.0801210. [DOI] [PubMed] [Google Scholar]
- 19.Fornage M, Jacobs DR, Steffes MW, Gross MD, Bray MS, Schreiner PJ. Inverse effects of the PPAR (gamma) 2 Pro12Ala polymorphism on measures of adiposity over 15 years in African Americans and whites. The CARDIA study. Metabolism. 2005;54:910–7. doi: 10.1016/j.metabol.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Tai ES, Corella D, Deurenberg-Yap M, Adiconis X, Chew SK, Tan CE, et al. Differential effects of the C1431T and Pro12Ala PPARgamma gene variants on plasma lipids and diabetes risk in an Asian population. J Lipid Res. 2004;45:674–85. doi: 10.1194/jlr.M300363-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Moffett SP, Feingold E, Barmada MM, Damcott CM, Marshall JA, Hamman RF, et al. The C161 →T polymorphism in peroxisome proliferator-activated receptor gamma, but not P12A, is associated with insulin resistance in Hispanic and non-Hispanic white women: Evidence for another functional variant in peroxisome proliferator-activated receptor gamma. Metabolism. 2005;54:1552–6. doi: 10.1016/j.metabol.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Chen J, Xu W. Association between C1431T polymorphism in peroxisome proliferator-activated receptor-γ gene and coronary artery disease in Chinese Han population. Mol Biol Rep. 2012;39:1863–8. doi: 10.1007/s11033-011-0931-y. [DOI] [PubMed] [Google Scholar]
- 23.Wan J, Xiong S, Chao S, Xiao J, Ma Y, Wang J, et al. PPARgamma gene C161T substitution alters lipid profile in Chinese patients with coronary artery disease and type 2 diabetes mellitus. Cardiovasc Diabetol. 2010;9:13. doi: 10.1186/1475-2840-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang LL, Hua Q, Liu RK, Yang Z. Association between two common polymorphisms of PPARgamma gene and metabolic syndrome families in a Chinese population. Arch Med Res. 2009;40:89–96. doi: 10.1016/j.arcmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Dongxia L, Qi H, Lisong L, Jincheng G. Association of peroxisome proliferator-activated receptorgamma gene Pro12Ala and C161T polymorphisms with metabolic syndrome. Circ J. 2008;72:551–7. doi: 10.1253/circj.72.551. [DOI] [PubMed] [Google Scholar]
- 26.Ogawa S, Urano T, Hosoi T, Miyao M, Hoshino S, Fujita M, et al. Association of bone mineral density with a polymorphism of the peroxisome proliferator-activated receptor gamma gene: PPARgamma expression in osteoblasts. Biochem Biophys Res Commun. 1999;260:122–6. doi: 10.1006/bbrc.1999.0896. [DOI] [PubMed] [Google Scholar]
- 27.Sanada K, Iemitsu M, Murakami H, Tabata I, Yamamoto K, Gando Y, et al. PPARγ2 C1431T genotype increases metabolic syndrome risk in young men with low cardiorespiratory fitness. Physiol Genomics. 2011;43:103–9. doi: 10.1152/physiolgenomics.00129.2010. [DOI] [PubMed] [Google Scholar]
- 28.Jiang J, Gajalakshmi V, Wang J, Kuriki K, Suzuki S, Nakamura S, et al. Influence of the C161T but not Pro12Ala polymorphism in the peroxisome proliferator-activated receptor-gamma on colorectal cancer in an Indian population. Cancer Sci. 2005;96:507–12. doi: 10.1111/j.1349-7006.2005.00072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang XL, Oosterhof J, Duarte N. Peroxisome proliferator-activated receptor gamma C161 →T polymorphism and coronary artery disease. Cardiovasc Res. 1999;44:588–94. doi: 10.1016/s0008-6363(99)00256-4. [DOI] [PubMed] [Google Scholar]
- 30.Andersen V, Christensen J, Ernst A, Jacobsen BA, Tjønneland A, Krarup HB, et al. Polymorphisms in NF-κB, PXR, LXR, PPARγ and risk of inflammatory bowel disease. World J Gastroenterol. 2011;17:197–206. doi: 10.3748/wjg.v17.i2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poulsen P, Andersen G, Fenger M, Hansen T, Echwald SM, Vølund A, et al. Impact of two common polymorphisms in the PPARgamma gene on glucose tolerance and plasma insulin profiles in monozygotic and dizygotic twins: Thrifty genotype, thrifty phenotype, or both? Diabetes. 2003;52:194–8. doi: 10.2337/diabetes.52.1.194. [DOI] [PubMed] [Google Scholar]
- 32.Dallongeville J, Iribarren C, Ferrières J, Lyon L, Evans A, Go AS, et al. Peroxisome proliferator-activated receptor gamma polymorphisms and coronary heart disease. PPAR Res. 2009;2009:543746. doi: 10.1155/2009/543746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orio F, Jr, Matarese G, Di Biase S, Palomba S, Labella D, Sanna V, et al. Exon 6 and 2 peroxisome proliferator-activated receptor-gamma polymorphisms in polycystic ovary syndrome. J Clin Endocrinol Metab. 2003;88:5887–92. doi: 10.1210/jc.2002-021816. [DOI] [PubMed] [Google Scholar]
- 34.Koch M, Rett K, Maerker E, Volk A, Haist K, Deninger M, et al. The silent PPARgamma exon 6 CAC (His)→CAT (His) polymorphism does not affect the plasma leptin levels in a collective of first degree relatives of type 2 diabetes patients from South West Germany. Exp Clin Endocrinol Diabetes. 2000;108:341–6. doi: 10.1055/s-2000-8126. [DOI] [PubMed] [Google Scholar]
- 35.dbSNP. (Database of Single Nucleotide Polymorphisms), National Center for Biotechnology Information, National Library of Medicine. (dbSNP) 2012. Available from: http://www.ncbi.nlm.nih.gov/SNP/2012 .
- 36.Gao L, Wang L, Yun H, Su L, Su X. Association of the PPARgamma2 gene Pro12Ala variant with primary hypertension and metabolic lipid disorders in Han Chinese of Inner Mongolia. Genet Mol Res. 2010;9:1312–20. doi: 10.4238/vol9-3gmr833. [DOI] [PubMed] [Google Scholar]
- 37.Li LL, Ma XL, Ran JX, Sun XF, Xu LM, Ren J, et al. Genetic polymorphism of peroxisome proliferator-activated receptor-gamma 2 Pro12Ala on ethnic susceptibility to diabetes in Uygur, Kazak and Han subjects. Clin Exp Pharmacol Physiol. 2008;35:187–91. doi: 10.1111/j.1440-1681.2007.04796.x. [DOI] [PubMed] [Google Scholar]
- 38.Kaydashev I, Rasin A, Shlykova O, Gorbas I, Smirnova I, Petrushov A, et al. Frequency of Pro12Ala-polymorphism of the Gene PPARγ2 in the Ukrainian population and its possible relation to the development of the metabolic syndrome. Cytol Genet. 2007;41:297–302. [Google Scholar]
- 39.Dragojevič J, Ostanek B, Mencej-Bedrač S, Komadina R, Preželj J, Marc J. PPARG gene promoter polymorphism is associated with non-traumatic hip fracture risk in the elderly Slovenian population: A pilot study. Clin Biochem. 2011;44:1085–9. doi: 10.1016/j.clinbiochem.2011.06.981. [DOI] [PubMed] [Google Scholar]
- 40.Kao WH, Coresh J, Shuldiner AR, Boerwinkle E, Bray MS, Brancati FL, et al. Pro12Ala of the peroxisome proliferator-activated receptor-gamma2 gene is associated with lower serum insulin levels in nonobese African Americans: The Atherosclerosis Risk in Communities Study. Diabetes. 2003;52:1568–72. doi: 10.2337/diabetes.52.6.1568. [DOI] [PubMed] [Google Scholar]
- 41.Farjadian S, Moqadam FA, Ghaderi A. HLA class II gene polymorphism in Parsees and Zoroastrians of Iran. Int J Immunogenet. 2006;33:185–91. doi: 10.1111/j.1744-313X.2006.00594.x. [DOI] [PubMed] [Google Scholar]


