Abstract
AIM:
The aim of this study is to analyze the association of TaqI vitamin D receptor (VDR) gene polymorphism with the chronic periodontitis (CP) in Dravidian ethnicity.
MATERIALS AND METHODS:
A total of 120 subjects were recruited for this study, which included 60 CP and 60 healthy controls. TaqI VDR gene polymorphism was analyzed using specific primers and amplified by polymerase chain reaction (PCR) and visualized under 2% agarose gel.
RESULTS:
Our study results showed that Tt and tt genotype had a higher frequency of occurrence in CP compared with controls. Similarly, t allele was found to be associated with CP.
CONCLUSION:
Our study concludes that TaqI VDR gene polymorphism is associated with CP in Dravidian ethnicity.
Keywords: Alleles, chronic periodontitis, polymorphism, TaqI, vitamin D receptor
Introduction
Periodontitis is a chronic inflammatory disease of multifactorial etiology. Although the presence of Gram-negative anaerobic species is essential in the initiation of periodontal destruction, many environmental and genetic factors could perpetuate the progression of the disease. The genetic influence plays a key role in determining the host susceptibility to periodontal destruction.[1]
Vitamin D has an important role in bone formation and preservation.[2] Several epidemiological studies have reported positive associations between osteoporosis or low bone density and alveolar bone and tooth loss, which suggests that poor bone quality is a risk factor for developing periodontal disease.[3,4,5] Recent findings suggest that the pathway involving bone mineral density (BMD) mediated effects is important for the development of periodontitis.[6] The vitamin D receptor (VDR) is involved in a variety of biological processes, such as bone metabolism and modulation of the immune response, in which it functions as a vitamin D3-dependent transcription factor.[7] Vitamin D influences the development of periodontal disease through both immunomodulatory effects and an effect on BMD.[8,9]
It is clear that mutations in functionally critical areas of the VDR gene can have profound effects on mineral metabolism and BMD.[10,11]
Many association studies have shown conflicting results of the relationship between VDR polymorphisms and susceptibility to aggressive and chronic periodontal disease.[12] The Dravidian ethnicity comprises of the people (Tamil, Malayalam, Telugu and Kannada) in the southern part of India. The Dravidian race is a genetically distinct race among the populations of Indian subcontinent.[13,14] To the best of our knowledge, there are no published data comparing the association of VDR gene polymorphisms with periodontitis in Dravidian ethnicity. Thus, the aim of the present study was to compare the association of VDR gene polymorphism with chronic periodontitis (CP) in Dravidian ethnicity.
Materials and Methods
This study employed a cross-sectional design involving individuals from the southern part of India belonging to the Dravidian ethnicity. A total of 120 individuals who reported to the Department of Periodontics, Saveetha Dental College, Chennai (Tamil Nadu) were included in this study. The subjects were stratified into a CP group (n = 60) and a control group (n = 60) based on the clinical examination of probing pocket depth, clinical attachment loss, bleeding on probing and radiographic analysis. The CP group contained 60 patients (male-29; female-31) with the mean age of 38.39 ± 6.47. The CP patients were recruited based on the criteria of American Association of Periodontology (AAP)-1999.[15] The control group contained 60 periodontally healthy subjects (male-33; female-27) with the mean age of 37.04 ± 5.16.
A detailed history of dental treatments, family history of periodontal diseases, smoking habits as well as general health concerns were obtained from the subjects. Except for the presence of periodontitis, the patients included in this study were systemically healthy. Smokers, pregnant or lactating mothers, immunocompromised individuals, subjects who underwent any periodontal therapy within the past 6 months were excluded from this study. Both patients and the control subjects belong to Dravidian ethnicity; others were excluded from this study. The study got approval from institutional ethical committee and Informed consent was obtained from all subjects.
Sample collection and deoxyribonucleic acid extraction
A volume of 5 ml of venous blood was collected by vein puncture and dispersed into a sterile tube containing a pinch of ethylene diamine tetra acetic acid (EDTA). It was mixed thoroughly to avoid clot formation. DNA isolation was performed according to the modified Miller et al. 1998 protocol.[16]
Polymerase chain reaction and restriction endonuclease digestion
VDR gene (TaqI) polymorphisms were assessed by PCR amplification and digestion. The sequences of PCR primers were
Forward primer: 5’-CAGAGCATGGACAGGGAGC
AAG-3’
Reverse primer: 5’-CACACTGCAGACGTACATCC-3’.
PCR was carried out in a total volume of 20 μl containing 2 μl of template DNA, a premixed buffer (×10 assay buffer - 2 μl, 50 mM MgCl2-2 μl, dNTPs mix - 1.5 μl, Taq polymerase - 0.2 μl) and forward and reverse primers each of 0.3 μl. The remaining 12.9 μl were autoclaved double distilled water. The amplification conditions consisted of 94°C for 3 min, followed by 35 cycles of 94°C for 30 s, 55°C for 35 s and 72°C for 30 s. The run was terminated by final elongation at 72°C for 5 min. Amplification was performed in an Eppendorf thermocycler. The products were digested with 5 U of TaqI at 65°C for 4 h and obtained 294 + 46 bp. The visualization was performed in a 3% agarose gel electrophoresis. The change is T-C at codon 352 in exon 9 of VDR gene.
Statistical analysis
All statistical analyses were performed using the Statistical Package for the Social Sciences Version 11 for Windows (SPSS Inc., Chicago, IL). The distributions of genotypes and allele frequencies in the disease and control groups were compared using the Chi-squared test. The risk associated with individual alleles or genotypes was calculated as the odds ratio (OR) with 95% confidence intervals (95% CI). Statistical significance in all tests was determined at P < 0.05.
Results
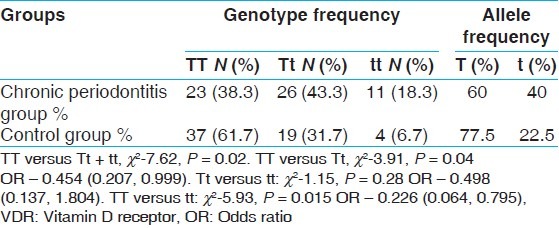
The clinical characteristics of the subjects in CP and control groups are shown in Table 1. The genotype and allele frequencies of the groups are shown in Table 2. The genotype frequency of VDR TaqI polymorphisms was consistent with Hardy-Weinberg equilibrium. Our study results showed that the prevalence of homozygous and heterozygous mutant genotypes (tt and Tt) were high in CP group compared with healthy control group. The association of VDR gene polymorphism with CP was highly significant (TT vs. Tt + tt) with P value 0.02. The detected frequency of Tt (43.3 vs. 31.7%) and tt (18.3 vs. 6.7%) genotypes were significantly higher in the CP group than in the healthy control subjects. The frequency of t allele was also significantly higher in the CP group (40.0 vs. 22.5%, P < 0.05). Individuals in the Dravidian population with the t allele seemed to be more likely to develop CP (TT vs. Tt + tt, χ2-7.62, P = 0.02: TT vs. Tt; χ2-3.91, P = 0.04, OR -0.454, CI 0.207, 0.999; TT vs. tt: χ2-5.93, P = 0.015 OR -0.226, CI 0.064,0.795).
Table 1.
Demographic data of chronic periodontitis and control groups
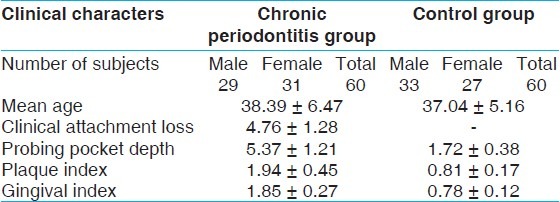
Table 2.
Genotype distributions and allele frequencies of TaqI VDR gene polymorphism of chronic periodontitis and control groups
Discussion
Genetic polymorphisms, like SNPs, may influence disease in a complex way, acting with other genetic variants and environmental factors to influence disease susceptibility and progression. Many studies have revealed that certain SNPs may be associated with susceptibility to periodontitis.[1]
The human VDR is a ligand-regulated transcription factor that mediates the actions of the 1,25-dihydroxyvitamin D3 hormone to effect bone mineral homeostasis. The VDR mediates the hormonal function of vitamin D and regulates a variety of downstream functions. VDR TaqI polymorphism is located at extron 9.
In our study smokers were excluded, because smoking is a major risk factor for periodontitis. Our study results showed that the Tt and tt genotype were more prevalent in CP patients compared with the control group and whereas TT genotype had a higher frequency in the healthy control group. There was a significant association of Tt and tt genotype with CP with P value equal to 0.04 and 0.015 respectively. There was also significant association of TT versus Tt + tt genotype and CP with P value equal to 0.02.
The frequency of the TaqI allele was significantly different in the CP patients and controls in our study. de Brito Júnior et al. reported that patients with t allele were 2.4 times more susceptible to periodontal disease than patients who lacked this allele. Our study results were in accordance with that of de Brito Júnior et al.[17] Another study performed with a Japanese population showed a significant correlation between VDR TaqI genotypes and CP.[18] However, Sun et al. determined genotypes of the TaqI VDR gene in 24 cases of CP, 37 cases of early-onset periodontitis and 39 healthy controls and found no difference in the distribution of VDR TaqI genotypes between CP patients and controls.[19] Our results were different from that of Sun et al. The t allele of the TaqI polymorphism had a higher frequency of occurrence in Caucasians and Asians (43 and 8%, respectively).[12] In our study, t allele had a higher frequency of occurrence in chronic periodontitis group compared with the healthy group. This is a distinct finding since t allele is more common in CP in Dravidian ethnicity compared with Asian population.
Although numerous association studies relating polymorphisms in this gene to periodontitis have been published, results are conflicting, possibly because of variations in study design, small sample sizes and heterogeneous populations, among other issues.
Accumulating evidence from basic and clinical research makes the association between the VDR gene polymorphism and periodontal disease biologically plausible. VDR gene polymorphisms have been strongly associated with BMD in many studies. Some investigators presumed that the VDR polymorphisms influence bone resorption and immune function.[7,10,11]
Thus, our study concludes that the VDR gene TaqI polymorphism is associated with CP in Dravidian ethnicity. Further studies are required to explore the interaction of TaqI VDR gene polymorphism with microbial and environmental factors in the Etiopathogenesis of periodontitis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Hassell TM, Harris EL. Genetic influences in caries and periodontal diseases. Crit Rev Oral Biol Med. 1995;6:319–42. doi: 10.1177/10454411950060040401. [DOI] [PubMed] [Google Scholar]
- 2.Specker BL, Ho ML, Oestreich A, Yin TA, Shui QM, Chen XC, et al. Prospective study of vitamin D supplementation and rickets in China. J Pediatr. 1992;120:733–9. doi: 10.1016/s0022-3476(05)80236-7. [DOI] [PubMed] [Google Scholar]
- 3.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 4.Tezal M, Wactawski-Wende J, Grossi SG, Ho AW, Dunford R, Genco RJ. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–8. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 5.Dervis E. Oral implications of osteoporosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:349–56. doi: 10.1016/j.tripleo.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich T, Joshipura KJ, Dawson-Hughes B, Bischoff-Ferrari HA. Association between serum concentrations of 25-hydroxyvitamin D3 and periodontal disease in the US population. Am J Clin Nutr. 2004;80:108–13. doi: 10.1093/ajcn/80.1.108. [DOI] [PubMed] [Google Scholar]
- 7.Uitterlinden AG, Fang Y, Van Meurs JB, Pols HA, Van Leeuwen JP. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338:143–56. doi: 10.1016/j.gene.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 8.D’Ambrosio D, Cippitelli M, Cocciolo MG, Mazzeo D, Di Lucia P, Lang R, et al. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J Clin Invest. 1998;101:252–62. doi: 10.1172/JCI1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krall EA, Wehler C, Garcia RI, Harris SS, Dawson-Hughes B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am J Med. 2001;111:452–6. doi: 10.1016/s0002-9343(01)00899-3. [DOI] [PubMed] [Google Scholar]
- 10.Lin NU, Malloy PJ, Sakati N, al-Ashwal A, Feldman D. A novel mutation in the deoxyribonucleic acid-binding domain of the vitamin D receptor causes hereditary 1,25-dihydroxyvitamin D-resistant rickets. J Clin Endocrinol Metab. 1996;81:2564–9. doi: 10.1210/jcem.81.7.8675579. [DOI] [PubMed] [Google Scholar]
- 11.Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest. 1997;99:297–304. doi: 10.1172/JCI119158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng H, Liu F, Pan Y, Jin X, Wang H, Cao J. BsmI, TaqI, ApaI, and FokI polymorphisms in the vitamin D receptor gene and periodontitis: A meta-analysis of 15 studies including 1338 cases and 1302 controls. J Clin Periodontol. 2011;38:199–207. doi: 10.1111/j.1600-051X.2010.01685.x. [DOI] [PubMed] [Google Scholar]
- 13.Garn SM, Coon . On the number of races of mankind. In: Garn S, editor. Readings on Race. Springfield: CC Thomas; 1982. [Google Scholar]
- 14.Jorde LB, Wooding SP. Genetic variation, classification and ‘race’. Nat Genet. 2004;36(Suppl 11):S28–33. doi: 10.1038/ng1435. [DOI] [PubMed] [Google Scholar]
- 15.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Brito Júnior RB, Scarel-Caminaga RM, Trevilatto PC, de Souza AP, Barros SP. Polymorphisms in the vitamin D receptor gene are associated with periodontal disease. J Periodontol. 2004;75:1090–5. doi: 10.1902/jop.2004.75.8.1090. [DOI] [PubMed] [Google Scholar]
- 18.Tachi Y, Shimpuku H, Nosaka Y, Kawamura T, Shinohara M, Ueda M, et al. Vitamin D receptor gene polymorphism is associated with chronic periodontitis. Life Sci. 2003;73:3313–21. doi: 10.1016/j.lfs.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 19.Sun JL, Meng HX, Cao CF, Tachi Y, Shinohara M, Ueda M, et al. Relationship between vitamin D receptor gene polymorphism and periodontitis. J Periodontal Res. 2002;37:263–7. doi: 10.1034/j.1600-0765.2002.01605.x. [DOI] [PubMed] [Google Scholar]


