Abstract
Purpose
To illustrate the ethical challenges that arose from investigating a novel treatment procedure, transcranial direct current stimulation (tDCS), in a research participant with aphasia.
Method
First, we reviewed the current evidence supporting the use of tDCS in aphasia research, highlighting methodological gaps in our knowledge of tDCS. Second, we examined the case of Mr. C, a person with chronic aphasia who participated in a research protocol investigating the impact of tDCS on aphasia treatment.
Results
We describe the procedures that he underwent and the resulting behavioral and neurophysiological outcomes bed. Finally, we share the steps that were taken to balance beneficence and nonmaleficence, and to ensure Mr. C’s autonomy. Conclusion: Researchers must consider not only the scientific integrity of their studies, but also potential ethical issues and consequences to the research participants.
Keywords: aphasia, brain stimulation, ethics, functional MRI, rehabilitation
Aphasia is an acquired disorder of language that impairs the understanding and expression of oral language, reading, and writing. It results from a wide range of neurologic disorders, but most commonly occurs after stroke. Studies have estimated that 20% to 38% of acute stroke patients experience aphasia and require speech and language therapy to reduce the symptoms.1,2 Although clinical outcomes from behavioral and environmental interventions are generally positive,3–5 patients continue to be left with residual deficits that affect their daily communication as well as quality of life.6
An area of research that shows promise for enhancing language recovery in individuals with stroke-induced aphasia is the direct application of stimulation to the cerebral cortex to facilitate brain plasticity. Methods of delivering cortical brain stimulation to modulate cortical excitability include direct epidural cortical stimulation, repetitive transcranial magnetic stimulation (rTMS), and transcranial direct current stimulation (tDCS).7 These methods have been studied in animal models and applied to the rehabilitation of motor deficits after stroke with promising results.8–10 Studies investigating the use of cortical stimulation in the rehabilitation of language problems after stroke are preliminary, but results suggest a potential role for cortical stimulation as an adjuvant strategy in aphasia rehabilitation.11,12
Of all the methods of cortical brain stimulation, tDCS has the most potential for clinical use in view of its noninvasive application, portability, ease of administration, and relative low cost.9,13 With tDCS, constant weak electrical currents are delivered to the cortex via 2 electrodes placed on the scalp: an active electrode on the site overlying the cortical target and a reference electrode usually placed over the contralateral supraorbital area. Studies involving the motor cortex have shown that the nature of the effect depends on the polarity of the current. In general, anodal tDCS has an excitatory effect likely from partial depolarization of superficial cortical axons; cathodal tDCS has the opposite effect, inducing inhibition likely via hyperpolarization.14–16 Thus, tDCS differs from other types of stimulation because it does not stimulate the axon and cause it to discharge action potentials. Instead, it biases the resting potential of cortical neurons; depolarization causes them to discharge more readily to other synaptic inputs (up-regulation), whereas hyperpolarization has the opposite effect (down-regulation).
Although tDCS has been used in psychiatric and motor system research for many years,17 research on tDCS and aphasia rehabilitation is only in its infancy. The first published study specifically investigating tDCS and aphasia appeared in 2008.18 Since then, there have been only 8 additional prospective studies (see Table 1). Results have been promising, and the use of tDCS in aphasia warrants further investigation. A fundamental ethical challenge for any clinical researcher is to promote high-quality scientific research in the interest of future patients, while at the same time safeguarding the rights and interests of the current research participants. Balancing risks and benefits becomes more difficult when the approach under investigation is novel, there are no standard procedures, safety guidelines are limited, and there are relatively few studies regarding risks, side effects, and efficacy in the specific population being studied. Furthermore, when the intervention targets the brain, a complex system of networks that scientists are far from understanding fully, potential serious consequences may result. Other ethical issues occur in aphasia research, including maintaining respect for the participants’ autonomy, because the informed consent process may be complicated by their language difficulty.19
Table 1.
Clinical Studies of Transcranial Direct Current Stimulation and Aphasia
| Study authors and design | Subjects | tDCS polarity | Targeted hemisphere and electrode location |
tDCS procedures per condition or per arm |
Concurrent Behavioral treatment |
Results |
|---|---|---|---|---|---|---|
| Monti et al, 200818 Crossover study; 4 subjects received anodal/sham conditions and 4 subjects received cathodal/sham conditions |
N=8 Chronic Nonfluent aphasia (4 global, 4 Broca’s). |
Anodal/sham Cathodal/sham |
Left Fronto-temporal area |
Single session 10 minutes at 2 mA. |
None | Significantly improved naming accuracy with cathodal stimulation |
| Fiori et al, 201026 Crossover study with 2 conditions |
N=3 Chronic Nonfluent aphasia classified as mild, moderate and severe. |
Anodal/sham | Left Wernicke’s area. |
5 Consecutive Daily sessions 20 minutes at 1mA |
Associative picture naming training of 2 different word lists | Improved naming accuracy in both conditions, but greater improvement in the anodic versus sham condition. |
| Baker et al, 201024 Crossover study with 2 conditions |
N=10 Chronic 6 fluent and 4 nonfluent aphasia |
Anodal/sham | Left Frontal cortex |
5 Consecutive Daily sessions 20 minutes at 1mA. |
Self-administered Computerized Anomia Treatment consisting of spoken word picture matching | Significantly improved naming of treated items after anodal tDCS vs sham tDCS. |
| Fridiriksson et al, 201127 Crossover study with 2 conditions |
N=8 Chronic Fluent aphasia with posterior cortical or subcortical lesions. |
Anodal/sham | Left Posterior cortex |
5 Consecutive Daily sessions 20 minutes at 1 mA. |
Self-administered Computerized Anomia Treatment consisting of spoken word-picture matching | Greater reduction of RT for naming trained nouns after anodal tDCS vs sham tDCS on immediate post-testing and at 3 weeks follow-up. |
| Kang et al, 201121 Crossover study with 2 conditions |
N=10 Chronic 3 global, 4 Broca’s, 1 transcortical motor, 2 anomic. |
Cathodal/sham | Right Broca’s homologue area. |
5 Consecutive Daily sessions 20 minutes at 2 mA. |
Word-retrieval Training provided by SLP | Improved response accuracy of picture naming relative to baseline with cathodal tDCS; sham stimulation had little effect. |
| You et al, 201120 RCT with 3 arms |
N= 21 Subacute (TPO: 16 – 38 days) Global aphasia. |
Anodal Cathodal Sham |
Both Left and Right (this RCT had three different study arms) Anodal tDCS to L superior temporal gyrus Cathodal tDCS to R superior temporal gyrus Sham tDCS to L superior temporal gyrus, |
10 Consecutive sessions, 5 times a week for 2 weeks 30 minutes at 2 mA |
“Conventional speech-language therapy” | Cathodal tDCS to right superior temporal area showed significantly greater improvements in auditory verbal comprehension than other arms. |
| Marangola et al, 201125 Crossover study with 2 conditions |
N=3 Chronic Nonfluent aphasia with severe apraxia of speech. |
Anodal/sham | Left Inferior frontal gyrus |
5 Consecutive Daily sessions 20 minutes at 1 mA |
Repetition of syllables and words. | Significant effect of training i.e. improvement in both anodal and sham conditions. Retention of achieved improvement only for the anodal condition. |
| Vines et al, 201123 Crossover study with 2 conditions |
N=6 Chronic Moderate - severe nonfluent Broca’s aphasia |
Anodal/sham | Right Posterior inferior frontal gyrus |
3 Consecutive Daily sessions 20 minutes at 1.2mA |
Melodic Intonation Therapy | Significantly greater improvement in verbal fluency with anodal tDCS than sham tDCS. condition. |
| Floel et al, 201122 Crossover study with 3 conditions |
N=12 Chronic 7 Brocas, 1 Wernickes, 2 amnestic, 1 global and 1 not classified |
Anodal, cathodal and sham | Right Temporoparietal cortex |
Two times each day for 3 days 20 minutes at 1 mA | Computer assisted naming therapy - 1 set of 15 objects trained in each condition 2 hours per day; the first 20 minutes of each hour was concurrent with tDCS | Main effect of stimulation with greatest improvement on anodal tDCS. Cathodal tDCS showed greater gains compared to sham immediately after treatment but effect not maintainedat follow-up. |
L = left; RT = response time; R = right; RCT = randomized controlled trial; SLP = speech-language pathologist; TPO = time post onset.
In this article, we illustrate some of the ethical challenges that arose when the use of tDCS in a research participant with aphasia was investigated. We begin by summarizing the current evidence supporting the use of tDCS in aphasia research, highlighting methodological gaps in our knowledge of the procedure. We present the case of Mr. C, a person with chronic aphasia who participated in a research protocol investigating the impact of tDCS on aphasia treatment, and describe the procedures that he underwent and the resulting behavioral and neurophysiological outcomes. We share the steps that we took to balance beneficence and nonmaleficence and to ensure Mr. C’s autonomy.
tDCS and Aphasia
Table 1 summarizes the 9 prospective studies in which tDCS was used to target language deficits across a total of 81 participants with aphasia. Outcomes are promising with few adverse events, but the heterogeneity of the research participants and the variability in the procedures are illustrated.
The majority of participants presented with chronic aphasia (more than 6 months post stroke); only 1 study recruited subjects with subacute aphasia (2–6 weeks post stroke).20 Nineteen study participants were described as fluent, while 61 participants were nonfluent (1 subject was not categorized). A large number of the nonfluent aphasia subjects were severely impaired, with 29 of them diagnosed as having global aphasia.
Perhaps the most important unanswered questions are how and where to stimulate (ie, whether to up-regulate or down-regulate the right or the left hemispheres) and where the stimulation should specifically occur within the identified hemisphere. Across different studies, both anodal and cathodal stimulation have been applied to both the right and left hemispheres. For example, 2 studies applied cathodal stimulation to the right hemisphere, with 1 study down-regulating right Broca’s homologue21 and the other study down-regulating the right superior temporal area.20 Both studies reported improvements in naming accuracy and auditory verbal comprehension, respectively. In contrast, anodal stimulation to the right hemisphere (ie, up-regulation) has also resulted in positive outcomes in naming22 and fluency.23 With regard to targeting the left hemisphere, 4 studies reported improvements with anodal stimulation; 2 studies stimulated the left frontal cortex,24,25 whereas 2 studies stimulated more posteriorly including Wernicke’s area.26,27 Only 1 study has reported greatest gains with cathodal stimulation to the left frontotemporal area.18
Although the mechanism of action of tDCS remains to be completely elucidated, computational models of brain current flow during tDCS derived from healthy individuals have shown that by varying tDCS parameters, such as electrode size and positioning, intensity and duration of stimulation, number of sessions per day, and intervals between sessions, different amounts of electric currents can be delivered with potentially diverse physiologic effects.28 In addition, anatomical differences, including the presence of lesions, may affect current flow through the cortex.29,30 In the aphasia studies, the greatest amount of tDCS was delivered in a randomized controlled trial in which participants received 30 minutes of tDCS at 2 mA for 10 daily sessions over a 2-week period.20 Five other studies were crossover studies in which participants received 20 minutes of tDCS for 5 consecutive daily sessions in each of 2 separate weeks, for a total of 10 sessions. Four of these studies delivered tDCS at 1 mA, whereas 1 study delivered it at 2 mA. No aphasia studies delivered tDCS for longer than 2 weeks.
All of the studies except 1 evaluated tDCS that was administered concurrent with speech and language therapy. Although there was no consistency regarding the optimal type and intensity of the language treatment, 5 studies focused on word-retrieval training, with subsequent outcome measures related to the rate and accuracy of naming. One study provided Melodic Intonation Therapy23 and another study25 provided an apraxia treatment comprising repetition of words and syllables. In contrast to administering a single treatment procedure, 1 study described the training as “conventional speech and language treatment” that included word retrieval training as well as auditory comprehension training and conversational skill training.20
Examining Ethical Perspectives in tDCS Clinical Research
As we investigate the application of tDCS in persons with aphasia, it is important to be cognizant of previous findings and to use that knowledge to inform our decisions. However, ethical analysis and decision making in conducting clinical research need to be made with consideration not only of the scientific data, but also of the individual and his or her personal situation. We present the case of Mr. C to highlight the practical and ethical considerations that were addressed during his participation in our research protocol with tDCS. Some decisions were made when developing the experimental protocol, whereas others were made at the point of service during the research study. In all cases, the course of action was selected by the entire research team following extensive dialogue. Having the knowledge and experience of persons representing a variety of disciplines and viewpoints allowed a more comprehensive discussion of the issues, potential solutions, and consequences to the individual participant, future participants, and the outcomes of the research study.
Methods
Participant
Mr. C was a 63-year-old right-handed man who had suffered an embolic stroke 17 years prior to beginning our study. Although he was born in China and was a native Cantonese speaker, he reportedly was fluent in English premorbidly, having studied it for 3 years in high school prior to moving to the United States in his late 20s. He had worked as a chef and a jewelry designer. He was a talented sketch artist, and had learned to draw well with his left hand since his stroke.
Following a comprehensive consenting process (which will be described later), a baseline assessment of language and communication skills was conducted. Mr. C presented with a severe mixed non-fluent aphasia.31 The Aphasia Quotient on the Western Aphasia Battery-Revised (WAB-R)32 was 27.1/100, and language output was limited to a few single words interspersed with non-words. Performance was good on nonverbal measures including the Construction subtest of the WAB (89/100) and the Spatial Span subtest of the Wechsler Memory Scales, third edition (WMS-III)33 (standard scores were 13 for Forward and 15 for Backward; 10 is average). Mr. C also performed in the above average range on measures of attention (omission and commission) on the Connors Continuous Performance Test II.34 When asked to rate his communication skills and confidence on 2 questionnaires, Mr. C gave himself a score of 33.75/100 on the Communicative Effectiveness Index (CETI)35 and 25/40 on the Rehabilitation Institute of Chicago’s Communication Confidence Rating Scale for Aphasia (CCRSA).36,37 Mr. C’s wife also completed the CETI, rating Mr. C ‘s communication skills as more impaired at 18.5/100. Table 2 shows the pretreatment test results. All language and communication assessments were conducted by a clinician who remained blind throughout the study to the location and type of modulation given to Mr. C.
Table 2.
Baseline, posttreatment, and follow-up test scores
| Baseline assessment (T1) |
Post treatment (T2) |
6-week follow-up (T3) |
|
|---|---|---|---|
| Western Aphasia Battery | |||
| Spontaneous Speech | 5/10 | 7/10 | 4/10 |
| Auditory Comprehension | 3.95/10 | 4/10 | 4.8/10 |
| Repetition | 2.1/10 | 2.9/10 | 2.5/10 |
| Naming | 2.5/10 | 2/10 | 1.7/10 |
| Aphasia Quotient | 27.1/100 | 31.8/100 | 26/100 |
| Reading | 37/100 | 39/100 | 32/100 |
| Writing | 48/100 | 49/100 | 48.5/100 |
| Language Quotient | 26/100 | 28.7/100 | 25.5/100 |
| Praxis | 44.00 | 43.00 | 40.00 |
| Construction Total | 89/100 | 95/100 | 91.5/100 |
| Ravens Progressive Matrices | 30/37 | 35/37 | 36/37 |
| Cortical Quotient | 42.23/100 | 45.37/100 | 41.67/100 |
| Boston Naming Test | 1/60 | 3/60 | 2/60 |
| Wechsler Memory Scale III | |||
| Spatial Span Forwarda | 13 | 12 | 12 |
| Spatial Span Backwarda | 15 | 10 | 10 |
| CETI (self-report) | 33.75/100 | 37.44/100 | 46.5/100 |
| CETI (caregiver-report) | 18.5/100 | 21.5/100 | 21.81.100 |
| CCRSA | 25/40 | 23/40 | 29/40 |
Note: CCRSA = Rehabilitation Institute of Chicago’s Communication Confidence Rating Scale for Aphasia; CETI = Communicative Effectiveness Index.
Standard scores.
Functional MRI
A functional MRI (fMRI) was conducted on a Siemens 3.0 Tesla Tim Trio (Malvern, Pennsylvania) whole-body system with a 32-channel head coil, before and after the 6 weeks of therapy. The pretreatment scan was used to determine eligibility, establish an imaging baseline, and identify the site of stimulation for the tDCS. The posttherapy scan was used to assess functional changes associated with therapy.
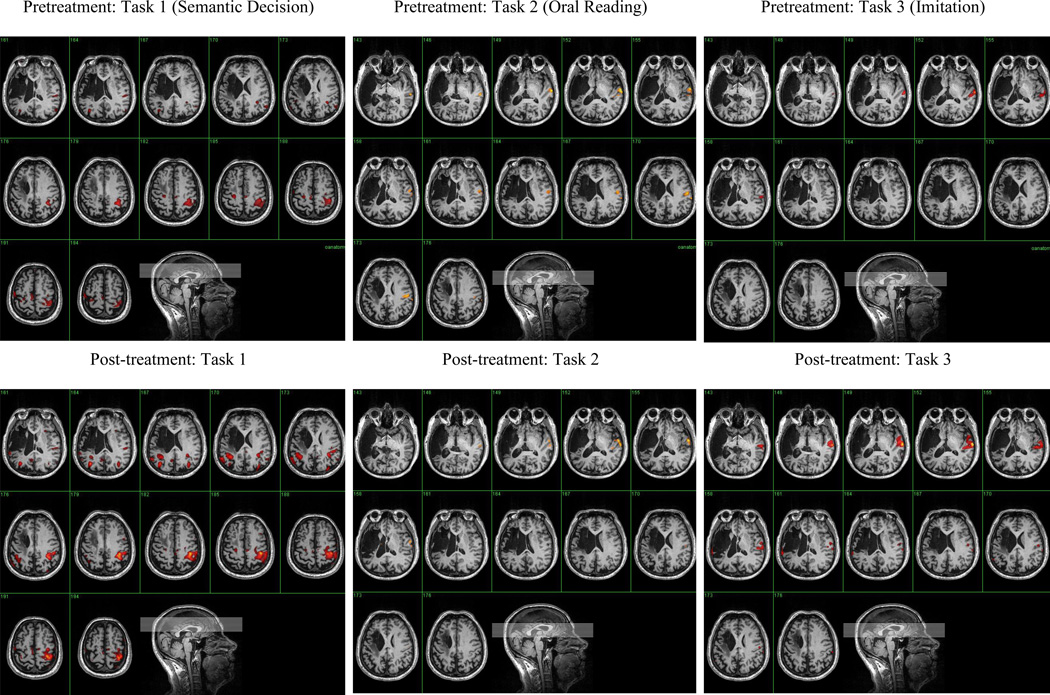
Three speech-language tasks were performed during the fMRI: (1) a semantic decision task; (2) a word reading task; and (3) imitation of consonant-vowel syllables. In the semantic decision task, 2 words were displayed simultaneously on the screen: a category name (eg, flower) and, underneath, an object label (eg, rose or chair). Mr. C indicated whether or not the object belonged to the category by pressing the yes or no buttons on a fiber-optic device, using his unimpaired left hand. In the word reading task, a 3- to 5-word sentence appeared on the screen. In half of the sentences, a content word changed from white to red print. Mr. C was instructed to read the word aloud if it turned red, but to remain silent if it did not change. For the third task, Mr. C. watched a video of a woman saying the syllables pa, fa, ta, and θa. He was asked to imitate the syllables. All 3 tasks were pretrained at the end of the language evaluations, then retrained just prior to Mr. C entering the MRI scanner. The Appendix includes details of how the fMRI images were analyzed. Figure 1 shows the pretreatment images that were obtained on each task.
Figure 1.
Pretreatment and posttreatment fMRI scans
Identifying site and type of stimulation
The fMRI scans were reviewed by all the investigators to determine placement of the electrodes and polarity. It was predetermined that the intersection of activation between any 2 tasks would be the preferred site for placement of the active electrode. Table 3 illustrates the options regarding selection of anodal or cathodal stimulation to the right or left hemispheres. For Mr. C, modulation of the left hemisphere was not an option given the extent of the lesion and minimal activity in the remaining perilesional areas. Prior research with rTMS had shown that inhibition of right hemisphere activity in persons with severe chronic aphasia resulted in improved naming skills.38–41 Therefore, cathodal stimulation to “hot spots” in the right hemisphere was selected, although there was trepidation regarding the possibility of inhibiting the activity of areas of the brain that supported both the language recovery that Mr. C had made to date and his preserved artistic abilities.
Table 3.
Selecting tDCS type and location: options and consequences of modulating each hemisphere
| Left hemisphere | Right hemisphere | |
|---|---|---|
| Anodal stimulation | The lesion was large with almost no activity on any of the tasks; it would be difficult to locate an area of activity to modulate. |
|
| Cathodal stimulation | The lesion was large with almost no activity on any of the tasks; it would be difficult to locate an area of activity to modulate. |
|
The fMRI images were co-registered with specific external bony markers using a neuronavigation eXimia image-guidance system (software version 4.3; Nexstim Ltd, Helsinki, Finland). The system identifies the scalp coordinates overlying the target cortex and then maps the computerized picture of the scalp to the physical location on the person’s head. The electrode location was marked on Mr. C’s scalp with small ink spots. Figure 2 shows the location for placement of the electrode and illustrates how 2 fMRI hot spots were incorporated into 1 electrode location with the proper orientation. The electrode was placed horizontally to facilitate stimulation of both spots.
Figure 2.
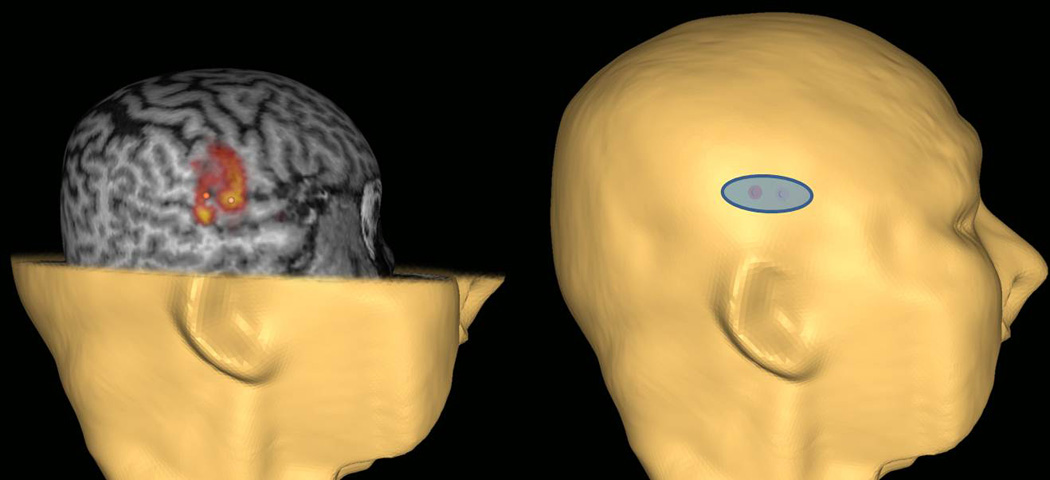
Determination of electrode placement using fMRI and the neuronavigation system. The electrode was placed horizontally to facilitate stimulation of 2 areas in the right hemisphere that were identified as areas of activation that intersected on 2 tasks.
Manual measurements were taken using external markers (eg, ears, nose) so that the target location could be reliably identified on treatment days without further use of the neuronavigation system. This was especially important because the ink spots were not permanent and could be removed with washing. For Mr. C, we measured from the external occipital protuberance at the back of the head to the nasion (the distinctly depressed area directly between the eyes, just superior to the bridge of the nose). From the mid-point of this measurement on the scalp, we identified the center of the electrode placement site as 6.5 cm medially to the right along the interaural line projected through the vertex between the tragus of each ear. To ensure inter- and intra-clinician reliability in daily placement of the electrodes, the treating clinician double-checked placement initially and throughout the first week against that of another investigator’s measurements and placement. Validation of the electrode placement site by the clinician with the neuronavigation system also occurred after the first week of the intervention.
Intervention: tDCS
tDCS (1mA for 13 minutes) was delivered using a constant current stimulator (Dupel Iontophoresis System, Empi, Minnesota) via an 8 cm2 oblong saline-soaked sponge cathode (Dupel B.L.U.E.; Empi, St. Paul, Minnesota) placed over the previously identified scalp location on the right temporal area. The density of the stimulation (voltage divided by the size of electrode pad) was 125 µA/cm2 and the charge density was 96 mC/cm2. A self-adhesive carbonized reference anode (48 cm2) was placed on the forehead above the contralateral orbit. These electrode sizes were chosen to enhance focality of the stimulation over target cortices and to minimize cortical effects under the reference electrode.42
During the first weeks of therapy, Mr. C complained of an itching/burning sensation at the reference electrode site during the ramping up of the tDCS and continuing throughout the application of the tDCS. The discomfort increased in intensity over days, and Mr. C would wince and attempt to touch or scratch at the reference electrode site. Therefore, in the second week we replaced the self-adhesive reference electrode with a round 28 cm2 saline-soaked electrode pad, which reduced the discomfort considerably.
At each treatment session, the 13 minutes of tDCS was applied concurrent with 15 minutes of speech-language treatment. The electrodes were then removed and Mr. C completed an additional 75 minutes of speech-language treatment. Therefore, Mr C received 90 minutes of daily language therapy in conjunction with 13 minutes of tDCS, 5 days a week for 6 weeks. The daily language treatment time was selected because results from motor studies indicated that the depolarization effects of 13 minutes of tDCS continue for 90 minutes after termination of tDCS.16 In another study, 1.5 mA tDCS was applied for 7 minutes, 5 times per week for 6 weeks without raising any safety concerns.43 By using a lower current for a longer period per session, we expected tDCS to be a safe and effective technique for modulating language area excitability.
To monitor the safety of the tDCS treatment, vital signs (blood pressure, heart rate, and temperature) and self-reported physical feelings/side effects were checked 3 times during each treatment session: immediately before the tDCS was initiated, 15 minutes later after completion of the tDCS had prior to the removal of the electrodes, and at the end of the entire session. We developed aphasia-friendly pictograms and drawings to assist Mr. C in expressing his feelings/side effects. Pictograms represented good, headache, dizzy, itchy on scalp, tired, nausea, dry mouth, and uncomfortable under electrodes. If Mr. C identified any side effects, he used a rating scale (ranging from 0 to 10) to express its severity.
Intervention: Language treatment
The speech-language treatment was a computer version of Oral Reading for Language in Aphasia (ORLA) in which the person with aphasia repeatedly reads aloud sentences and paragraphs, first in unison with the virtual therapist on the screen and then independently.44,45 Mr. C worked with sentences that were 3 to 5 words long. ORLA has been shown to be efficacious when delivered either by a clinician or via computer.46,47 Administration via computer ensured fidelity of treatment from session to session.
Treatment probes
Language probes were taken 3 times during the week prior to the start of treatment (baseline probes), once a week during the 6-week treatment period, and at both the posttreatment and follow-up assessments. Three probe tasks were used: picture naming, oral reading of trained stimuli, and oral reading of untrained stimuli. For the naming probe, picture stimuli were randomly chosen from a group of 50 simple line drawings. For the oral reading probes, sentences were randomly chosen from groups of trained ORLA sentences, untrained sentences of the same length, and untrained sentences at the next level of difficulty (ie, 10 trained 3- to 5- word sentences, 10 untrained 3- to 5-word sentences, and 10 untrained 8- to 10-word sentences). Probe tasks were selected based on preliminary information, suggesting that ORLA may improve oral reading accuracy and rate in individuals with nonfluent aphasia,45,46 while tDCS has been shown to improve naming.21,22,24,26,27
Probe task performance was audiotaped, timed, and scored using the following 5-point scale for each word: 5 = accurate and immediate response; 4 = accurate but delayed or self-corrected response; 3 = appropriate and intelligible response but with a minor error, 2 = semantic or verbal paraphasia (table for chair), or apraxic errors in half the word; 1 = unintelligible or unrelated response, or apraxic errors in over 50% of the word; 0 = no response. Confrontation naming was scored as percent accuracy. For oral reading of sentences, measures were obtained for accuracy and rate of production of recognizable words (ie, words obtaining a score of 3 or greater).
Results
Behavioral results
Standardized test results are shown in Table 2. There was a 4.7-point improvement on Mr. C’s WAB AQ from baseline (T1) to post treatment (T2), which is just shy of the 5-point change considered clinically significant.48 However, the WAB AQ score at follow-up testing (T3) was 1.1 points lower than at T1. Although a small, steady improvement in the Auditory Comprehension subtest was noted from T1 (3.95) to T3 (4.8), Naming subtest scores decreased from 2.5 at T1 to 1.7 at T3.
Performance on the Raven’s Progressive Matrices, the Conners' Continuous Performance Test II, and the Spatial Span subtest of the Wechsler Memory Test was examined from pre treatment to post treatment to determine whether there was any decrease in performance that may be attributable to deleterious effects from the prolonged and repetitive tDCS. Most scores remained stable across testing times (eg, block design) or even increased from pretreatment (T1) to follow-up (T3) (eg, Ravens Progressive Matrices), supporting the notion that the prolonged and repetitive tDCS was not harmful to these nonlinguistic functions. However, it should be noted that the drawing score on the WAB-R decreased at each successive test session.
With regard to probe data collected at each assessment and then weekly during treatment, small but negative effect sizes were obtained for naming accuracy. From pre treatment to post treatment, generally positive but small effective sizes were obtained for oral reading of trained sentences and longer, untrained sentences. From pre treatment to the 6-week follow-up, effect sizes were overall negative for trained sentences but positive for untrained sentences of increased length. Table 4 shows these effect sizes, which were calculated using the formula recommended by Beeson and Robey.49
Table 4.
Effect sizes for oral reading and naming probes
| Trained 3–5 word sentence |
Untrained 3–5 word sentences |
Untrained 8–10 word sentences |
Naming | ||||
|---|---|---|---|---|---|---|---|
| wpm | % accuracy |
wpm | % accuracy |
wpm | % accuracy |
% accuracy |
|
| Effect size (baseline to post treatment) | 1.36 | 1.69 | −0.83 | −0.71 | 0.67 | 0.29 | −0.89 |
| Effect size (baseline to 6 week follow-up) | −0.88 | −0.68 | −0.05 | −0.45 | 0.98 | 4.68 | −0.33 |
Note: wpm = recognizable words per minute.
fMRI results
Table 5 shows the number of activated voxels in the right and left hemispheres at pre treatment and post treatment. Following cathodal tDCS to the right hemisphere, there was an increase in the number of activated voxels in the left hemisphere on every task, with much of the increase occurring perilesionally. At the same time, the number of activated voxels in the right hemisphere also increased in 2 of the 3 tasks. The pre-post scans for each task are included in Figure 1.
Table 5.
Number of activated voxelsa for each fMRI task before and after treatment
| Semantic decisions | Oral reading | Imitation | ||||
|---|---|---|---|---|---|---|
| Pre treatment |
Post treatment |
Pre treatment |
Post treatment |
Pre treatment |
Post treatment |
|
| Right hemisphere | 2540 | 5120 | 1260 | 910 | 242 | 942 |
| Left hemisphere | 1523 | 3202 | 15 | 90 | 23 | 119 |
| Perilesional | 45 | 135 | 14 | 12 | 0 | 70 |
Note: fMRI = functional MRI.
Voxel size is 1.72 × 1.72 × 3 mm.
Safety
Mr. C’s vital signs were within normal limits for blood pressure, heart rate, and temperature throughout the 6 weeks of treatment. As mentioned previously, Mr. C reported itching/burning sensation at the reference electrode site, perceived on the severity scale ranging from 5/10 to 7/10. After this electrode was replaced with a saline-soaked electrode, the itchy/burning sensation decreased to between 1/10 and 3/10, and there were no more attempts to scratch at the electrode site. Mr. C also reported occasional tiredness; on further discussion, it was determined that this was related to poor sleep during the night, a problem that had been ongoing prior to starting the research project. Intermittently, Mr. C reported dry mouth from the repeated oral productions required during ORLA, and water was provided during each session. Once, on separate occasions, he reported dizziness (2/10) and headache (4/10), but these discomforts did not continue beyond mentioning on these occasions.
To monitor potential side effects after the end of the treatment period, we conducted weekly phone calls to Mr. C for 6 weeks. He was asked to respond to the same questions using the same pictograms that he was familiar with from his treatment sessions. We asked that his wife be on the phone call to verify his pointing responses and relate them to us. Mr. C reported feeling good each week with no side effects or discomfort. He also was asked about his perception of changes in talking, understanding, reading, and writing (ie, whether they improved, decreased, or stayed the same), and he responded using pictograms. During several weeks (weeks 2, 3, 4, 5, and 6), he indicated his reading was better and his writing was better (weeks 2, 3, 5, and 6). Talking and understanding were the same each week during the 6-week follow-up period.
Discussion
Several ethical issues and practical concerns arose at each step of the research protocol. Table 6 summarizes the key concerns related to each stage of Mr. C’s participation in this research study. Although these concerns were specific to Mr. C, they will need to be addressed in any study investigating the application of tDCS in persons with aphasia.
Table 6.
Research-related procedural decisions and associated ethical consideration
| Time period | Procedure | Questions/decisions | Ethical principles/ considerations |
|---|---|---|---|
| Pre enrollment | Obtaining informed consent |
|
Autonomy Therapeutic misconceptions |
| Assessment | Determining tDCS procedures |
|
Nonmaleficence |
| Treatment | tDCS treatment Behavioral treatment |
|
Beneficence |
| tDCS treatment Behavioral treatment |
|
||
| Safety monitoring | Short-term monitoring: during the treatment sessions |
|
Nonmaleficence |
| Long-term monitoring: during the follow-up period |
|
||
| Post treatment | Reviewing and interpreting results |
|
Therapeutic misconception |
Note: L = left; R = right; tDCS = transcranial direct current stimulation.
Informed consent
The first step with any research participant is the consenting procedure. Given the nature of deficits with aphasia, we instituted additional measures to ensure comprehension of the consent form and understanding of the research protocol.19 The consenting clinician slowly read each paragraph of the consent form aloud as Mr. C followed along with the written text. Supportive conversation techniques such as drawing and writing key words were implemented as needed to facilitate comprehension. Mr. C’s wife was present during the consenting process. She asked many questions, which were answered by extensive discussion and explanations. The clinician ensured that Mr. C was included in these discussions and that he understood the information conveyed in the responses. The evaluation procedures were carefully explained (approximately 3–4 hours of testing) as well as potential risks such as fatigue and frustration with tasks that might be difficult. Common concerns with fMRI procedures (ie, claustrophobia, extended time period without moving) were also explained. Treatment procedures were discussed, and Mr. C was shown the tDCS device as well as the ORLA program. Mr. C was shown pictograms illustrating potential risks. It was repeatedly emphasized that this was a research study and that there may not be any direct benefit to participating in the study. It was also emphasized that participation was voluntary and that Mr. C could withdraw at any time. Based on our modifications to create an informed consent procedure that was “aphasia-friendly” (see Table 7), we felt Mr. C had a good understanding of the research protocol and its associated risks and could make an informed and autonomous decision. To further ensure that this decision was Mr. C’s and not his wife’s, Mr. C was asked again about his willingness to participate when his wife was not present; he was given the opportunity to withdraw, which he declined.
Table 7.
Aphasia-friendly consent process
| Modify presentation |
|
| Emphasize and repeat important points |
|
| Use supported conversation techniques |
|
| Probe understanding frequently |
|
Throughout the consenting process, we highlighted the risks and disadvantages of participating in the research study in an attempt to avoid any therapeutic misconception. Although there is still disagreement about the concept of therapeutic misconception, it has been defined by the National Bioethics Advisory Commission as “the belief that the purpose of a clinical trial is to benefit the individual patient rather than to gather data for the purpose of contributing to scientific knowledge.”50 Lack of understanding of the risks of research participation and failure to differentiate their consequences from those of regular treatment raise an ethical concern; subjects may be motivated to take part in a research study because of their beliefs that they will be receiving individualized care and that they are meant to benefit from the study, even though the consent form might indicate differently.51 To minimize the potential for therapeutic misconception, Kimmelman52 recommends that studies be designed to ensure maximum therapeutic benefit for enrolled subjects. In view of this recommendation, we designed our study to provide a behavioral speech-language therapy (ORLA) that has been shown to be efficacious in persons with aphasia.44–47 Furthermore, even though selection of the therapy was not individualized for Mr. C, the level of difficulty could be assigned according to his ability (ie, 3- to 5-word sentences rather than 8- to 10-word sentences). Although the therapeutic outcome of the tDCS was uncertain, the fact that it was administered concurrently with an evidence-based behavioral treatment maximized the possibility that Mr. C would benefit, regardless of the type of tDCS he would receive. Rather than therapeutic misconception, Mr. C and his wife displayed “therapeutic optimism,” which is considered to be acceptable “because hope does not compromise the autonomy of a decision to participate in research.”53(p12)
Probably the most important ethical questions we grappled with were related to selection of location and type of stimulation (ie, anodal or cathodal stimulation to the left or to the right hemisphere), given the conflicting data in the literature on tDCS treatment and aphasia. As discussed previously and illustrated in Table 3, the fMRI scans showed that there was little activity in the left hemisphere during the language tasks, precluding modulation of this hemisphere. The decision of whether to use anodal or cathodal stimulation to the right hemisphere was based on prior studies that found that down-regulation of right hemisphere activity that may be maladaptive improved naming skills in persons with severe chronic aphasia.38–41 Our major concern related to the harm that could occur if the homologous right hemisphere language areas were indeed supporting the little language skills that Mr. C had recovered. Further, we were concerned about harming right hemisphere areas that were responsible for Mr. C’s retained artistic skills. It was necessary to carefully consider the risks to Mr. C in the context of this being a research study that was not necessary for his care. After lengthy discussions and review of the literature on rTMS, tDCS, and aphasia, the research team chose to provide cathodal modulation to the right hemisphere. In reaching this decision, the team members were cognizant of their motivation to enroll Mr. C as a research subject for the benefit of the study and the professional benefits to them as researchers. They carefully weighed benefits to the study as a whole with the potential risks to Mr. C before coming to a final decision.
To reduce risk, procedures to monitor physical side effects together with careful observation of language skills were implemented as described previously. These safety monitoring procedures were important because the dose and duration of the tDCS were greater than what had been given in prior studies. Over the 6 weeks, Mr. C. received a total of 390 minutes of 1mA tDCS (13 minutes, 5 times a week for 6 weeks). Other published studies provided between 150 and 300 cumulative minutes of tDCS. As described, Mr. C reported only minimal side effects during the treatment period and no side effects during the 6-week follow-up period. This is consistent with previous studies that have indicated that tDCS is a relatively safe procedure.54,55
Not only did Mr. C receive more tDCS than participants of other studies, but he also received more language therapy – both concurrent with the tDCS (on-line) and immediately after (off-line). The research team considered this to be a benefit to Mr. C, with fatigue being the only risk. To address this, Mr. C was given a 10-minute rest break during the ORLA treatment to walk around, get a drink, or use the bathroom. Although additional breaks could be taken as needed, Mr. C did not indicate a need for additional rest breaks during the program.
Even though the tDCS was deemed to be safe for Mr. C, the results of the study were generally disappointing. Objective language testing showed some improvement from pre treatment to post treatment, but these gains were not maintained during the 6-week follow-up. The language gains from pre treatment to post treatment were accompanied by increased left hemisphere activity, which we hypothesized would occur based on previous studies. There was also increased right hemisphere activity on 2 of the 3 language tasks, mostly in the same regions that had been active before treatment. It is difficult to interpret whether these changes in cortical activation were positive or negative. Furthermore, we could not ascertain whether the change in cortical activation was temporary or permanent because we did not repeat the fMRIs at the 6-week follow-up time. In summary, the objective data show that while Mr. C may not have benefitted from participating in the research, neither did he experience any harm.
We can only speculate on the reasons why the standardized test results were disappointing. The selected outcome measures may not have been sensitive enough to capture important clinical change. Alternatively, there may not have been any important change because Mr. C was not a good candidate for this treatment approach (eg, considering severity of aphasia, time post onset) or the dosing of the tDCS and/or behavioral treatments may not have been optimal. Regardless of the reasons, the key question is whether further investigation of tDCS with other persons with aphasia is warranted.
The patient-reported data tell a somewhat different story. Mr. C’s self-ratings on the CETI indicated an increase of almost 13 points from pre treatment to the 6-week maintenance. Similarly, his communication confidence, self-reported on the CCRSA, improved from pre treatment to follow-up. We attribute these increases in self-report scores to his perceived improvements in reading and writing skills reported during the weekly phone calls. Mr. C’s wife also reported gains, albeit much smaller, on the CETI from pre to post-treatment. From Mr. C’s perspective, he benefitted from participating in the treatment study. However, the confounding influence of a placebo effect (ie, the expectation of a positive result) cannot be ruled out without also administering a period of “sham” tDCS and comparing the results.
Scientific questions regarding tDCS in aphasia treatment abound, and scientific investigations are continuing.56 Scientific integrity must extend beyond the mere collection and interpretation of valid and reliable data. We must consider the participants who willingly volunteer in research studies. As we move forward with additional research, the case of Mr. C reminds us of the challenges we face to ensure sound ethical practices, including those of participant autonomy, nonmaleficence, and beneficence.
Acknowledgments
This study was supported by the National Institute on Deafness and Other Communication Disorders award number 5R21DC9876 (to L.R.C.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders or the National Institutes of Health.
APPENDIX
fMRI Analyses
T1-weighted anatomical images were acquired with the following parameters: voxel size, 1×1x1 mm; TR/TE, 2300/2.97 ms; flip angle = 9°; matrix resolution, 176 256 × 256. During the functional tasks, high-resolution gradient echo echo-planar imaging (EPI) data were acquired with the following parameters: TR/TE, 2200/20 ms; flip angle = 80°; in-plane matrix resolution, 128 116; voxel size, 1.721.723 mm; and a total of 37 slices for whole brain coverage. The duration of each task varied slightly with 284 volumes collected during the category task, 230 volumes for the oral reading task, and 285 volumes for the imitation task.
The first 6 volumes of each run were discarded to allow the MR signal to reach equilibrium. Images were analyzed using SPM8. Functional images were realigned with the first volume, resliced, co-registered to the T1 anatomical image, spatially smoothed with an 6-mm full-width at half-maximum Gaussian kernel, and high-pass filtered (cutoff period was 256 s for category and oral reading task and 128 s for imitation task). Fixed effects analyses were conducted on each task using a generalized linear model. Each acquisition block was modeled independently and convolved with the canonical hemodynamic response function combined with time and dispersion derivatives. Individual activation maps (F-contrast) for the category and imitation tasks were contrasted between baseline and task; for the oral reading task, activation maps were contrasted between overt and covert conditions. P < .05 with family-wise error (FWE) correction was considered significant activations.
The number of activated voxels was counted for both left and right hemispheres using FSLSTATS (part of FSL; http://www.fmrib.ox.ac.uk/fsl/). The manually segmented lesion masks based on anatomical images were spatially transformed to the individual’s functional space using FLIRT toolbox (part of FSL). Perilesional masks were created by dilating the lesion masks using a 4 mm Gaussian kernel. Activated voxels within the perilesional masks on the left hemisphere were obtained to indicate the level of perilesional brain activation.
REFERENCES
- 1.Dickey L, Kagan A, Lindsay P, et al. Incidence and profile of inpatient stroke-induced aphasia in Ontario, Canada. Arch Phys Med Rehabil. 2010;91:196–202. doi: 10.1016/j.apmr.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Pedersen PM, Vinter K, Olsen TS. Aphasia after stroke: type, severity and prognosis. Cerebrovasc Dis. 2004;17:35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- 3.Robey RR. A meta-analysis of clinical outcomes in the treatment of aphasia. J Speech Lang Hear Res. 1998;41:172–187. doi: 10.1044/jslhr.4101.172. [DOI] [PubMed] [Google Scholar]
- 4.Cherney LR, Robey RR. Aphasia treatment: recovery, prognosis and clinical effectiveness. In: Chapey R, editor. Language Intervention Strategies in Adult Aphasia. 4th ed. Baltimore: Williams & Wilkins; 2001. pp. 148–172. [Google Scholar]
- 5.Cherney LR, Robey RR. Aphasia treatment: recovery, prognosis and clinical effectiveness. In: Chapey R, editor. Language Intervention Strategies in Adult Aphasia. 4th ed. Baltimore: Williams & Wilkins; 2008. pp. 186–202. [Google Scholar]
- 6.Le Dorze G, Brassard C. A description of the consequences of aphasia on aphasic persons and their relatives and friends, based on the WHO model of chronic diseases. Aphasiology. 1995;9:239–255. [Google Scholar]
- 7.Lefaucheur J-P. Methods of therapeutic cortical stimulation. Clin Neurophysiol. 2009;39:1–14. doi: 10.1016/j.neucli.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Harvey RL, Nudo RJ. Cortical brain stimulation: a potential therapeutic agent for upper limb motor recovery following stroke. Top Stroke Rehabil. 2007;14:54–67. doi: 10.1310/tsr1406-54. [DOI] [PubMed] [Google Scholar]
- 9.Harvey R, Stinear JW. Cortical stimulation as an adjuvant to upper limb rehabilitation after stroke. Phys Med Rehabil. 2010;2:S269–S278. doi: 10.1016/j.pmrj.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Talelli P, Rothwell J. Does brain stimulation after stroke have a future? Curr Opin Neurol. 2006;19:543–550. doi: 10.1097/WCO.0b013e32801080d1. [DOI] [PubMed] [Google Scholar]
- 11.Cherney LR, Erickson RK, Small SL. Epidural cortical stimulation as adjunctive treatment for non-fluent aphasia: preliminary findings. J Neurol Neurosurg Psychiatry. 2012;81(9):1014–1021. doi: 10.1136/jnnp.2009.184036. [DOI] [PubMed] [Google Scholar]
- 12.Hamilton RH, Chrysikou EG, Coslett B. Mechanisms of aphasia recovery after stroke and the role of noninvasive brain stimulation. Brain Lang. 2011;118:40–50. doi: 10.1016/j.bandl.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holland R, Crinion J. Can tDCS enhance treatment of aphasia after stroke? Aphasiology. 2012;26:1169–1191. doi: 10.1080/02687038.2011.616925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarization induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114:600–604. doi: 10.1016/s1388-2457(02)00412-1. [DOI] [PubMed] [Google Scholar]
- 15.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57:1899–1901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 17.Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1:206–223. doi: 10.1016/j.brs.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Monti A, Cogiamanian F, Marceglia S, et al. Improved naming after transcranial direct current stimulation in aphasia. J Neurol Neurosurg Psychiatry. 2008;79(4):451–453. doi: 10.1136/jnnp.2007.135277. [DOI] [PubMed] [Google Scholar]
- 19.Stein J, Brady-Wagner LC. Is informed consent a “Yes or No” response? Enhancing the shared decision-making process for persons with aphasia. Top Stroke Rehabil. 2006;13(4):42–46. doi: 10.1310/tsr1304-42. [DOI] [PubMed] [Google Scholar]
- 20.You D-S, Kim D-Y, Chun MH, Jung SE, Park SJ. Cathodal transcranial direct current stimulation of the right Wernicke’s area improves comprehension in subacute stroke patients. Brain Lang. 2011;119:1–5. doi: 10.1016/j.bandl.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Kang EK, Kim YK, Sohn HM, Cohen LG, Paik N-J. Improved picture naming in aphasia patients treated with cathodal tDCS to inhibit the right Broca's homologue area. Restor Neurol Neurosci. 2011;29:141–152. doi: 10.3233/RNN-2011-0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Floel A, Meinzer M, Kitstein R, Nijhof S, Deppe M, Knecht S, Breitenstein C. Short-term anomia training and electrical brain stimulation. Stroke. 2011;42:2065–2067. doi: 10.1161/STROKEAHA.110.609032. [DOI] [PubMed] [Google Scholar]
- 23.Vines BW, Norton AC, Schlaug G. Non-invasive brain stimulation enhances the effects of melodic intonation therapy. Front Psychol. 2011;2:230. doi: 10.3389/fpsyg.2011.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker JM, Rorden C, Fridriksson J. Using transcranial direct-current stimulation to treat stroke patients with aphasia. Stroke. 2010;41:1229–1236. doi: 10.1161/STROKEAHA.109.576785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marangolo P, Marinelli CV, Bonifazi S, Fiori V, Ceravolo MG, Provinciali L, Tomaiuolo F. Electrical stimulation over the left inferior frontal gyrus (IFG) determines long-term effects in the recovery of speech apraxia in three chronic aphasics. Behav Brain Res. 2011;225:498–504. doi: 10.1016/j.bbr.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Fiori V, Coccia M, Marinelli CV, et al. Transcranial direct current stimulation improves word retrieval in healthy and non- fluent aphasic subjects. J Cogn Neurosci. 2010;23:2309–2323. doi: 10.1162/jocn.2010.21579. [DOI] [PubMed] [Google Scholar]
- 27.Fridriksson J, Richardson JD, Baker JM, Rorden C. Transcranial direct current stimulation improves naming reaction time in fluent aphasia: a double-blind, sham-controlled study. Stroke. 2011;42:819–821. doi: 10.1161/STROKEAHA.110.600288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranda PC, Lomarev M, Hallett M. Modeling the current distribution during transcranial direct current stimulation. Clin Neurophysiol. 2006;117:1623–1629. doi: 10.1016/j.clinph.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Wagner T, Fregni F, Fecteau S, Grodzinsky A, Zahn M, Pascual-Leone A. Transcranial direct current stimulation: a computer-based human model study. NeuroImage. 2007;35:1113–1124. doi: 10.1016/j.neuroimage.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Datta A, Baker JM, Bikson M, Fridriksson J. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011;4:169–174. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2nd ed. Philadelphia, PA; Lea & Febiger; 1983. [Google Scholar]
- 32.Kertesz A. Western Aphasia Battery Revised. San Antonio, TX: Harcourt Assessment; 2006. [Google Scholar]
- 33.Wechsler D. Wechsler Memory Scale—Third Edition (WMS–III) Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997. [Google Scholar]
- 34.Connors CK. Continuous Performance Test II. Toronto: Multihealth Systems Inc; 2002. [Google Scholar]
- 35.Lomas J, Pickard L, Bester S, Elbard H, Finlayson A, Zoghaib C. The Communicative Effectiveness Index: development and psychometric evaluation of a functional measure for adult aphasia. J Speech Hear Dis. 1989;54:113–124. doi: 10.1044/jshd.5401.113. [DOI] [PubMed] [Google Scholar]
- 36.Cherney LR, Babbitt EM, Semik P, Heinemann AW. Psychometric Properties of the Communication Confidence Rating Scale for Aphasia (CCRSA): phase 1. Top Stroke Rehabil. 2011;18:352–360. doi: 10.1310/tsr1804-352. [DOI] [PubMed] [Google Scholar]
- 37.Babbitt EM, Heinemann AW, Semik P, Cherney LR. Psychometric Properties of the Communication Confidence Rating Scale for Aphasia (CCRSA): phase 2. Aphasiology. 2011;25:727–735. doi: 10.1310/tsr1804-352. [DOI] [PubMed] [Google Scholar]
- 38.Martin PI, Naeser MA, Ho M, et al. Research with transcranial magnetic stimulation in the treatment of aphasia. Curr Neurol Neurosci Rep. 2009;9(6):451–458. doi: 10.1007/s11910-009-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Naeser MA, Martin PI, Nicholas M, et al. Improved naming after TMS treatments in a chronic, global aphasia patient - case report. Neurocase. 2005;11(3):182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 41.Naeser MA, Martin PI, Treglia E, et al. Research with rTMS in the treatment of aphasia. Restor Neurol Neurosci. 2010;28:511–529. doi: 10.3233/RNN-2010-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nitsche MA, Doemkes S, Karaköse T, et al. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97(4):3109–3117. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- 43.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SGB. Combined transcranial direct curent stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25:9–15. [PubMed] [Google Scholar]
- 44.Cherney LR. Efficacy of oral reading in the treatment of two patients with chronic Broca's aphasia. Top Stroke Rehabil. 1995;2(1):57–67. doi: 10.1080/10749357.1995.11754055. [DOI] [PubMed] [Google Scholar]
- 45.Cherney L, Merbitz C, Grip J. Efficacy of oral reading in aphasia treatment outcome. Rehabil Lit. 1986:112–119. [PubMed] [Google Scholar]
- 46.Cherney LR. Oral Reading for Language in Aphasia (ORLA): impact of aphasia severity on cross-modal outcomes in chronic nonfluent aphasia. Semin Speech Lang. 2010;31:42–51. doi: 10.1055/s-0029-1244952. [DOI] [PubMed] [Google Scholar]
- 47.Cherney LR. Oral Reading for Language in Aphasia (ORLA): evaluating the efficacy of computer-delivered therapy in chronic nonfluent aphasia. Top Stroke Rehabil. 2010;17(6):423–431. doi: 10.1310/tsr1706-423. [DOI] [PubMed] [Google Scholar]
- 48.Katz RC, Wertz RT. The efficacy of computer-provided reading treatment for chronic aphasic adults. J Speech Lang Hear Res. 1997;40:493–507. doi: 10.1044/jslhr.4003.493. [DOI] [PubMed] [Google Scholar]
- 49.Beeson PM, Robey RR. Evaluating single-subject treatment research: lessons learned from the aphasia literature. Neuropsychol Rev. 2006;16(4):161–169. doi: 10.1007/s11065-006-9013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.National Bioethics Advisory Commission. Ethical and Policy Issues in International Research: Clinical Trials in Developing Countries. Volume I, Report and Recommendations of the National Bioethics Advisory Commission. 2001 Apr;:vii. http://bioethics.georgetown.edu/nbac/clinical/Vol1.pdf. [PubMed]
- 51.Lidz CW, et al. Therapeutic misconception and the appreciation of risks in clinical trials. Soc Sci Med. 2004;58:1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- 52.Kimmelman J. The therapeutic misconception at 25: treatment, research, and confusion. Hastings Center Report. 2007;27(6):36–42. doi: 10.1353/hcr.2007.0092. [DOI] [PubMed] [Google Scholar]
- 53.Horng S, Grady C. Misunderstanding in clinical research: distinguishing therapeutic misconception, therapeutic misestimation, & therapeutic optimism. IRB. 2003;25(1):11–16. [PubMed] [Google Scholar]
- 54.Poreisz C, Boros K, Antal A, Paulus W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res Bull. 2007;72:208–214. doi: 10.1016/j.brainresbull.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Brunoni AR, Amadera J, Berbel B, Volz MS, Rizzerio BG, Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int J Neuropsychopharmacol. 2011;14:1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- 56.Brunoni AR, Nitsche MA, Bolognini N, et al. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]