Abstract
Synovitis is a key feature in osteoarthritis and is associated with symptom severity. Synovial membrane inflammation is secondary to cartilage degradation which occurs in the early stage and is located adjacent to cartilage damage. This inflammation is characterized by the invasion and activation of macrophages and lymphocytes, the release in the joint cavity of large amounts of pro-inflammatory and procatabolic mediators, and by a local increase of synovial membrane vascularity. This latter process plays an important role in the chronicity of the inflammatory reaction by facilitating the invasion of the synovium by immune cells. Therefore, synovial membrane angiogenesis represents a key target for the treatment of osteoarthritis. This paper is a narrative review of the literature referenced in PubMed during the past 5 years. It addresses in particular three questions. What are the mechanisms involved in synovium blood vessels invasion? Are current medications effective in controlling blood vessels formation and invasion? What are the perspectives of research in this area?
Keywords: angiogenesis, arthritis, synovium, vascularization
Introduction
Osteoarthritis (OA) is a disease affecting a whole organ, the joint. This disease is characterized by marked alterations in the metabolism, structure and function of multiple joints and periarticular tissues like cartilage, meniscus, synovial membrane (SM) and subchondral bone. OA symptoms are mainly mechanical pain, joint deformity and swelling, stiffness and cracks at motion. The natural evolution of OA may be interrupted by inflammatory flares which are generally associated with joint swelling, sudden increase of pain, pain at rest and a worsening stiffness.
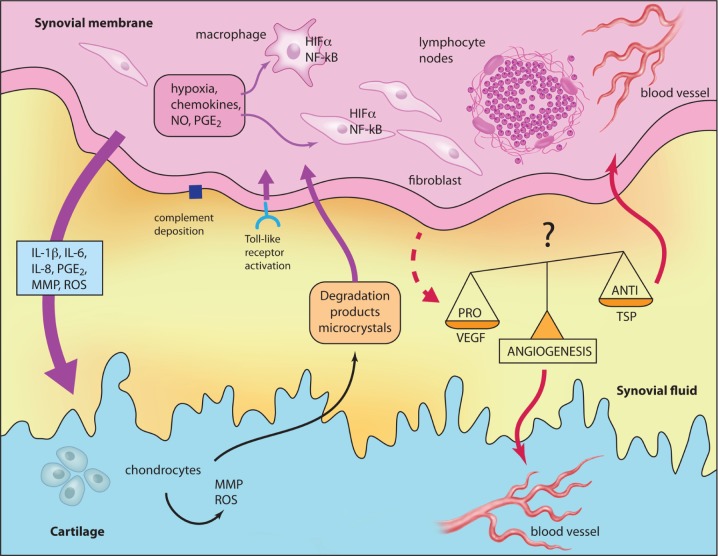
The inflammation targets SM (synovitis) in the early and late stages of OA. In the early stage, its distribution is confined to areas adjacent to sites of chondropathy and associated with an acceleration of cartilage degradation (chondrolysis) [Ayral et al. 2005]. This finding suggests that inflammation is brought about by cartilage breakdown. In advanced OA, synovitis has invaded across the SM, and progresses to fibrosis and villi hypertrophy [Shibakawa et al. 2003]. The pathophysiological schema generally described is as follows: mechanical stress directly damages cartilage or activate chondrocytes to produce abnormal levels of matrix metalloproteinases (MMPs) and reactive oxygen species (ROS) responsible for cartilage breakdown and the release in the joint cavity of microcrystals, osteochondral fragments and products of extracellular matrix degradation. These fragments and products trigger the secretion by cells of the inflamed synovium (synoviocytes, macrophages, lymphocytes) of cytokines, chemokines, lipidic mediators, ROS and MMP which can directly degrade the cartilage matrix components or dysregulate chondrocyte metabolism leading to an imbalance between cartilage matrix degradation and synthesis. Cartilage breakdown products, but also pro-inflammatory mediators released by chondrocytes and other joint cells, in turn amplify the SM inflammation, creating a vicious circle (Figure 1). These mediators may also trigger a systemic inflammatory response with consequent elevation of inflammatory serum biomarkers such as C-reactive protein (CRP). In OA, CRP is associated with clinical severity, the degree of inflammatory cell infiltration of the SM, disability, the number of involved joints and pain level [Stannus et al. 2013].
Figure 1.
Schematic representation of relationships between inflammation, angiogenesis and cartilage degradation in OA. Illustration courtesy of Alessandro Baliani. Copyright © 2014. Reproduced from Yves Henrotin’s personal slide. HIF, hypoxia-induced factor; IL, interleukin; MMP, matrix metalloprotease; NF-κB, nuclear factor-κB; NO, nitric oxide; PGE2, prostaglandin E2; ROS, reactive oxygen species; TSP, thrombospondin.
The relationship between cartilage degradation and synovitis was investigated in a study of soluble biochemical markers Coll2-1NO2 and ultrasensible CRP. Coll2-1NO2 is the nitrated form of an epitope specific of type II collagen molecule located in the triple helix. Coll2-1NO2 reflects the oxidative stress occurring in the inflammatory joint. Interestingly, Coll2-1 and Coll2-1NO2 were found to be elevated in the serum of patients with knee OA, but only Coll2-1NO2 was correlated with ultrasensible CRP, providing evidence of the relationship between inflammation and chondrolysis [Deberg et al. 2005].
This review focuses on one particular aspect of OA synovitis, the SM vascularization. Other aspects of synovitis have been described in detail in previous systematic reviews [Sellam and Berenbaum, 2010; De Lange-Brokaar et al. 2012; Berenbaum, 2013]. Herein, we discuss the recent advances in the understanding of: (1) pro-angiogenic phenotype expressed by OA synovial cells; (2) pathways promoting SM angiogenesis in OA; (3) the effects of current drugs on these pathways; and 4) therapeutic perspectives.
Method
A PubMed/Medline search was performed for articles published between January 2008 and July 2013 by combining the search terms related to OA [‘arthrosis’ OR ‘arthritis’ OR ‘osteoarthrosis’ OR ‘osteoarthritis’], to synovium [‘synovial membrane’ OR ‘synovium’ OR ‘synovitis’] and angiogenesis [‘angiogenesis’ OR ‘blood vessels’ OR ‘vascularization’]. Only articles in English were taken into account.
Structure and function of normal SM
Under normal physiological conditions, the synovial lining consists of a thin layer of cells with phenotypic characteristics of macrophages or fibroblasts. These cells are a major source of synovial fluid components which are directly involved in maintaining the cartilage integrity by lubricating the cartilage surface as well as by modulating chondrocyte metabolism. Two important molecules produced by synovial lining cells, lubricin and hyaluronic acid, contribute to protect articular cartilage surfaces in diarthrodial joints. In addition, lubricin reduces pathological deposition of protein at the cartilage surface and protects articular surface [Rhee et al. 2005; Ludwig et al. 2012]. Moreover, the SM provides nutrients that are essential for maintaining chondrocyte activity and which participate in the removal of products of chondrocytes metabolism and articular matrix turnover. Normal SM also acts as a semipermeable membrane, controlling molecular traffic into and out of the joint space and maintaining the composition of synovial fluid. Beside ‘fibroblastic-like’ and ‘macrophage-like’ cells, the SM also contains mesenchymal stem cells with multipotency which are able to differentiate into multiple mature cell lineages including cartilage, bone, muscle or adipose tissue [Gullo and de Bari, 2013].
SM characteristics in OA
Cellular aspects of the SM
T cells, B cells and monocytes/macrophages are the main immune cells found in OA inflammatory SM. T cells that infiltrate the synovium are mainly represented by CD4+ and CD8+ cells. The organization of T cells in the synovium becomes angiocentric, mainly in the perivascular areas forming nodes visible in SM intima [Lambert et al. 2012]. T cells appear to be activated in situ in the SM after exposure to antigen , which may be an autoantigen of cartilage. Possible auto-antigens released from cartilage are chitinase-3-like protein 2 (also known as YKL-39) and type II collagen peptides [Kim et al. 1999]. Patients with OA also seem to express cellular immunity to proteoglycan link protein. Low numbers of mast cells, B cells, natural killer cells and dendritic cells have been also found by several authors; neutrophils were almost never found. All these cellular aspects have been recently reviewed [De Lange-Brokaar et al. 2012].
Soluble catabolic and inflammatory mediators
Recent data suggest that microcrystals, complement components, matrix fragments and products of cell death and matrix catabolism can activate the innate immune response via pattern-recognition Toll-like receptors expressed by macrophages and other synovial lining cells. The binding of this receptor leads to the activation of specific transcription factors, with nuclear factor κB (NF-κB) playing a key role. NF-κB activation leads to the production by SM cells of cytokines, chemokines, ROS and MMPs that can cause local tissue damage, recruitment and activation of immune cells (macrophages, lymphocytes, granulocytes) but also driving osteophytosis and angiogenesis. These aspects were well documented in a recent review by Sokolove and Lepus [Sokolove and Lepus, 2013].
A broad spectrum of cytokines, chemokines, ROS, lipids, lipidic mediators, complement pathway components and MMPs is secreted by activated SM cells and found to be increased in the synovial fluid of OA patients [Goldring et al. 2011; Kosinska et al. 2013; Ritter et al. 2013]. Cytokines such as interleukin 1β (IL-1β), tumour necrosis factor-α (TNFα) and IL-6 have been largely investigated and presented as prominent cytokines in the pathogenesis of OA [McNulty et al. 2013]. However, this statement has been challenged since IL-1 and TNFα inhibitor trials failed to demonstrate significant efficacy [Chevalier et al. 2005; Magnano et al. 2007]. Particular attention was therefore paid to the expression and activity of cytokines involved in lymphocytes biology in OA synovium. IL-15 was consistently detectable and elevated in the serum of patients with early stage OA, compared with end-stage patients undergoing total knee replacement [Scanzello et al. 2009]. Serum IL-15 detected by a proteomic approach was associated with the presence and progression of radiographic OA [Gonzalez-Alvaro et al. 2011]. IL-15 may stimulate MMP production and recruitment or survival of CD8+ T cells within the OA joint. Another pro-inflammatory cytokine, IL-17, induces OA synovial fibroblasts and chondrocytes to produce pro-angiogenic factors including vascular endothelium growth factor (VEGF) [Honorati et al. 2002] as well as chemokines such as IL-8 and growth-regulated α protein (GRO-α) [Honorati et al. 2007]. Chemokines are also largely expressed in the joint tissues of patients with OA. The OA SM is also a source of adipokines and neuropeptides which may also directly or indirectly be involved in cartilage degradation and SM inflammation [De Boer et al. 2012]. Among the most investigated adipokines, adiponectin, visfatin and leptin seem to be the more active. Recently, positive significant correlation between serum levels of resitin and the histological severity of synovial inflammation were found, suggesting that this adipokine might modulate synovitis [De Boer et al. 2012]. Plasma leptin correlated with the severity of knee OA according to Ahlback’s radiographic classification [Staikos et al. 2013]. Chronic synovitis is associated with a marked change in the sensory innervation, and the synthesis and release of neurotransmitters and neuromodulators [Takeshita et al. 2012]. In addition to their role in pain, neuropeptides are involved in vasodilation, inflammation (by activating inflammatory infiltrating cells and by producing proinflammatory cytokines), osteoclast formation, and synoviocyte proliferation and activation. Particular attention has been paid to substance P and nerve growth factor (NGF). Substance P stimulates synoviocytes proliferation and the production by these cells of prostaglandin E2 (PGE2) and collagenase. NGF can stimulate proliferation of synoviocytes.
Vascular aspects of SM
Angiogenesis is the formation of new capillaries from pre-existing blood vessels. It has been associated with inflammation and inflammatory diseases. Inflammatory cells produce pro-angiogenic factors and promote the formation and invasion of new blood vessels, which facilitate inflammatory cell infiltration [Bonnet and Walsh, 2005]. In OA, angiogenesis contributes to the persistence rather than the initiation of inflammation.
Angiogenesis results from a sequence of events. Angiogenic factors produced by various cell types in the synovium activate local endothelial cells which, in turn, release proteolytic enzymes. These enzymes degrade the endothelial basement membrane and the perivascular extracellular matrix. Endothelial cells then proliferate and migrate into the intertitial tissue forming a ‘primary sprout’. The lumen formation within these sprouts leads to the formation of ‘capillary loops’ followed by synthesis of a new basement membrane and ultimately capillary formation.
Lessons from histology
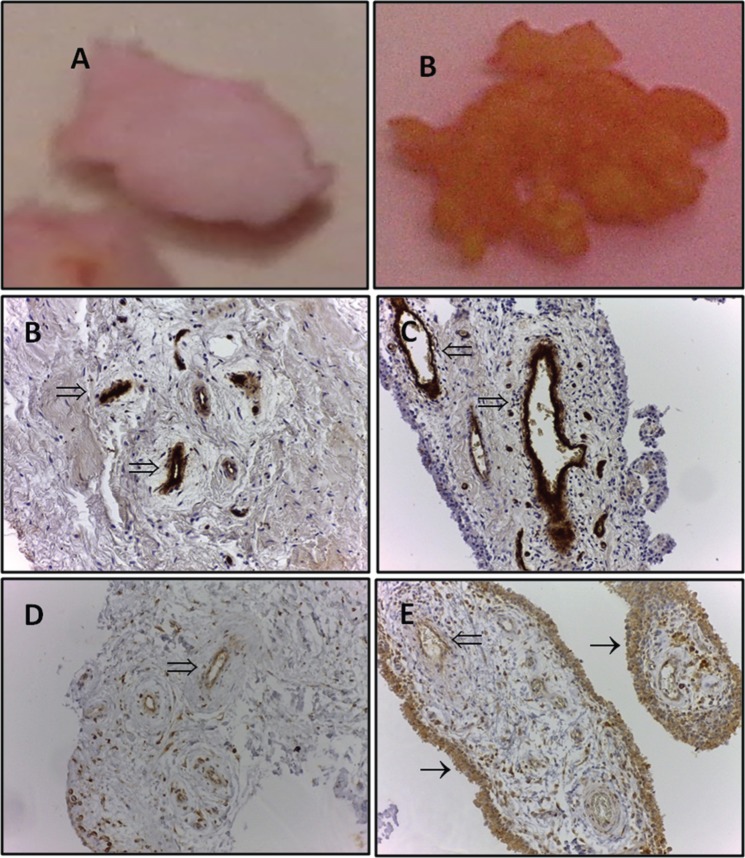
In OA, the SM undergoes multiples structural, metabolic and functional changes that can be investigated by imaging, biochemical markers, macroscopically or microscopically. A standardized macroscopic classification based in part on SM vascularization was established by Ayral and colleagues [Ayral et al. 1996] for the arthroscopic evaluation of the SM. This scoring system distinguishes three different grades: normal SM; reactive SM; and inflammatory SM (Table 1). Figure 2 shows representative photomicrographs depicting SM histopathological changes observed in inflammatory (I) compared with the normal/reactive (N/R) area of OA SM. The histological changes observed in the SM in OA generally include a range of abnormalities indicative of an inflammatory synovitis such as synovial lining hyperplasia, infiltration of inflammatory cells (mainly macrophages and T lymphocytes), and an increase in vascularity and fibrosis. Histological severity of synovitis in OA is low grade in comparison with the high grade synovitis of rheumatoid arthritis (RA), more focal than the widespread synovitis seen in RA, with synovitis abutting cartilage or meniscal lesions [Krenn et al. 2006; Pessler et al. 2008; Slansky et al. 2010]. We have investigated the blood vessels density in N/R and I synovial biopsies using antibody against von Willebrand’s factor. The analysis showed that OA blood vessels were distributed throughout the depth of the SM without preferential distribution in lining cells. Vascular density and vessels size were higher in I than in N/R biopsies. A staining for VEGF was observed in perivascular and sublining cells in both N/R and I biopsies. An acute positive staining was observed in the lining layer of I but not N/R biopsies, indicating that lining cells are key actors in the OA SM angiogenesis process.
Table 1.
Arthroscopic scoring system established by Ayral et al.
| Normal synovial membrane (grade 0) |
| ✓ Few translucent, slender villi with a fine vascular network can be clearly seen. |
| ✓ Proliferation of opaque villi. |
| Reactive synovial membrane (grade 0.5) |
| ✓ Villi have normal morphology or somewhat thicker and squat appearance. |
| ✓ Vascular network not seen due to loss translucence. |
| Inflammatory synovial membrane (grade 1) |
| ✓ Hypervascularization of synovial membrane and/or proliferation of hypertrophic and hyperemic villi are apparent. |
Figure 2.
Macroscopic appearance of N/R (A) and I (B) synovial biopsies. Immunohistochemical detection of von Willebrand’s factor in N/R (C) and I (D) synovial biopsies. N/R and I synovial biopsies were stained with anti-von Willebrand factor antibody. The presented images are representative of the obtained results. Immunohistochemical detection of VEGF in N/R (E) and I (F) synovial biopsies. N/R and I synovial biopsies were stained with anti-VEGF antibody. The presented images are representative of the obtained results. Magnification ×20.
I, inflammatory; N/R, normal/reactive; VEGF, vascular endothelial growth factor; (⇒), blood vessels; (→), intima lining.
Kennedy and colleagues investigated blood vessels stability in synovial tissue obtained from RA, psoriatric arthritis and OA patients using α-smooth muscle actin, a pericyte marker indicating vessel maturity [Kennedy et al. 2010]. Sections from patients with inflammatory arthritis demonstrated a mixture of immature vessels, vessels acquiring pericytes, and stable vessels, which showed close alignment of endothelial cells and pericytes. In OA tissue, all vessels had acquired pericytes and thus undergone full maturation and stabilization. This finding explains in part the persistence of inflammation in OA synovium.
Lessons from synovial cell cultures
In OA synovium, angiogenic factors are primarily released by macrophages, endothelial cells and synoviocytes. These factors include mainly growth factors, pro-inflammatory cytokines, chemokines, extracellular matrix protein, low oxygen tension, matrix-degrading proteolytic enzymes and cellular adhesion molecules [Szekanecz et al. 2010].
Recently, our group has developed an original methodology which compares inflammation and angiogenesis in the SM with different grades of synovitis. We used the Ayral’s macroscopic synovitis score to select, in the same OA SM, biopsies coming from N/R synovial or I areas [Lambert et al. 2012]. Synovial cells were isolated and cultured separately and the production of pro-inflammatory factors by synovial cells from N/R and I areas compared. Interestingly, cells from the I area produced more IL-6, IL-8 and VEGF, but less thrombospondin (TSP)-1 (an anti-angiogenic factor) than cells coming from the N/R area. In addition, VEGF levels were strongly correlated with IL-6 and IL-8 levels, confirming the relationship between inflammation and angiogenesis in OA. A significant negative correlation was obtained between TSP-1 and the pro-inflammatory factors IL-6 and IL-8. These results suggested a shift in the balance of angiogenic factors in favour of the development of new blood vessels. We also examined the effects of IL-1β (1 ng/ml) on the gene expression of five pro-angiogenic factors – VEGF, basic fibroblast growth factor (bFGF), NGF, angiopoietin-1 (Ang1) and MMP-2 – and three anti-angiogenic factors – vascular endothelium growth inhibitor (VEGI), TSP-1 and TSP-2. After 24 h treatment, IL-1β stimulated pro-angiogenic gene expression and strongly depressed anti-angiogenic gene expression. With regards to angiogenesis, VEGF is of outstanding importance. VEGF is probably the key regulator of neovascularization in inflammation. VEGF induces endothelial cell proliferation and migration, and also stimulates angiogenesis [Gao et al. 2013].
Local hypoxia is a major feature of the inflammatory tissue that also triggers angiogenesis in SM. Hypoxia stimulates the expression of hypoxia inducible factor (HIF)-1α and HIF-2α which act predominantly via upregulation of VEGF. The direct link between accumulation of HIF-αs and overexpression of VEGF, and the important role of the VEGF angiogenic pathway in arthritis, suggests the central role of HIF-αs in the pathogenesis of OA [Giatromanolaki et al. 2003]. A significant cytoplasmic and nuclear overexpression of HIF-1α and HIF-2α was noted in the synovial lining and stromal cells of OA synovium relative to normal. Overexpression of HIF-α was related to high microvessel density, high platelet-derived endothelial cell growth factor (PD-ECGF) expression and high VEGF/kinase insert domain protein receptor (KDR) receptor activation, suggesting HIF-α dependent synovial angiogenesis in OA [Giatromanolaki et al. 2003].
As well as observed with hypoxia and HIFs, other angiogenic mediators including hepatocyte growth factor (HGF), prostaglandins and nitric oxide (NO) also act through stimulation of VEGF production during neovascularization [Lin et al. 2012]. Interaction between VEGF and angiopoietin-1 (Ang1/Tie2) is critical for the stabilization of newly formed vessels [Szekanecz and Koch, 2008a, 2008b].
Some chemokines and chemokine receptors have also been implicated in synovial inflammation and angiogenesis [Szekanecz and Koch, 2008a]. Most CXC chemokines containing the glutamyl–leucyl–arginyl (ELR) amino acid sequence stimulate neovascularization while chemokines lacking this motif suppress neovascularization. Among ELR+ chemokines, we can mention IL-8/CXCL8 or connective tissue activating protein-III (CTAP-III/CXCL6) [Szekanecz and Koch, 2001]. The most important endothelial receptor for ELR+ angiogenic CXC chemokines is represented by CXCR2.
Pro-inflammatory cytokines may also either directly induce neovascularisation or may act by stimulating VEGF production. Among these cytokines, TNF-α, IL-1, IL-6, IL-15, IL-17, IL-18, oncostatin M, macrophage migration inhibitory factor (MIF), granulocyte (G-CSF) and granulocyte-macrophage colony stimulating factors (GM-CSF) are involved in angiogenesis, as well as OA synovitis [Vergunst et al. 2005; De Lange-Brokaar et al. 2012].
Lessons from animal models
Intra-articular gene transfer of TSP-1 in Wistar rats with OA induced by anterior cruciate ligament transection reduces microvessel density and macrophage infiltration in the synovium, and decreases macroscopic and histologic cartilage lesions [Hsieh et al. 2010]. In parallel, IL-1β levels in synovium tissue extracts decrease while transforming growth factor-β (TGF-β) is increased suggesting the involvement of these factors in the TSP-1 effects. Collectively, these data indicate that the local overexpression of an anti-angiogenic factor suppresses synovium inflammation, osteophytes formation and cartilage degradation. This highlights the key role played by angiogenesis in the OA pathogenesis and that targeting angiogenesis could be a useful strategy to control disease progression.
PPI-2458, an anti-angiogenic fumagillin analogue, reduces synovitis, bone and cartilage damage in animal models of arthritis [Bainbridge et al. 2007; Lazarus et al. 2008]. It exerts its effects by inhibiting methionine aminopeptidase type 2 (MetAp-2), triggering growth arrest of endothelial cells in the late G1 phase of the cell cycle, inhibiting endothelial cell proliferation and angiogenesis without affecting inflammatory cytokines release [Griffith et al. 1997]. In OA induced in male Lewis rats by meniscal transection, PPI-2458 reduced synovial and osteochondral angiogenesis, synovial inflammation, cartilage damage, osteophyte size and pain behaviour as evaluated by weight bearing asymmetry [Ashraf et al. 2011]. This also suggests that the effects of angiogenesis in inflammation are independent of inflammatory cytokines. Again, this demonstrates the key role played by angiogenesis in OA synovitis, structural damages and pain. Inhibition of angiogenesis therefore offers a potential novel therapeutic strategy for OA.
Lessons from Doppler ultrasonography
Recently, Gok and colleagues investigated the relationship between ultrasonographic findings and synovial angiogenesis modulators in 13 Behcet’s disease, 15 spondylarthropathy, 21 RA and 15 OA patients [Gok et al. 2013]. Synovial fluid angiostatin and bFGF levels were significantly higher in patients with inflammatory arthritis than with OA, while no significant difference was found for angiopoietin, endostatin and TSP-1. It was also noted that angiogenesis markers seemed not to be useful in discriminating between different forms of inflammatory arthritis. Synovial hypertrophy scores were positively correlated with angiostatin and bFGF and negatively correlated with TSP-1. No correlation was found between power Doppler ultrasonography scores and modulators. This is probably due to the small sample used in this study. Indeed, in knee arthritis, a power Doppler signal is difficult to detect, and it has been reported that an intra-articular power Doppler signal can be found in approximately 20% of all knee arthritis [Riente et al. 2010].
Are the current treatments of OA anti-angiogenic?
It is clear that angiogenesis is a key process in OA synovium inflammation and that SM inflammation is related to disease activity. Therefore, targeting SM inflammation is the goal of the current pharmacological and nonpharmacological treatments. The most frequently recommended and used oral pharmacological agents are acetaminophen, nonsteroidal anti-inflammatory drug (NSAIDs), glucosamine and chondroitin sulfate/HCl, avocado/soybean unsaponifiables and diacerein [Zhang et al. 2005, 2007, 2008, 2010; Hochberg et al. 2012]. Hyaluronic acid and glucocorticoids are recommended as intra-articular treatment. With the exception of acetominophen, all these agents have been demonstrated to reduce the production of pro-inflammatory and catabolic mediators (cytokines, prostanoids, MMPs or ROS) by joint cells. These aspects have been well documented in some recent reviews [Henrotin et al. 2010, 2011, 2012]. Herein, we overview the potential anti-angiogenic properties of these compounds.
NSAIDs
NSAIDs are among the most widely drugs for the suppression of inflammation and pain. However, their use is limited because they induce significant negative side effects, most notably in the gastrointestinal tract. Recently, it was suggested that the gastrointestinal adverse effects could be induced, at least partially, by the inhibitory effect of NSAIDs on the production of pleiotrophin by intestinal epithelial cells [Silver et al. 2012]. Pleiotrophin is a heparin-binding growth factor known to participate to angiogenesis. Pleiotrophin is expressed in embryonic but not mature cartilage, suggesting a role in cartilage development. Recently, pleiotrophin has been identified in OA cartilage and subchondral bone, suggesting its re-expression in pathological condition. This finding also designated pleiotrophin as a promising therapeutic target to control angiogenesis in OA [Kaspiris et al. 2013]. In vitro, NSAIDs are potent inhibitor of endothelial cells growth [Kjaer et al. 2010]. NSAIDs are also considered as inhibitors of tumour or retinal angiogenesis, notably through the inhibition of cyclooxygenase isoenzyme activity [Pakneshan et al. 2008; Yanni et al. 2010]. In male Lewis rats with knee OA induced by meniscal transection, indomethacin reduced synovial angiogenesis 35 days after meniscal transection [Ashraf et al. 2011].
Avocado/soybean unsaponifiables
Avocado/soybean unsaponifiables (ASU) are derived from unsaponifiable residues of avocado and soybean oils, and commonly mixed at a ratio one-third to two-thirds, respectively. ASU contains many compounds including fat-soluble vitamins, sterols, triterpene alcohols and possible furan fatty acids. The major components of ASU by weight are the phytosterols β-sisterol, campesterol and stigmasterol. ASUs have been studied for more than 20 years and show anti-OA properties [Christensen et al. 2008; Henrotin et al. 2011]. ASUs have been well investigated on chondrocytes and showed beneficial effects on cartilage degradation by stimulating aggrecan synthesis and by reducing catabolic and pro-inflammatory mediators. Some of these effects have been suggested to be secondary to an increase of TGF-β production by chondrocytes [Boumediene et al. 1999; Altinel et al. 2007]. In contrast, the effects of ASUs on synovial cells have been poorly investigated, with some findings even suggesting that ASUs could have anti-inflammatory effects. ASUs reduced IL-1β and TNF-α gene expression by lipopolysaccharide-stimulated monocytes/macrophage-like (THP-1) cell line [Au et al. 2007]. This is important since it was demonstrated that ASUs decreased cell infiltration in the SM of dog with OA induced by anterior cruciate ligament transection [Boileau et al. 2009]. We lack evidence to support a potential effect of ASUs on SM angiogenesis. However, this hypothesis should be explored since a study showed that ASUs strongly inhibited the production of MMP-2 by IL-1β stimulated gingival fibroblasts [Kut-Lasserre et al. 2001].
Diacerein
Diacerein, and its active metabolite rhein, is an anthraquinone derivate that refrains the expression of IL-1 in lipopolysaccharide-activated human OA chondrocytes and synoviocytes [Yaron et al. 1999]. Surprisingly, diacerein inhibited IL-1β-stimulated NF-κB activation in synoviocytes and chondrocytes, but increased cyclooxygenase-2 (COX-2) protein expression and PGE2 synthesis [Pelletier et al. 1998; Sanchez et al. 2003; Alvarez-Soria et al. 2008]. The impact of this PGE2 overexpression induced by diacerein needs to be clarified, particularly on joint tissue neovascularization. The release of PGE2 at high concentrations to the inflammatory site would contribute to inflammatory-related angiogenesis. However, rhein has been demonstrated to inhibit VEGF-stimulated human umbilical vein endothelial cell (HUVEC) tube formation, proliferation and migration under normoxic and hypoxic conditions [Fernand et al. 2011].
Chondroitin sulfate
Chrondroitin sulfate (CS) is recommended by the European League Against Rheumatism (EULAR) and the Osteoarthritis Research Society International (OARSI) as a symptomatic slow-acting drug for the treatment of knee and hip OA. In chondrocytes culture, CS acts by blocking NF-κB nuclear translocation, and as a consequence, the production of pro-inflammatory and procatabolic mediators like inducible nitric oxide synthetase (iNOS). The anti-inflammatory properties of CS have been observed in different animal models. In these models, CS administrated under a pretreatment regimen was able to reduce synovitis significantly, particularly cell infiltration, and the production of pro-inflammatory cytokines by joint cells [Omata et al. 2000; Cho et al. 2004]. However, no information is given about the vascular aspect of the SM after treatment with CS.
Using a microarray technique we have investigated the effects of CS on the expression of gene coding for pro- and anti-angiogenic factors. In a first set of experiments, we compared gene expression pattern of primary synoviocytes coming from the inflammation (I) area of OA SM cultured for 7 days with or without highly purified bovine CS (200 µg/ml, Bioiberica SA, Barcelona, Spain) and in low glucose. A total of 219 genes were identified as differentially expressed between I and I-CS conditions. Among them, we identified a number of genes implicated in angiogenesis and cell migration pathway. Endothelial cell-specific molecule-1 (ESM-1), transmembrene-4-L-six-family-1 (TMESF1), 5′-ectonucleotidase (NTS5E) and growth arrest-specific gene 6 (GAS6) were downregulated by CS. In a second set of experiments, we compared the effect of CS on IL-1β treated human synoviocytes coming from OA SM. A total of 3308 genes were identified as differentially expressed genes between control and IL-1 conditions. The most pro-angiogenic upregulated gene was stanniocalcin-1 (STC1). Interestingly, CS tended to decrease this factor (personal communication with Y Henrotin). Using real-time polymerase chain reaction (RT-PCR), a more sensitive and gene targeted method, we investigated the effect of CS (200 mg/ml) on the expression of selected pro- and anti-angiogenic genes by IL-1β treated synoviocytes. The stimulating effect IL-1β on VEGF, bFGF, NGF, Ang-1 and MMP-2 was unaffected by CS. After 24 h treatment, CS counteracted the inhibitory effect of IL-1 on VEGI and TSP-1. This effect was confirmed at the protein level by immunoassays [Lambert et al. 2012]. As an inhibitor of angiogenesis, TSP-1 overexpression decreases inflammation and blood vessel density in the SM. It also reduces cartilage lesion in rats where OA is induced by anterior cruciate ligament transection [Hsieh et al. 2010].
Recently, Calamia and colleagues studied the secretome of IL-1 treated human articular chondrocytes cultured with or without CS, employing a quantitative proteomic approach. They identified 75 different proteins in the secretome of human articular chondrocytes. Of these, 18 were modulated by bovine CS (200 µg/ml, Bioiberica SA, Barcelona, Spain) with statistical significance (6 increased and 12 decreased). Among these proteins, TSP-1, an angiogenic inhibitor, was strongly increased by CS [Calamia et al. 2012]. The anti-angiogenic action of CS was confirmed by the reduction in lactadherin, a protein that promotes VEGF-dependent vascularization and MMP-2, a MMP promoting tissue invasion by newly formed blood vessels.
Hyaluronic acid
Several studies underline hyaluronic acid (HA) involvement in endothelial cell proliferation, migration and new vessel formation [Cui et al. 2009; Matou-Nasri et al. 2009]. The mechanism of HA-induced angiogenesis involves the receptor for HA-mediated motility (RHAMM) and TGF-β receptor I (TGFBRI) [Park et al. 2012]. It has been demonstrated that the plasmatic HA level is associated with a significant enhancement in coronary collateralization, suggesting that circulating HA could also promote angiogenesis [Xi et al. 2010]. This should be considered along with the recent discovery that HA degradation generates small oligosaccharides that are able to increase pro-inflammatory (IL-1, TNFα) and pro-angiogenic cytokines (IL-18) production by synovial fibroblasts (RASF) obtained from mice subjected to collagen induced arthritis (CIA) [Campo et al. 2012a, 2012b]. This effect is mediated by activating both CD44 and the toll-like receptor 4 (TLR-4). CD44 and TLR-4 stimulation in turn activate the NF-βB that induces the production of these cytokines. Inversely, high molecular weight HA decreases toll-like receptor 2 and 4 cartilage expression in the same experimental arthritis model [Campo et al. 2011].
New perspectives
Accumulated evidence links COX-2, an enzyme involved in inflammation and arthritis, with angiogenesis, suggesting that drugs inhibiting COX-2 and prostanoid-related signalling cascades might be effective anti-angiogenic agents. Severe adverse effects associated with NSAIDs and selective inhibitors of COX-2 (COXIBs) have limited their long-term use in chronic diseases like OA. The current focus is therefore on the development of a new generation of NSAIDs targeting effectors downstream of COX such as prostanoid receptors (EP, TP, IP) and possibly several prostanoid activated peroxisome proliferator-activated receptors (PPARs) [Salvado et al. 2012]. PPARs can bind and be activated by a variety of prostanoids. PPARα has well-characterized roles in endothelial cells, demonstrating antiproliferative and anti-angiogenic properties in a variety of in vitro and in vivo models. Recent reviews confirm that the PPARγ pathway is a potential therapeutic target for cancer and several other disorders in which excessive angiogenesis is implicated [Menendez-Gutierrez et al. 2012; Ammazzalorso et al. 2013].
Some new natural compounds investigated for their anti-tumour effects could be effective in limiting tissue neovascularization in arthritic joints. Among these, curcumin and resveratrol are particularly interesting because they have been demonstrated to be active on SM cells and chondrocytes [Shakibaei et al. 2007; Lee et al. 2009; Henrotin et al. 2013]. Curcumin and resveratrol inhibit NF-κB activation and translocation induced by IL-1β and the consequent expression of NF-κB induced pro-inflammatory and pro-angiogenic genes like COX-2, IL-8 and VEGF [Csaki et al. 2009].
Other natural steroids with demonstrated anti-angiogenic effects on cancer cell lines could also be explored. From these, two warrant special mention: deltonin, a steroidal saponin isolated from Dioscorea Zingiberensis Wright (DZW) and α-chanonine, a naturally occurring steroidal glycoalkaloid in potato sprouts [Lu et al. 2010; Tong et al. 2011]. These agents are particularly active in blocking blood vessel formation. Shikonin, an naphthoquinone derivative isolated from plants, showed anti-angiogenic activities by inhibiting endothelial cells migration and proliferation through the inhibition of VEGF production [Lee et al. 2008].
Treatment with infliximab, a monoclonal antibody against TNF-α, in combination with methotrexate reduced synovial VEGF expression and vascularity [Lainer-Carr and Brahn, 2007]. The anti-IL-6 receptor antibody tocilizumab was shown to decrease serum levels of VEGF in RA [Nakahara et al. 2003]. Thalidomide is a potent TNF-α antagonist and angiogenesis inhibitor which shows anti-angiogenic VEGF-independent effect in arthritic rats [Lainer-Carr and Brahn, 2007]. Regarding human arthritis, thalidomide showed little efficacy in RA [Lainer-Carr and Brahn, 2007].
Biotherapy targeting VEGF have also been tested in cancers and in arthritis. There have been several attempts to target VEGF by using synthetic VEGF and VEGF receptor inhibitors, anti-VEGF antibodies and inhibitors of VEGF and VEGFR signalling, primarily in colorectal, lung, renal and liver cancers [Szekanecz et al. 2010]. Some have also been tested in arthritis. A soluble VEGFR1 chimeric protein dose-dependently inhibited the proliferation of synovial endothelial cells [Manley et al. 2002]. A VEGFR protein kinase inhibitor, vatalanib, also inhibited knee arthritis in rabbits [Grosios et al. 2004]. Soluble FAS ligand (CD178) inhibited production of the 165 amino acid form of VEGF (VEGF165) by RA synovial fibroblasts, as well as neovascularization [Lainer-Carr and Brahn, 2007]. Hypoxia-HIF-mediated neovascularization may also be targeted. Palictaxel, an anticancer agent which diminishes HIF-1α expression and activity, was effective and safe in a phase I clinical trial including RA patients [Lainer-Carr and Brahn, 2007]. Vitaxian, a humanized antibody to αVB3 integrin, inhibited synovial angiogenesis in an animal model of arthritis but showed very little efficacy in a phase II RA trial [Lainer-Carr and Brahn, 2007; Szekanecz and Koch, 2008b].
Finally some angiogenic inhibitors have demonstrated interesting effects in arthritic animal models. Among these compounds, angiostatin and endostatin block αVβ3 integrin dependent angiogenesis. Endostatin interferes with VEGF receptor signalling. The administration of either these compounds suppressed arthritis in various rodent models [Szekanecz et al. 2010]. A peptide derived from TSP-1 supressed synovial inflammation and angiogenesis in peptidoglycan-polysaccharide-induced rat arthritis [Park et al. 2004].
Conclusion
Angiogenesis plays a key role in synovium inflammation and cartilage damage accompanying OA and seems to be a critical mechanism in the persistence of OA. Angiogenesis facilitates the invasion of inflammatory cells and increases pain receptors locally. In OA, the SM vascularization process differs in some aspects from that observed in RA. The blood vessel density and stability and the levels of synovial angiogenesis modulators are higher in RA than in OA. Additional studies are required to identify the specific pathways involved in angiogenesis of OA synovium. Therefore, the inhibition of angiogenesis represents a promising avenue to control inflammation and pain in OA. Among the currently used pharmacological agents in OA, chondroitin sulphate shows in vitro anti-angiogenic properties mainly by controlling the balance between pro- and anti-angiogenic factors. However, this potential anti-angiogenic effect needs to be confirmed in vitro in a functional model of endothelial cell proliferation and migration and in vivo in OA animal models. Some new molecules are under investigation for their anti-inflammatory and anti-angiogenic properties and they may offer a new opportunity to block chronic pain and inflammation in OA.
Footnotes
Funding: This work was supported by the Bioiberica SA through unrestricted educational grants to the Bone and Cartilage Research Unit, University of Liège, to support research on joint tissues angiogenesis.
Conflict of interest statement: Y.H. is the founder and the chairman of the board of Artialis SA and Synolyne, two spin-off companies of the University of Liège. C.L. is supported by an unrestricted educational grant from Bioiberica.
Contributor Information
Yves Henrotin, Bone and Cartilage Research Unit, Institute of Pathology, CHU Sart-Tilman, University of Liège, 4000 Liège, Belgium.
Laurence Pesesse, Bone and Cartilage Research Unit, Institute of Pathology, University of Liège, Liège, Belgium.
Cecile Lambert, Bone and Cartilage Research Unit, Institute of Pathology, University of Liège, Liège, Belgium.
References
- Altinel L., Saritas Z., Kose K., Pamuk K., Aksoy Y., Serteser M. (2007) Treatment with unsaponifiable extracts of avocado and soybean increases TGF-β1 and TGF-β2 levels in canine joint fluid. Tohoku J Exp Med 211: 181–186 [DOI] [PubMed] [Google Scholar]
- Alvarez-Soria M., Herrero-Beaumont G., Sanchez-Pernaute O., Bellido M., Largo R. (2008) Diacerein has a weak effect on the catabolic pathway of human osteoarthritis synovial fibroblast–comparison to its effects on osteoarthritic chondrocytes. Rheumatology 47: 627–633 [DOI] [PubMed] [Google Scholar]
- Ammazzalorso A., De Filippis B., Giampietro L., Amoroso R. (2013) Blocking the peroxisome proliferator-activated receptor (PPAR): an overview. ChemMedChem. Epub ahead of print 12 August 2013. 10.1002/cmdc.201300250 [DOI] [PubMed] [Google Scholar]
- Ashraf S., Mapp P., Walsh D. (2011) Contributions of angiogenesis to inflammation, joint damage, and pain in a rat model of osteoarthritis. Arthritis Rheum 63: 2700–2710 [DOI] [PubMed] [Google Scholar]
- Au R., Al-Talib T., Au A., Phan P., Frondoza C. (2007) Avocado soybean unsaponifiables (ASU) suppress TNF-α, IL-1β, COX-2, INOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthritis Cartilage 15: 1249–1255 [DOI] [PubMed] [Google Scholar]
- Ayral X., Mayoux-Benhamou A., Dougados M. (1996) Proposed scoring system for assessing synovial membrane abnormalities at arthroscopy in knee osteoarthritis. Br J Rheumatol 35(Suppl. 3): 14–17 [DOI] [PubMed] [Google Scholar]
- Ayral X., Pickering E., Woodworth T., Mackillop N., Dougados M. (2005) Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13: 361–367 [DOI] [PubMed] [Google Scholar]
- Bainbridge J., Madden L., Essex D., Binks M., Malhotra R., Paleolog E. (2007) Methionine aminopeptidase-2 blockade reduces chronic collagen-induced arthritis: potential role for angiogenesis inhibition. Arthritis Res Ther 9: R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum F. (2013) Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage 21: 16–21 [DOI] [PubMed] [Google Scholar]
- Boileau C., Martel-Pelletier J., Caron J., Msika P., Guillou G., Baudouin C., et al. (2009) Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res Ther 11: R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet C., Walsh D. (2005) Osteoarthritis, angiogenesis and inflammation. Rheumatology 44: 7–16 [DOI] [PubMed] [Google Scholar]
- Boumediene K., Felisaz N., Bogdanowicz P., Galera P., Guillou G., Pujol J. (1999) Avocado/soya unsaponifiables enhance the expression of transforming growth factor β1 and β2 in cultured articular chondrocytes. Arthritis Rheum 42: 148–156 [DOI] [PubMed] [Google Scholar]
- Calamia V., Lourido L., Fernandez-Puente P., Mateos J., Rocha B., Montell E., et al. (2012) Secretome analysis of chondroitin sulfate-treated chondrocytes reveals anti-angiogenic, anti-inflammatory and anti-catabolic properties. Arthritis Res Ther 14: R202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo G., Avenoso A., D’Ascola A., Scuruchi M., Prestipino V., Calatroni A., et al. (2012a) 6-Mer hyaluronan oligosaccharides increase IL-18 and IL-33 production in mouse synovial fibroblasts subjected to collagen-induced arthritis. Innate Immun 18: 675–684 [DOI] [PubMed] [Google Scholar]
- Campo G., Avenoso A., D’Aascola A., Scuruchi M., Prestipino V., Nastasi G., et al. (2012b) The inhibition of hyaluronan degradation reduced pro-inflammatory cytokines in mouse synovial fibroblasts subjected to collagen-induced arthritis. J Cell Biochem 113: 1852–1867 [DOI] [PubMed] [Google Scholar]
- Campo G., Avenoso A., Nastasi G., Micali A., Prestipino V., Vaccaro M., et al. (2011) Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim Biophys Acta 1812: 1170–1181 [DOI] [PubMed] [Google Scholar]
- Chevalier X., Giraudeau B., Conrozier T., Marliere J., Kiefer P., Goupille P. (2005) Safety study of intraarticular injection of interleukin 1 receptor antagonist in patients with painful knee osteoarthritis: a multicenter study. J Rheumatol 32: 1317–1323 [PubMed] [Google Scholar]
- Cho S., Sim J., Jeong C., Chang S., Choi D., Toida T., et al. (2004) Effects of low molecular weight chondroitin sulfate on type II collagen-induced arthritis in DBA/1J mice. Biol Pharm Bull 27: 47–51 [DOI] [PubMed] [Google Scholar]
- Christensen R., Bartels E., Astrup A., Bliddal H. (2008) Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage 16: 399–408 [DOI] [PubMed] [Google Scholar]
- Csaki C., Mobasheri A., Shakibaei M. (2009) Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of IL-1β-induced NF-kappab-mediated inflammation and apoptosis. Arthritis Res Ther 11: R165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Xu H., Zhou S., Zhao T., Liu A., Guo X., et al. (2009) Evaluation of angiogenic activities of hyaluronan oligosaccharides of defined minimum size. Life Sci 85: 573–577 [DOI] [PubMed] [Google Scholar]
- Deberg M., Labasse A., Christgau S., Cloos P., Bang Henriksen D., Chapelle J., et al. (2005) New serum biochemical markers (Coll 2–1 and Coll 2–1 NO2) for studying oxidative-related type II collagen network degradation in patients with osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage 13: 258–265 [DOI] [PubMed] [Google Scholar]
- De Boer T., Van Spil W., Huisman A., Polak A., Bijlsma J., Lafeber F., et al. (2012) Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthritis Cartilage 20: 846–853 [DOI] [PubMed] [Google Scholar]
- De Lange-Brokaar B., Ioan-Facsinay A., Van Osch G., Zuurmond A., Schoones J., Toes R., et al. (2012) Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage 20: 1484–1499 [DOI] [PubMed] [Google Scholar]
- Fernand V., Losso J., Truax R., Villar E., Bwambok D., Fakayode S., et al. (2011) Rhein inhibits angiogenesis and the viability of hormone-dependent and -independent cancer cells under normoxic or hypoxic conditions in vitro. Chem Biol Interact 192: 220–232 [DOI] [PubMed] [Google Scholar]
- Gao W., Sweeney C., Walsh C., Rooney P., Mccormick J., Veale D., et al. (2013) Notch signalling pathways mediate synovial angiogenesis in response to vascular endothelial growth factor and angiopoietin 2. Ann Rheum Dis 72: 1080–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giatromanolaki A., Sivridis E., Maltezos E., Athanassou N., Papazoglou D., Gatter K., et al. (2003) Upregulated hypoxia inducible factor-1α and -2α pathway in rheumatoid arthritis and osteoarthritis. Arthritis Res Ther 5: R193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gok M., Erdem H., Gogus F., Yilmaz S., Karadag O., Simsek I., et al. (2013) Relationship of Ultrasonographic findings with synovial angiogenesis modulators in different forms of knee arthritides. Rheumatol Int 33: 879–885 [DOI] [PubMed] [Google Scholar]
- Goldring M., Otero M., Plumb D., Dragomir C., Favero M., El Hachem K., et al. (2011) Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater 21: 202–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alvaro I., Ortiz A., Alvaro-Gracia J., Castaneda S., Diaz-Sanchez B., Carvajal I., et al. (2011) Interleukin 15 levels in serum May predict a severe disease course in patients with early arthritis. PLoS One 6: e29492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith E., Su Z., Turk B., Chen S., Chang Y., Wu Z., et al. (1997) Methionine aminopeptidase (type 2) is the common target for angiogenesis inhibitors AGM-1470 and ovalicin. Chem Biol 4: 461–471 [DOI] [PubMed] [Google Scholar]
- Grosios K., Wood J., Esser R., Raychaudhuri A., Dawson J. (2004) Angiogenesis inhibition by the novel VEGF receptor tyrosine kinase inhibitor, PTK787/ZK222584, causes significant anti-arthritic effects in models of rheumatoid arthritis. Inflamm Res 53: 133–142 [DOI] [PubMed] [Google Scholar]
- Gullo F., de Bari C. (2013) Prospective purification of a subpopulation of human synovial mesenchymal stem cells with enhanced chondro-osteogenic potency. Rheumatology 52: 1758–68. [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Lambert C., Couchourel D., Ripoll C., Chiotelli E. (2011) Nutraceuticals: Do they represent a new era in the management of osteoarthritis? A narrative review from the lessons taken with five products. Osteoarthritis Cartilage 19: 1–21 [DOI] [PubMed] [Google Scholar]
- Henrotin Y., Mathy M., Sanchez C., Lambert C. (2010) Chondroitin sulfate in the treatment of osteoarthritis: from in vitro studies to clinical recommendations. Ther Adv Musculoskelet Dis 2: 335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y., Mobasheri A., Marty M. (2012) Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res Ther 14: 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrotin Y., Priem F., Mobasheri A. (2013) Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus 2: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg M., Altman R., April K., Benkhalti M., Guyatt G., Mcgowan J., et al. (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64: 465–474 [DOI] [PubMed] [Google Scholar]
- Honorati M., Bovara M., Cattini L., Piacentini A., Facchini A. (2002) Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthritis Cartilage 10: 799–807 [DOI] [PubMed] [Google Scholar]
- Honorati M., Cattini L., Facchini A. (2007) VEGF production by osteoarthritic chondrocytes cultured in micromass and stimulated by IL-17 and TNF-Alpha. Connect Tissue Res 48: 239–245 [DOI] [PubMed] [Google Scholar]
- Hsieh J., Shen P., Shiau A., Jou I., Lee C., Wang C., et al. (2010) Intra-articular gene transfer of thrombospondin-1 suppresses the disease progression of experimental osteoarthritis. J Orthop Res 28: 1300–1306 [DOI] [PubMed] [Google Scholar]
- Kaspiris A., Mikelis C., Heroult M., Khaldi L., Grivas T., Kouvaras I., et al. (2013) Expression of the growth factor pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta in the serum, cartilage and subchondral bone of patients with osteoarthritis. Joint Bone Spine: [DOI] [PubMed] [Google Scholar]
- Kennedy A., Ng C., Biniecka M., Saber T., Taylor C., O’sullivan J., et al. (2010) Angiogenesis and blood vessel stability in inflammatory arthritis. Arthritis Rheum 62: 711–721 [DOI] [PubMed] [Google Scholar]
- Kim H., Kim W., Cho M., Lee S., Youn J., Kim S., et al. (1999) Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum 42: 2085–2093 [DOI] [PubMed] [Google Scholar]
- Kjaer B., Struve C., Friis T., Engel A., Beyer N., Hojrup P., et al. (2010) Synthesis and anti-angiogenic effect of conjugates between serum albumin and non-steroidal anti-inflammatory drugs. Protein Pept Lett 17: 121–130 [DOI] [PubMed] [Google Scholar]
- Kosinska M., Liebisch G., Lochnit G., Wilhelm J., Klein H., Kaesser U., et al. (2013) A lipidomic study of phospholipid classes and species in human synovial fluid. Arthritis Rheum: [DOI] [PubMed] [Google Scholar]
- Krenn V., Morawietz L., Burmester G., Kinne R., Mueller-Ladner U., Muller B., et al. (2006) Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology 49: 358–364 [DOI] [PubMed] [Google Scholar]
- Kut-Lasserre C., Miller C., Ejeil A., Gogly B., Dridi M., Piccardi N., et al. (2001) Effect of Avocado and soybean unsaponifiables on gelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase (TIMP-1 and TIMP-2) secretion by human fibroblasts in culture. J Periodontol 72: 1685–1694 [DOI] [PubMed] [Google Scholar]
- Lainer-Carr D., Brahn E. (2007) Angiogenesis Inhibition as a therapeutic approach for inflammatory synovitis. Nat Clin Pract Rheumatol 3: 434–442 [DOI] [PubMed] [Google Scholar]
- Lambert C., Mathy-Hartert M., Dubuc J., Montell E., Verges J., Munaut C., et al. (2012) Characterization of synovial angiogenesis in osteoarthritis patients and its modulation by chondroitin sulfate. Arthritis Res Ther 14: R58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus D., Doyle E., Bernier S., Rogers A., Labenski M., Wakefield J., et al. (2008) An inhibitor of methionine aminopeptidase type-2, PPI-2458, ameliorates the pathophysiological disease processes of rheumatoid arthritis. Inflamm Res 57: 18–27 [DOI] [PubMed] [Google Scholar]
- Lee J., Huh J., Jeon G., Yang H., Woo H., Choi D., et al. (2009) Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int Immunopharmacol 9: 268–276 [DOI] [PubMed] [Google Scholar]
- Lee H., Magesh V., Nam D., Lee E., Ahn K., Jung M., et al. (2008) Shikonin, acetylshikonin, and isobutyroylshikonin inhibit VEGF-induced angiogenesis and suppress tumor growth in Lewis lung carcinoma-bearing mice. Yakugaku Zasshi 128: 1681–1688 [DOI] [PubMed] [Google Scholar]
- Lin Y., Huang Y., Fong Y., Tsai C., Chou M., Tang C. (2012) Hepatocyte growth factor increases vascular endothelial growth factor-A production in human synovial fibroblasts through C-met receptor pathway. PLoS One 7: e50924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Chen P., Shih Y., Chang Y., Huang E., Liu C., et al. (2010) Alpha-chaconine inhibits angiogenesis in vitro by reducing matrix metalloproteinase-2. Biol Pharm Bull 33: 622–630 [DOI] [PubMed] [Google Scholar]
- Ludwig T., Mcallister J., Lun V., Wiley J., Schmidt T. (2012) Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum 64: 3963–3971 [DOI] [PubMed] [Google Scholar]
- Magnano M., Chakravarty E., Broudy C., Chung L., Kelman A., Hillygus J., et al. (2007) A Pilot study of tumor necrosis factor inhibition in erosive/inflammatory osteoarthritis of the hands. J Rheumatol 34: 1323–1327 [PubMed] [Google Scholar]
- Manley P., Martiny-Baron G., Schlaeppi J., Wood J. (2002) Therapies directed at vascular endothelial growth factor. Expert Opin Invest Drugs 11: 1715–1736 [DOI] [PubMed] [Google Scholar]
- Matou-Nasri S., Gaffney J., Kumar S., Slevin M. (2009) Oligosaccharides of hyaluronan induce angiogenesis through distinct CD44 and rhamm-mediated signalling pathways involving CDC2 and gamma-adducin. Int J Oncol 35: 761–773 [DOI] [PubMed] [Google Scholar]
- McNulty A., Rothfusz N., Leddy H., Guilak F. (2013) Synovial fluid concentrations and relative potency of interleukin-1 α and β in cartilage and meniscus degradation. J Orthop Res 31: 1039–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez-Gutierrez M., Roszer T., Ricote M. (2012) Biology and therapeutic applications of peroxisome proliferator-activated receptors. Curr Top Med Chem 12: 548–584 [DOI] [PubMed] [Google Scholar]
- Nakahara H., Song J., Sugimoto M., Hagihara K., Kishimoto T., Yoshizaki K., et al. (2003) Anti-interleukin-6 receptor antibody therapy reduces vascular endothelial growth factor production in rheumatoid arthritis. Arthritis Rheum 48: 1521–1529 [DOI] [PubMed] [Google Scholar]
- Omata T., Itokazu Y., Inoue N., Segawa Y. (2000) Effects of chondroitin sulfate-C on articular cartilage destruction in murine collagen-induced arthritis. Arzneimittelforschung 50: 148–153 [DOI] [PubMed] [Google Scholar]
- Pakneshan P., Birsner A., Adini I., Becker C., D’Amato R. (2008) Differential suppression of vascular permeability and corneal angiogenesis by nonsteroidal anti-inflammatory drugs. Invest Ophthalmol Vis Sci 49: 3909–3913 [DOI] [PubMed] [Google Scholar]
- Park D., Kim Y., Kim H., Kim K., Lee Y., Choe J., et al. (2012) Hyaluronic acid promotes angiogenesis by inducing RHAMM-TGFβ receptor interaction via CD44-PKCdelta. Mol Cells 33: 563–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., Kang Y., Butterfield J., Detmar M., Goronzy J., Weyand C. (2004) Thrombospondin 2 functions as an endogenous regulator of angiogenesis and inflammation in rheumatoid arthritis. Am J Pathol 165: 2087–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Mineau F., Fernandes J., Duval N., Martel-Pelletier J. (1998) Diacerhein and rhein reduce the interleukin 1β stimulated inducible nitric oxide synthesis level and activity while stimulating cyclooxygenase-2 synthesis in human osteoarthritic chondrocytes. J Rheumatol 25: 2417–2424 [PubMed] [Google Scholar]
- Pessler F., Dai L., Diaz-Torne C., Gomez-Vaquero C., Paessler M., Zheng D., et al. (2008) The synovitis of ‘non-inflammatory’ orthopaedic arthropathies: a quantitative histological and immunohistochemical analysis. Ann Rheum Dis 67: 1184–1187 [DOI] [PubMed] [Google Scholar]
- Rhee D., Marcelino J., Baker M., Gong Y., Smits P., Lefebvre V., et al. (2005) The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J Clin Invest 115: 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riente L., Delle Sedie A., Filippucci E., Scire C., Iagnocco A., Gutierrez M., et al. (2010) Ultrasound imaging for the rheumatologist XXVII. Sonographic assessment of the knee in patients with rheumatoid arthritis. Clin Exp Rheumatol 28: 300–303 [PubMed] [Google Scholar]
- Ritter S., Subbaiah R., Bebek G., Crish J., Scanzello C., Krastins B., et al. (2013) Proteomic analysis of synovial fluid from the osteoarthritic knee: comparison with transcriptome analyses of joint tissues. Arthritis Rheum 65: 981–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvado M., Alfranca A., Haeggstrom J., Redondo J. (2012) Prostanoids in tumor angiogenesis: therapeutic intervention beyond COX-2. Trends Mol Med 18: 233–243 [DOI] [PubMed] [Google Scholar]
- Sanchez C., Mathy-Hartert M., Deberg M., Ficheux H., Reginster J., Henrotin Y. (2003) Effects of rhein on human articular chondrocytes in alginate beads. Biochem Pharmacol 65: 377–388 [DOI] [PubMed] [Google Scholar]
- Scanzello C., Umoh E., Pessler F., Diaz-Torne C., Miles T., Dicarlo E., et al. (2009) Local Cytokine profiles in knee osteoarthritis: elevated synovial fluid interleukin-15 differentiates early from end-stage disease. Osteoarthritis Cartilage 17:1040–1048 [DOI] [PubMed] [Google Scholar]
- Sellam J., Berenbaum F. (2010) The Role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6: 625–635 [DOI] [PubMed] [Google Scholar]
- Shakibaei M., John T., Seifarth C., Mobasheri A. (2007) Resveratrol inhibits IL-1β induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann NY Acad Sci 1095: 554–563 [DOI] [PubMed] [Google Scholar]
- Shibakawa A., Aoki H., Masuko-Hongo K., Kato T., Tanaka M., Nishioka K., et al. (2003) Presence of pannus-like tissue on osteoarthritic cartilage and its histological character. Osteoarthritis Cartilage 11: 133–140 [DOI] [PubMed] [Google Scholar]
- Silver K., Desormaux A., Freeman L., Lillich J. (2012) Expression of pleiotrophin, an important regulator of cell migration, is inhibited in intestinal epithelial cells by treatment with non-steroidal anti-inflammatory drugs. Growth Factors 30: 258–266 [DOI] [PubMed] [Google Scholar]
- Slansky E., Li J., Haupl T., Morawietz L., Krenn V., Pessler F. (2010) Quantitative determination of the diagnostic accuracy of the synovitis score and its components. Histopathology 57: 436–443 [DOI] [PubMed] [Google Scholar]
- Sokolove J., Lepus C. (2013) Role of Inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis 5: 77–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staikos C., Ververidis A., Drosos G., Manolopoulos V., Verettas D., Tavridou A. (2013) The association of adipokine levels in plasma and synovial fluid with the severity of knee osteoarthritis. Rheumatology 52: 1077–1083 [DOI] [PubMed] [Google Scholar]
- Stannus O., Jones G., Blizzard L., Cicuttini F., Ding C. (2013) Associations between serum levels of inflammatory markers and change in knee pain over 5 years in older adults: a prospective cohort study. Ann Rheum Dis 72: 535–540 [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Besenyei T., Paragh G., Koch A. (2010) New insights in synovial angiogenesis. Joint Bone Spine 77: 13–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. (2001) Chemokines and angiogenesis. Curr Opin Rheumatol 13: 202–208 [DOI] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. (2008a) Targeting angiogenesis in rheumatoid arthritis. Curr Rheumatol Rev 4: 298–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekanecz Z., Koch A. (2008b) Vascular involvement in rheumatic diseases: ‘vascular rheumatology’. Arthritis Res Ther 10: 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita M., Nakamura J., Ohtori S., Inoue G., Orita S., Miyagi M., et al. (2012) Sensory innervation and inflammatory cytokines in hypertrophic synovia associated with pain transmission in osteoarthritis of the hip: a case-control study. Rheumatology 51: 1790–1795 [DOI] [PubMed] [Google Scholar]
- Tong Q., Qing Y., Shu D., He Y., Zhao Y., Li Y., et al. (2011) Deltonin, a steroidal saponin, inhibits colon cancer cell growth in vitro and tumor growth in vivo via induction of apoptosis and anti-angiogenesis. Cell Physiol Biochem 27: 233–242 [DOI] [PubMed] [Google Scholar]
- Vergunst C., van de Sande M., Lebre M., Tak P. (2005) The role of chemokines in rheumatoid arthritis and osteoarthritis. Scand J Rheumatol 34: 415–425 [DOI] [PubMed] [Google Scholar]
- Xi W., Zhou Y., Lv S., Gao Q., Bu G., Wang Y., et al. (2010) Plasma hyaluronan and collateral development in patients with coronary artery disease. Coron Artery Dis 21: 228–232 [DOI] [PubMed] [Google Scholar]
- Yanni S., Clark M., Yang R., Bingaman D., Penn J. (2010) The effects of nepafenac and amfenac on retinal angiogenesis. Brain Res Bull 81: 310–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaron M., Shirazi I., Yaron I. (1999) Anti-interleukin-1 effects of diacerein and rhein in human osteoarthritic synovial tissue and cartilage cultures. Osteoarthritis Cartilage 7: 272–280 [DOI] [PubMed] [Google Scholar]
- Zhang W., Doherty M., Arden N., Bannwarth B., Bijlsma J., Gunther K., et al. (2005) EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 64: 669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Doherty M., Leeb B., Alekseeva L., Arden N., Bijlsma J., et al. (2007) EULAR evidence based recommendations for the management of hand osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 66: 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Doherty M., Peat G., Bierma-Zeinstra M., Arden N., Bresnihan B., et al. (2010) EULAR evidence-based recommendations for the diagnosis of knee osteoarthritis. Ann Rheum Dis 69: 483–489 [DOI] [PubMed] [Google Scholar]
- Zhang W., Moskowitz R., Nuki G., Abramson S., Altman R., Arden N., et al. (2008) OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage 16: 137–162 [DOI] [PubMed] [Google Scholar]