Abstract
Recent findings suggest that hypercholesterolemia may contribute to the onset of Alzheimer’s disease (AD)-like dementia but the underlying mechanisms remain unknown. In this study, we evaluated the cognitive performance in rodent models of hypercholesterolemia in relation to neuroinflammatory changes and amyloid precursor protein (APP) processing, the two key parameters of AD pathogenesis. Groups of normal C57BL/6 and low density lipoprotein receptor (LDLR)-deficient mice were fed a high fat/cholesterol diet for an 8-week period and tested for memory in a radial arm maze. It was found that the C57BL/6 mice receiving a high fat diet were deficient in handling an increasing working memory (WM) load compared to counterparts receiving a control diet while the hypercholesterolemic LDLR−/− mice showed impaired WM regardless of diet. Immunohistochemical analysis revealed the presence of activated microglia and astrocytes in the hippocampi from high fat-fed C57BL/6 mice and LDLR−/− mice. Consistent with a neuroinflammatory response, the hyperlipidemic mice showed increased expression of cytokines/mediators including TNFα, IL-1β, IL-6, NOS2 and COX2. There was also an induced expression of the key APP processing enzyme i.e., BACE1 in both high fat/cholesterol-fed C57BL/6 and LDLR−/− mice accompanied by an increased generation of C-terminal fragments (CTFs) of APP. Although ELISA for Aβ failed to record significant changes in the non-transgenic mice, a 3-fold increase in Aβ-40 accumulation was apparent in a strain of transgenic mice expressing wt hAPP on high fat/cholesterol diet. The findings link hypercholesterolemia with cognitive dysfunction potentially mediated by increased neuroinflammation and APP processing in a non-transgenic mouse model.
Keywords: Cholesterol, LDLR, working memory, neuroinflammation, APP processing, BACE1
INTRODUCTION
A number of findings suggest a link between abnormal cholesterol metabolism and AD pathogenesis (Puglielli et al. 2003; Casserly and Topol 2004; Sambamurti et al. 2004). First, several AD-associated genes (i.e., ApoE, cyp46, ABCA1) that show disease-specific polymorphisms are otherwise normal participants in cholesterol metabolism (Roses 1996; Bjorkhem et al. 1998; Katzov et al. 2004). Second, clinical studies have indicated that individuals with increased cholesterol are more susceptible to AD and that elevated levels of low-density lipoprotein (LDL) cholesterol correlate well with AD incidence compared to asymptomatic cases (Puglielli et al. 2003). Third, animal studies using New Zealand white rabbits (Sparks et al. 2000) and transgenic mouse models of AD (Refolo et al. 2000; Levin-Allerhand et al. 2002) have demonstrated that diet-induced hypercholesterolemia could enhance brain Aβ accumulation. Cholesterol has also been shown to directly modulate the processing of the APP protein in neuronal cell cultures (Bodovitz and Klein 1996; Simons et al. 1998; Frears et al. 1999; Ehehalt et al. 2003). Many such, but not all, observations have suggested that cholesterol may play a prominent role in AD pathogenesis and that lowering cholesterol may benefit disease prognosis. In fact, retrospective studies have demonstrated that cholesterol lowering drugs i.e., statins, could lower the incidence of AD (Wolozin et al. 2000). However, the statin activity most likely involves mechanisms other than inhibiting cholesterol synthesis, a prominent one being the drug’s anti-inflammatory effects (Liao and Laufs 2005; Wolozin et al. 2006). In this regard, it is now clear that atherosclerosis, AD as well as many other neurodegenerative diseases all have in common, an inflammatory component. While inflammation is central to atherosclerosis involving changes in the endothelium caused by oxidized lipids/lipoproteins, monocyte/macrophage accumulation and ultimately resulting in sclerotic plaque formation (Steinberg 2002), there is ample evidence for an activation of inflammatory processes and the presence of cytokines and other mediators in pathologically vulnerable regions of the AD brain (Akiyama et al. 2000; Wyss-Coray and Mucke 2002; Heneka and O’Banion 2007).
It is likely that a compromised cholesterol homeostasis, resulting either from dietary practice or genetic imposition, facilitates an exacerbated neuroinflammatory response in association with increased Aβ generation leading to AD-associated degeneration. To test this idea, we administered a high fat/cholesterol diet to groups of 4-month-old C57BL/6 and the hypercholesterolemic strain i.e., LDL receptor-deficient (LDLR-/) mice for an 8-week period, followed by an assessment of working and reference memory function in relation to neuroinflammatory and AD-related biochemical changes. Importantly, these evaluations were all performed in the same animals, allowing correlational assessments between the cognitive, physiological and neurobiological variables. The results presented implicate a link between diet-induced neuroinflammation and AD-associated cognitive impairment. Parts of this study have previously been reported in abstract form --
MATERIALS AND METHODS
Animals and treatment
Normal C57BL/6 mice, LDLR−/− mice (ldlr KO strain, B6.12957-Ldlrtm1Her) and a transgenic line i.e., PDGFB-APPwt, were obtained from Jackson Labs (Bar Harbor, ME). The three strains of mice (4-month old) were divided into two groups (each n=6): group 1, normal chow (5% fat; 0.05% chol) and group 2, a high fat (21%), high cholesterol (1.25%) diet (Teklad), resulting in a total of six treatment groups. The mice were on these diets for 2 months. During the last 2 weeks of this 2 month diet, mice were tested for memory performance in an 8-arm water maze (description below) before sacrifice. Following behavioral testing, to obtain tissue samples, the animals were anesthetized with an intraperitoneal (IP) injection of sodium pentobarbital (50mg/kg) and transcardially perfused with phosphate-buffered saline (PBS, 4°C). Next, the brains were removed and divided sagittally. One hemibrain was post-fixed in phosphate-buffered 4% paraformaldehyde, pH 7.4 at 4°C for 48 hours for sectioning; the other was rapidly dissected for hippocampus and cortical tissues, snapfrozen and stored at −70°C for biochemical analyses. All animal procedures were carried out in accordance with the USPHS policy on the Humane Care and Use of Laboratory Animals.
Immunohistochemistry
The fixed tissue was serially sectioned at 40micron with a freezing microtome (Microm HM400) and the sections used for immunohistochemical analysis of activated glia i.e., microglia (stained with anti-CD45 antibodies) and astrocytes (stained for GFAP). A kit (Vector ABC Elite kit) from Vector Laboratories (Burlingame, CA) was used along with 3′3′ diaminobenzoic acid (DAB) as a chromogen to develop the reaction in the presence of H202. For quantitative measurements of staining intensities, images of stained areas were captured with a Nikon Eclipse E-600 microscope equipped with a CCD camera and analyzed using NIH Image® Software. Measurements from 4-6 sections per brain were averaged to obtain one value per subject.
Aβ ELISA
Known amounts of cortex samples were homogenized in 10 volumes of PBS and centrifuged at 100,000 ×g for an hr to collect membranes. The membrane pellets were then extracted in 10 vol of a basic solution containing 0.2% diethylamine (DEA; Sigma-Aldrich), 50mM NaCl, protease inhibitor cocktail (Roche, IN) and 5mM (O-phenanthroline (Sigma-Aldrich). The DEA extract (100μl) was neutralized with 50mM Tris-Hcl, pH 6.8, centrifuged at 100,000 xg/1h and the supernatant diluted in the ELISA buffer for further analysis. Aβ40 and 42 levels were measured by a Sandwich ELISA using kits from IBL-America (Minneapolis, MN). The antibodies used for the Sandwich ELISA were specific for the Aβ40 (1A10) and Aβ42 (affinity purified polyclonal) ends for capture and a monoclonal antibody (12B2) against Aβ residues 11-28 coupled to horseradish peroxidase for detection. The target sequence is conserved in rodents (except residue 13) and the antibody detects rodent Aβ peptide as efficiently as human Aβ as reported by the manufacturer.
Western blot
For Western blot analysis of APP and CTFs, the homogenates representing equal amounts of protein were loaded on a 10-20% gradient gel (BioRad Criterion). The antibody used i.e., 0443, was raised in rabbits against the C-terminal 20 residues of APP (Pinnix et al. 2001b). This anti-body detects APP (from a variety of species from fish to humans) with an intact cytoplasmic domain, which includes full-length APP, CTFα (C-terminal 83 residues) and CTFβ (C-terminal 99 residues). The antibody against the cytoplasmic domain of BACE used in this study has been described before (Pinnix et al. 2001a) and detects a 62kDA protein in mouse and guinea pig brain extracts. Its specificity has been characterized by Western analysis and blocking experiments using cell extracts from CHO cells transfected with recombinant BACE (Venugopal and Sambamurti, unpublished studies).
QRT-PCR
Total RNA was isolated using Trizol reagent (Invitrogen). The mRNAs were reverse transcribed into cDNAs using M-MLV reverse transcriptase (Invitrogen). Specfic primers for the mouse cytokines i.e., IL-1β, TNF-α, IL-6 and GAPDH were as described (Kitazawa et al. 2005). The primers used for iNOS were: 5′-CAGCTGGGCTGTACAAACCTT; 5′-CATTGGAAGTGAAGCGTTTCG and those for COX2 were: 5′-ACACTCTATCACTGGCACCC; 5′-GAAGGGACACCCCTTCACAT. The primer sets for mouse BACE was purchased from SuperArray (Frederick, MD). Real-time RT-PCR analysis was carried out on a Bio-Rad iCycler system using a SYBR Green qPCR Supermix kit. At the end of the runs, melting curves were obtained to make sure that there are no primer-dimer artifacts. The products were verified by agarose gel analysis. Threshold cycle (Ct) values were calculated with MyiQ software (BioRad) and quantitative fold changes in mRNA were determined as relative to GAPDH or 18S mRNA levels in each treatment group.
Behavioral testing
Cognitive testing was performed using the water radial-arm maze. The win-shift radial-arm maze utilizes water escape onto hidden platforms as the reinforcer, thereby avoiding food deprivation (Bimonte and Denenberg 2000; Bimonte et al. 2000). The 8 arm maze had hidden escape platforms in the ends of 4 arms. Each subject had different platform locations that were semi-randomly determined and that remained fixed throughout the experiment. A subject had 3 minutes to locate a platform. If the allotted time expired, the subject was guided to the nearest available platform. Once a platform was found, the animal remained on it for 15s, and was then returned to its heated home cage for 30s until its next trial. During the interval, the just-chosen platform was removed from the maze. The animal was then placed again into the start alley and allowed to locate another platform. A daily session consisted of this sequence of events repeated until all four platforms were located. Consequently, for each animal a daily session consisted of four trials per session, with the number of platformed arms reduced by one on each subsequent trial. Hence, the working memory system was increasingly taxed as trials progressed. This version is similar to the land version of the radial-arm maze in that animals had to avoid arms that never contained a reinforcer (reference memory) and enter only once into arms that contained a reinforcer (working memory).
The following quantification and blocking procedures are based upon previous studies using the water radial-arm maze (Hyde et al. 1998; Bimonte and Denenberg 2000; Bimonte et al. 2000; Hyde et al. 2000). Each subject was given one session a day for 15 consecutive days. Day 1 was considered a training session because the animal had no previous experience in the maze. Days 2-15 were testing sessions, which were blocked into 2-day blocks for analyses. Errors were quantified for each daily session using the orthogonal measures of working and reference memory errors (Jarrard et al. 1984), as done previously in studies using the water escape radial-arm maze (Bimonte et al. 2000; Hyde et al. 2000; Hunter et al. 2003). Working Memory Correct errors were the number of first and repeat entries into any arm from which a platform had been removed during that session. Reference Memory errors were the number of first entries into any arm that never contained a platform. Working Memory Incorrect errors were the number of repeat entries into an arm that never contained a platform in the past (thus, repeat entries into a reference memory arm). Data were analyzed using repeated measures ANOVA, with Genotype and Diet as between subjects variables, and Block and Trial as repeated measures (Statview 5.0).
RESULTS
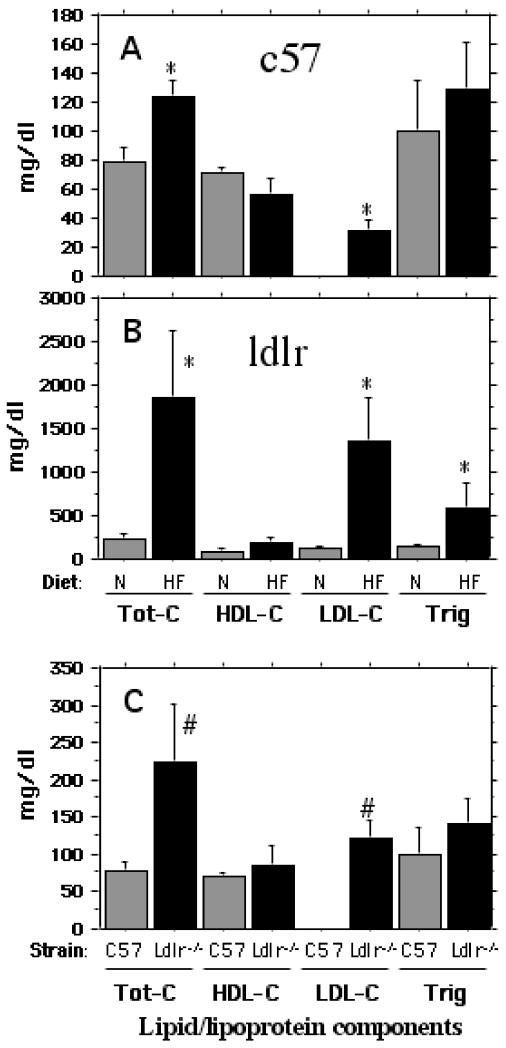
The high fat/high cholesterol diet did not alter weight gain at any point during the two-month period. However, as shown in Fig 1, there were significant increases in plasma levels of total and LDL-associated cholesterol in both C57BL/6 mice (A) and in the LDLR-deficient mice (B). While the high fat diet fed C57BL/6 mice showed an increase of 150% over normal chow-fed controls for total cholesterol, there was a 10-fold increase in the case of the hypercholesterolemic LDLR−/− mice. The excess cholesterol was almost exclusively associated with plasma LDL, the level of which was undetectable in the C57BL/6 mice on a normal diet. There was also a significant increase in plasma level of triglycerides in LDLR-deficient mice. Interestingly, the LDLR−/− mice on a ‘normal’ diet contained significantly higher (over 3-fold) level of cholesterol compared to normally fed C57BL/6 mice (Fig C). Again, this increase was reflected in a high level of LDL cholesterol.
Fig 1.
Plasma cholesterol and triglyceride levels in C57BL/6 and LDLR−/− mice fed a normal chow vs. a high fat/high cholesterol diet. The plasma lipid profiles of different treatment groups were determined in the GCRC facility at MUSC. A. Total cholesterol, HDL-cholesterol, LDL-cholesterol and triglyceride levels of plasma samples from C57BL/6 mice fed either normal chow or a high fat diet. B. Total cholesterol, HDL-cholesterol, LDL-cholesterol and triglyceride levels of plasma from LDLR−/− on either normal chow or the high fat diet. C. A comparison of total cholesterol, HDL-cholesterol, LDL-cholesterol and triglyceride levels of plasma derived from normally fed C57BL/6 and LDLR−/− mice. * p < 0.05 versus the normally fed control group. # represents significant (p < 0.05) difference of normally fed LDLR−/− group from normally fed C57BL/6 (control) group.
The high-fat, high cholesterol diet impairs working memory in normal mice
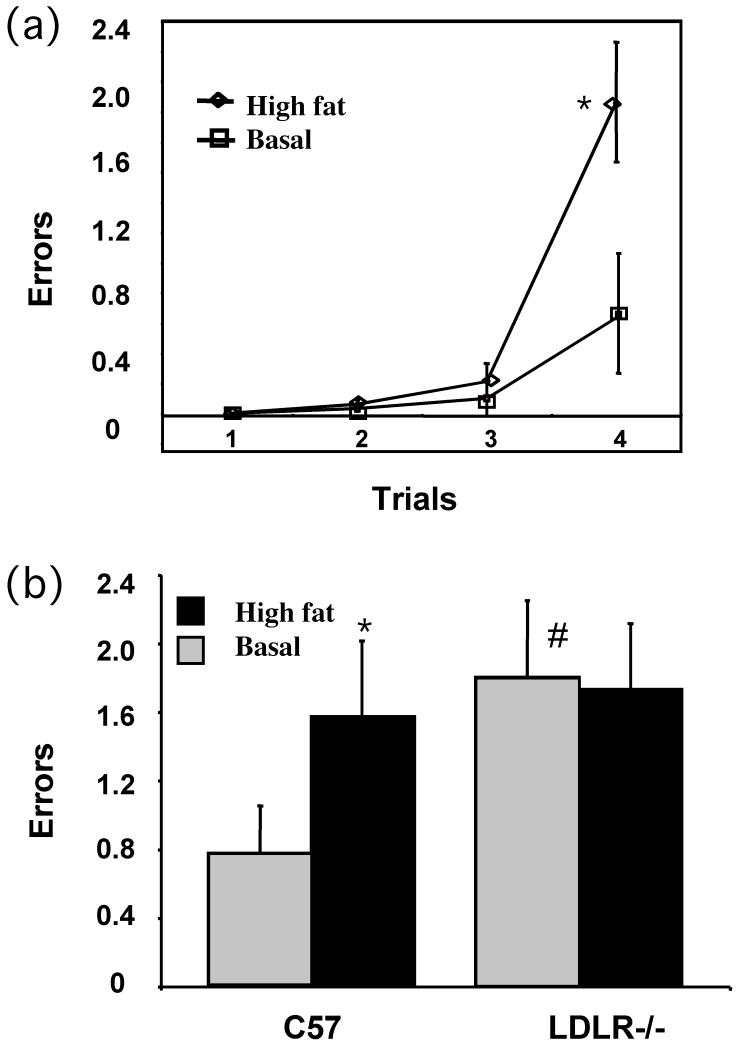
During weeks 7 and 8 of the feeding protocol, groups of C57BL/6 and LDLR−/− mice fed either the normal chow or the high fat/high cholesterol diet for the entire two month period, were cognitively tested on the working and reference memory water radial-arm maze as described under Methods. As shown in Fig 2A, C57BL/6 mice receiving the high fat diet were less able to successfully handle an increasing working memory (WM) load compared to counterparts receiving a control diet. Further, while a high fat diet impaired overall WM incorrect errors for C57BL/6 mice, LDLR−/− mice showed impaired WM regardless of diet (Fig 2B).
Fig 2.
Behavioral analysis of working memory. Mean (± SE) number of working memory incorrect errors for each trial committed on the water radial maze for normally fed and high fat fed C57BL/6 mice were determined as described under Methods. B. Working memory incorrect ± SE for each treatment group i.e., C57BL/6 and LDLR−/− mice fed either normal or high fat diet. * p < 0.05 versus the normally (basal diet) fed control group. # represents significant (p < 0.05) difference of normally fed LDLR−/− group from normally fed C57BL/6 (control) group.
The high-fat, high cholesterol diet induces neuroinflammation characterized by glial activation and cytokine expression
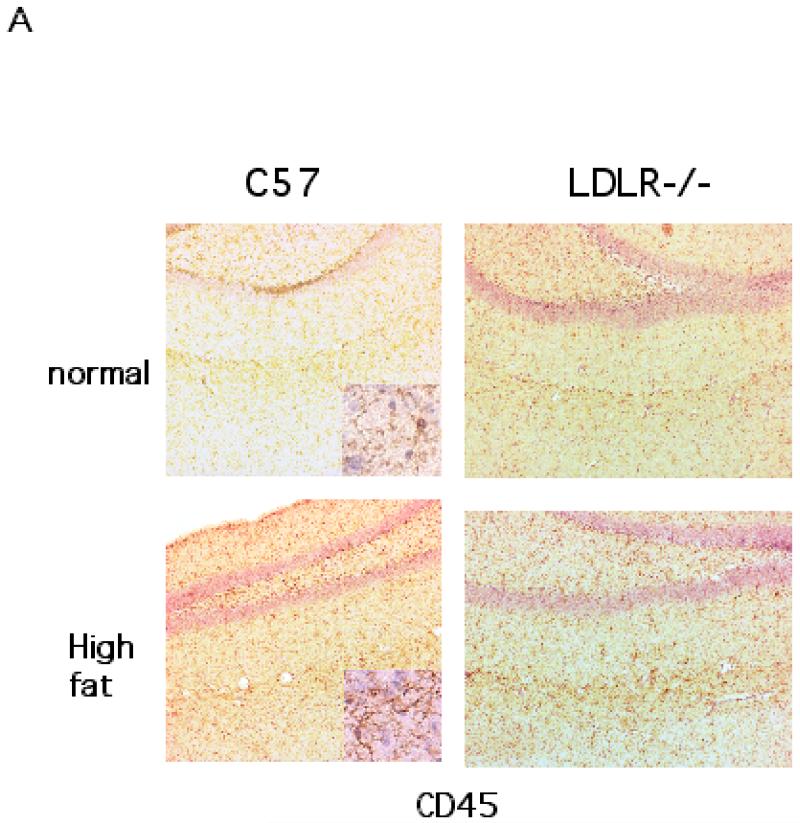
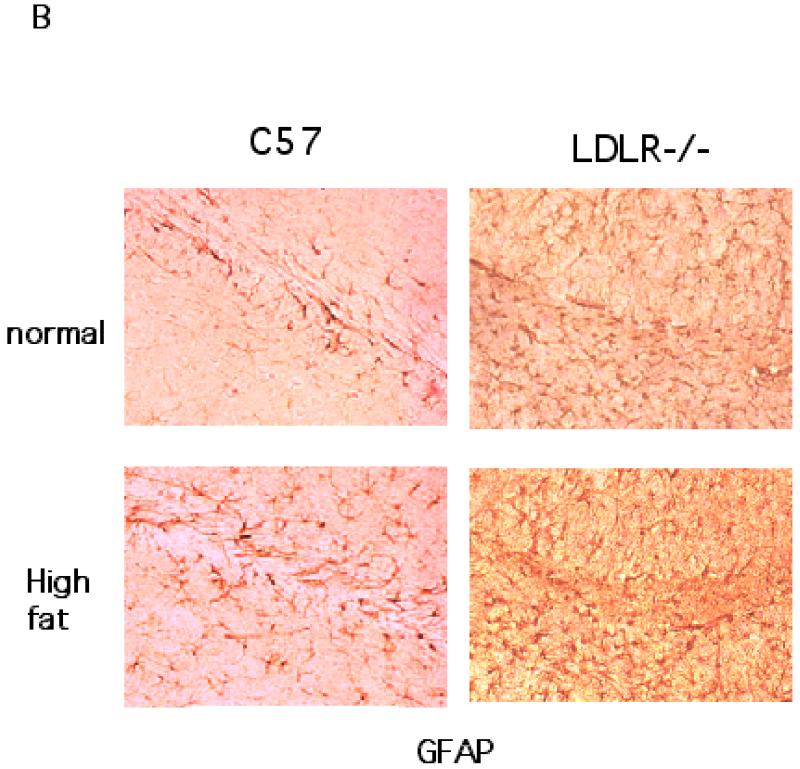
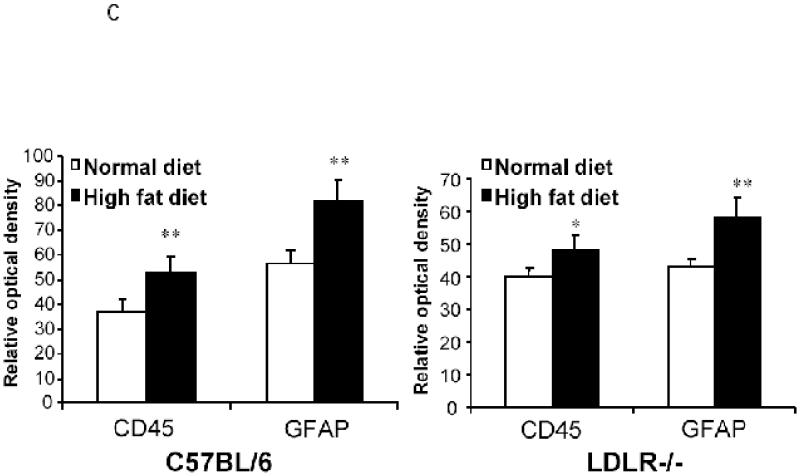
The brain sections obtained from the two groups each of C57BL/6 and LDLR−/− mice fed either a high fat/cholesterol diet or normal chow were processed for immunohistochemistry to detect signs of neuroinflammation. The sections were stained for CD45, a microglial marker and GFAP, a marker for reactive astrocytes. As shown in Fig 3A, the hippocampi from high fat-fed C57BL/6 mice elicited increased immunostaining for CD45 compared to basal diet fed C57BL/6 animals. Sections from LDLR−/− mice seemed to show increased CD45 staining irrespective of the diet indicating that the LDLRKO phenotype may predispose the mice to increased neuroinflammatory response. High fat diet also induced the expression of immunoreactive GFAP, indicating an activation of astrocytes in C57BL/6 mice (Fig 3B). As in the case of CD45, there was an increased basal GFAP-immunoreactivity in the LDLR−/− mice that was further enhanced by high fat/cholesterol diet (Fig 3B). A quantitative analysis of these immunohistochemical data is shown in Fig 3C.
Fig 3.
Immunohistochemical analysis of high fat/cholesterol diet-induced glial activation. A shows CD45 staining of microglia in the hippocampus of C57BL/6 and LDLR−/− mice fed normal chow or the high fat/cholesterol diet. B. GFAP-immunoreactive astrocytes in the hippocampus of C57BL/6 and LDLR−/− mice fed normal chow or the high fat/cholesterol diet. C. Quantitation of CD45 and GFAP stainings of C57BL/6 (left panel) and LDLR−/− (right panel) mice fed normal and high fat diet. The images (4 sections per animal) were captured and quantified using the NIH Image software package. * p < 0.05 versus the normally (basal diet) fed control group. ** p < 0.01 versus the normally (basal diet) fed control group.
Induction of brain expression of proinflammatory cytokines in hypercholesterolemic mice
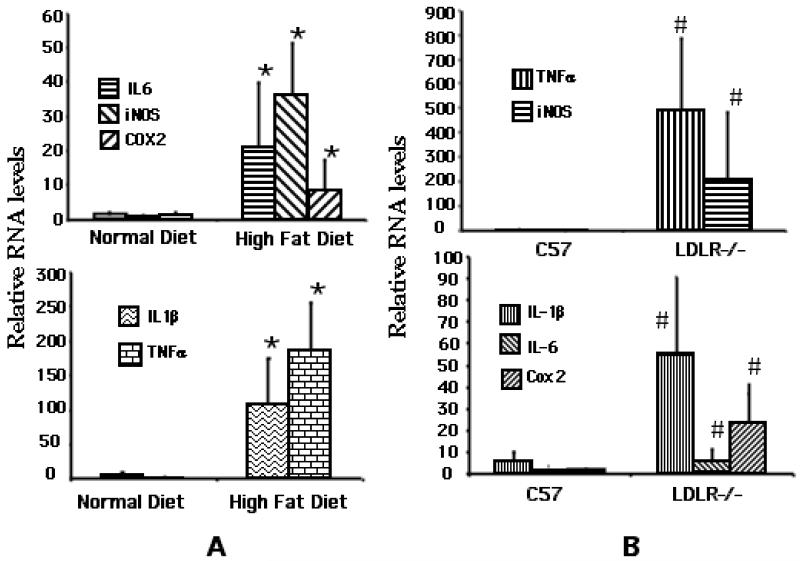
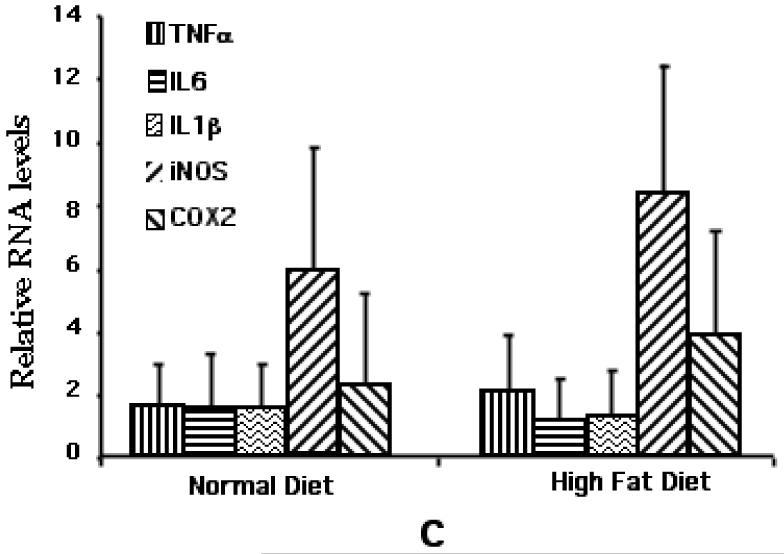
Real-time qPCR was carried out to determine the relative expression levels of the cytokines i.e., IL-1β, IL-6 and TNFα as well as the two proinflammatory enzymes i.e., cycoxygenase (Cox)-2 and inducible nitric oxide synthase (iNOS), in the hippocampi dissected from the C57BL/6 and LDLR−/− fed either normal chow or the high-fat diet for 8 weeks. GAPDH message was used to normalize the values. As shown in Fig 4A, there was a marked increase in the expression of the mediators tested. While the cytokines, TNFα and IL-1β showed increases of between 100-200-fold, there was a 30-fold increase in the expression of IL-6 in high fat fed C57BL/6 mice over normally fed controls. There was also a significant increase in the expression of the two enzymes i.e., iNOS (35-fold) and Cox-2 (6-fold). Interestingly, LDLR−/− mice fed a normal diet had markedly elevated levels of all these mediators relative to normally fed C57BL/6 mice (Fig 4B). Feeding of these mice with the high fat diet did not result in a significant increase in their levels over already elevated basal levels (Fig 4C).
Fig 4.
qPCR analysis of high fat/cholesterol-induced proinflammatory mediator expression. A. Relative levels of cytokine/mediator mRNAs in C57BL/6 mice fed either normal or high fat diet. B. Relative cytokine/mediator mRNA levels in normally fed C57BL/6 and LDLR−/− mice. * p < 0.05 versus the normally fed control group. # represents significant (p < 0.05) difference of normally fed LDLR−/− group from normally fed C57BL/6 (control) group. C. Relative levels of cytokine/mediator mRNAs in LDLR−/− mice fed either normal or high fat diet.
BACE1 expression, APP processing and Aβ generation in response to high fat/cholesterol feeding
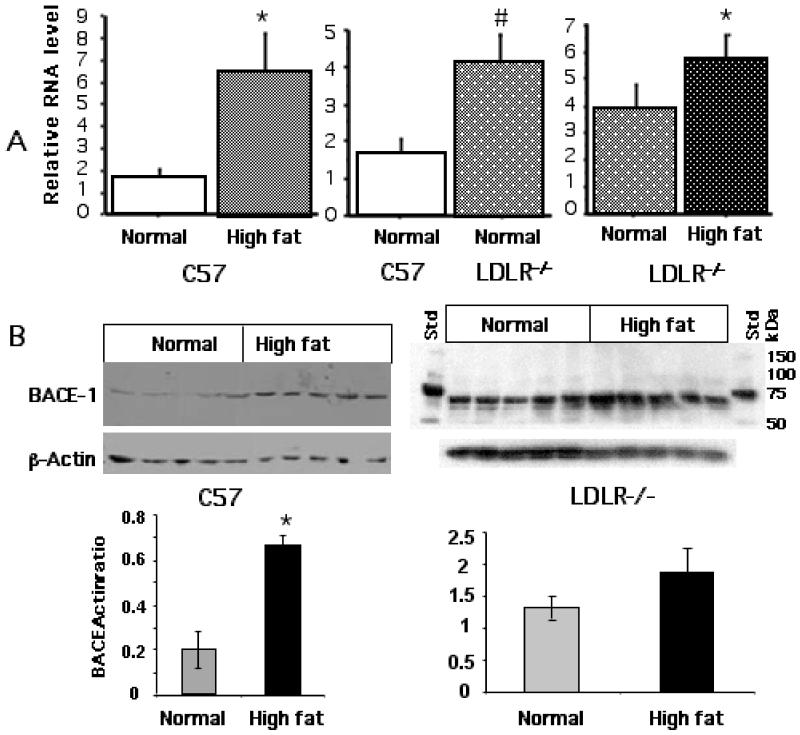
We next analyzed parameters associated with APP processing including the expression of BACE1, a key enzyme involved in this process. Fig 5A shows the qPCR data for the expression of BACE1 mRNA in response to cholesterol-enriched diet. There was over 4-fold increase in the levels of BACE1 message in high fat fed C57BL/6 mice over normally fed mice while the basal level of BACE1 expression in the LDLR−/− mice was about 3-fold higher than the C57BL/6 mice. The LDLR−/− showed a further increase when fed a high cholesterol diet. Fig 5B shows the expression of the enzyme protein as determined by Western blot, again confirming the induction of the enzyme as a result of cholesterol feeding in both C57BL/6 and LDLR−/− mice.
Fig 5.
High fat/cholesterol-induced expression of BACE1. A. Total RNA was isolated from the corteces of C57BL/6 and LDLR−/− mice fed the two diet regimen and analysed by qPCR for BACE1 mRNA. * p < 0.05 versus the normally fed control group. # represents significant (p < 0.05) difference of normally fed LDLR−/− group from normally fed C57BL/6 (control) group. B. Corresponding tissue extracts were analysed by immunoblot for BACE1 protein levels using BACE1-specific antibodies. The line graphs below show the relative BACE1 protein levels.
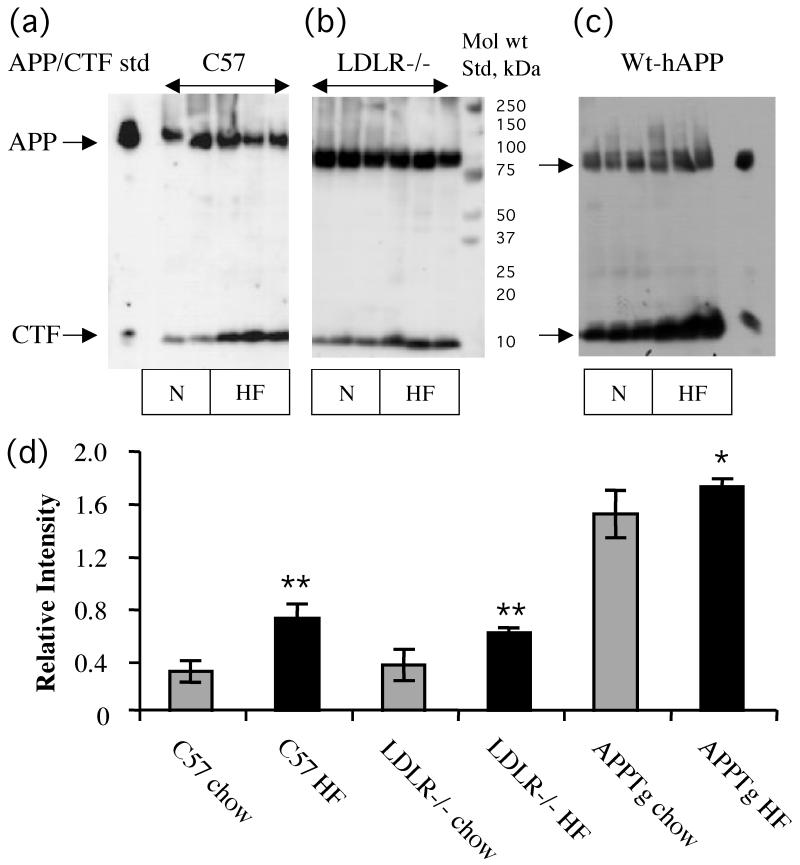
The protein extracts were also used for Western analysis of APP processing using the antibody 0443. Fig 6A,B represent Western blot analysis showing increased levels of CTFs in high cholesterol-fed C57BL/6 and LDLR−/− mice, respectively compared to normal chow-fed counterparts. We also analyzed APP processing products in brain samples derived from wt hAPP mice fed normal chow and high fat diet. As shown in Fig 6C, the increased CTF bands seem to contain both α and β forms in the high fat fed samples. Fig 6D gives quantitative data obtained from a densitometric analysis of the Western blots of all the samples.
Fig 6.
Western blot analysis of APP processing. A. Representative blots of APP/CTFs in tissue samples from C57BL/6, LDLR−/− and APPTg mice fed either a normal chow or the high fat/cholesterol diet. B. Densitometric analysis of CTF relative to APP. All three strains of high fat/cholesterol fed mice showed significantly higher CTF:APP ratio compared to normal chow fed counter part animals.**p<0.01; *P<0.05
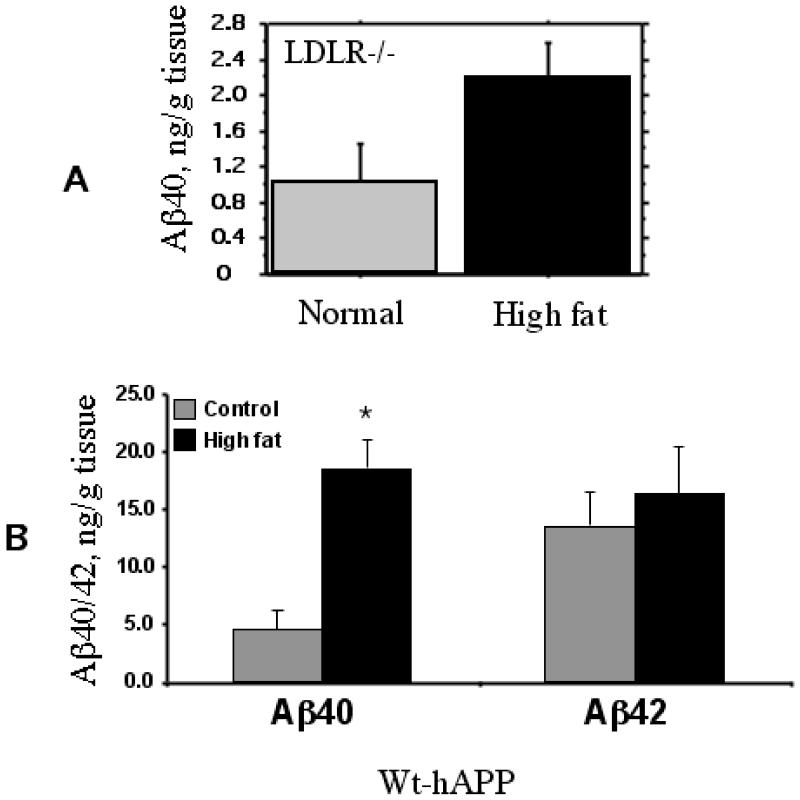
We next determined the changes in the levels of Aβ40 and Aβ42 by ELISA. Despite the observed increases in the production of CTFs (above), the ELISA assay failed to detect significant changes in Aβ levels in high fat fed C57BL/6 mice (data not shown). However, LDLR−/− mice fed a high fat diet had an appreciable increase in the level of Aβ40 (Fig 7A) but not Aβ42 (not shown). Since the transgenic mice expressing human APP produce Aβ that can be easily detected by the available antibodies, we tested the effects of the high cholesterol diet on the production of Aβ in the wt hAPP mice, the brain extracts from which showed higher levels of CTFs, as above (Fig 6C). As shown in Fig 7B, the levels of Aβ were much higher than in the normal mice. Moreover, there was a significant, 3-fold increase in the production of Aβ40 in response to high fat/cholesterol feeding although Aβ42 did not show a further increase over the basal levels.
Fig 7.
Aβ generation in LDLR−/− mice (A) and hAPP tg mice (B) fed either the normal chow or the high fat/cholesterol diet. The tissue extracts were prepared as described under Methods and analysed for Abet species using a sensitive ELISA. The levels of Ab42 were very low in the LDLR−/− mice. The levels of AB40 as well as 42 were very low in the normal C57BL/6 mice. * p < 0.05 versus the normally fed control group.
DISCUSSION
In this study, we have examined the influence of hypercholesterolemia on the cognitive performance in mice in relation to neuroinflammatory changes and APP processing, the two common traits associated with Alzheimer’s dementia. The studies employed normal C57BL/6 mice fed a high fat, high cholesterol diet as well as mice with a targeted disruption of the LDL receptor gene (Ldlr−/−) that develop moderate hypercholesterolemia that can be greatly exacerbated by cholesterol feeding (Ishibashi et al. 1993). We found that both high fat/high cholesterol fed C57BL/6 mice and the LDLR−/− mice experience significant memory deficits in an 8-arm radial water maze test. In the case of LDLR−/− mice the memory deficit occurred regardless of diet most likely due to basally elevated cholesterol levels. However, the findings with the LDLR−/− mice must be viewed with the caveat that although their genetic background is the same as that of the control strain, i.e., C57BL/6 used for comparison, it may not be identical as could have been with the use of littermate controls. In C57BL/6 mice, the atherogenic diet induced a neuroinflammatory response characterized by an activation of glia (i.e., microglia and astrocytes) and increased expression of a number of inflammatory cytokines/mediators. In contrast, the CNS of LDLR−/− mice on a ‘normal’ diet, showed basal activation of glia and an upregulation of the expression of inflammatory markers. Feeding of high fat/cholesterol diet to these mice resulted in an increased glial activation but this effect was not accompanied by a further induction of the expression of inflammatory mediators. The reason for this discrepancy is not clear but may suggest a dissociation of reactive phenotype from mediator expression or may reflect differential transcriptional vs. post-transcriptional mechanisms of marker/mediator expression under the two conditions. Nonetheless, the elevated pro-inflammatory response and behavioral deficits observed in C57BL/6 mice on a high cholesterol diet the LDLR-deficient mice correlated well with the high plasma cholesterol status of these mice. There was also an induction of the expression of BACE1, an enzyme responsible for the amyloidogenic processing of APP. APP processing itself showed changes with an increased production of CTFs in both C57BL/6 and LDLR-deficient mice fed a high cholesterol diet. There was also an appreciable (but not reaching significance with n=6) increase in Aβ-40 but not Aβ-42 levels in hypercholesterolemic LDLR−/− mice and a significant increase in Aβ40 levels in an hAPPwt transgenic strain fed high cholesterol. The main findings of the present studies are summarized in Table 1.
Table 1. Summary of the relative effects of a high fat/high cholesterol diet on biochemical and behavioral changes in C57BL/6 and LDLR−/− mice.
| Genotype | Diet | Plasma cholesterol |
Gliosis | Mediator expression |
BACE-1 expression |
APP processing |
Behavior # errors |
|---|---|---|---|---|---|---|---|
| C57BL/6 | Normal High fat |
Normal Increased |
Minimal Increased |
Minimal Increased |
Low Increased |
Low Increased |
Low Increased |
| LDLR−/− | Normal High fat |
Elevated Very high |
Increased Further increase |
Increased No further increase |
Higher Further increase |
Higher Further increase |
Higher No further increase |
The most significant and novel finding of our studies is the occurrence of diet-induced cognitive impairment in the normal mice. This represents an advance over previous reports indicating cholesterol-induced memory deficits in the transgenic mice expressing human mutant APP (Li et al. 2003). There have also been reports of hypercholesterolemia-induced memory dysfunction in the LDLR−/− mice (Mulder et al. 2004; Buga et al. 2006) similar to our findings and an aggravation of learning deficits in association with amyloid deposition in the APPTg with LDLR-deficiency (Cao et al. 2006). Another important aspect of our findings pertains to neuroinflammatory changes in the hypercholesterolemic mice that show a close correlation with behavioral changes suggesting that cholesterol-induced neuroinflammation may play a causal role in memory dysfunction observed in our model. There is now convincing evidence that inflammatory cascades mainly mediated by activated microglia play a significant role in AD-like changes (Akiyama et al. 2000; Wyss-Coray and Mucke 2002; Heneka and O’Banion 2007). In AD and AD models, a common trigger of inflammation is thought to be an activation of glia by plaque-associated Aβ, and hence, neuroinflammation represents a secondary consequence of AD-associated amyloidogenesis (Hardy and Selkoe 2002). Although elevated membrane cholesterol might induce production of extracellular Aβ (see below) potentially contributing to enhanced microglial activation, the mechanisms underlying cholesterol-induced neuroinflammation may be different and likely involve neurovascular changes. A damaged or dysfunctional cerebrovasculature under an hypercholesterolemic condition may trigger an activation of perivascular microglia involved in barrier and scavenger functions in the brain (Perry et al. 2003). The vascular pathology may also promote systemic macrophage invasion into the brain parenchyma. It is possible that some of the CD45-immunoreactive cells in our analysis might represent invading macrophages. The recruitment of local immune cells to the inflamed brain arterioles could then initiate a cascade of events leading to an altered amyloid processing, neurodegeneration and synaptic/cognitive dysfunction. Although cognitive studies have not been feasible, the rabbit model of hypercholesterolemia does provide supporting evidence for the vascular mechanism of neuroinflammation including increased expression of VCAM1 (Streit and Sparks 1997; Sparks et al. 2000). There is also evidence for an activation of microglia (Streit and Sparks 1997) as well as an occurrence of increased Aβ immunoreactivity (intraneuronal and occasional extracellular) in the hippocampus and cortical regions (Sparks et al. 1994). A recent study by the same group found that microglial activation was restricted to the hippocampus indicating its occurrence independent of increased amyloid production (Xue et al. 2007). Instead, it was thought that high levels of serum cholesterol would induce vascular changes similar to early inflammatory lesions of atherosclerosis but, in addition, would induce BBB permeability and localized neuroinflammatory changes observed. Another study by (Ghribi et al. 2006b) with the rabbit model shows cholesterol-induced Aβ and iron deposition in the cortex, more so than in the hippocampus, in association with BBB disruption. The connection between cerebrovascular (including region-specific) changes and neuroinflammation in our model remains to be further explored. It is possible that an inflamed vasculature might have contributed substantially to the increased levels of the cytokines/mediator observed. In support of the occurrence of vascular inflammation in AD itself, brain microvessels isolated from AD brain do contain high levels of cytokines and chemokines (Grammas et al. 2006) thereby supporting a cerebrovascular contribution to AD pathogenesis via neuroinflammatory processes (Zlokovic 2005). Of related interest, a prominent role played by inflammation in inducing behavioral deficits in a model of cerebral microvascular amyloid has been emphasized in a study using minocycline (Fan et al. 2007). The anti-inflammatory drug was able to correct/reverse cognitive deficits along with reduction in the number of activated microglia and cytokine (i.e., IL-6) levels but without altering the accumulation and distribution of Aβ.
Our results show that in addition to several proinflammatory cytokines, BACE1 expression is induced in hypercholesterolemic mice. This finding is in line with a recent report indicating an upregulation of BACE1 expression in the rabbit model (Ghribi et al. 2006a). It is known that, the regulation of BACE-1 and other processing enzymes can occur not only at the level of their activities but also in the expression of these enzymes, especially, in response to inflammation and oxidative stress. The expression of BACE-1, in particular, is subject to regulation by cytokines such as TNFα, IL-1β and IFNγ (Sastre et al. 2006), oxidants (Tamagno et al. 2005) and hypoxia (Sun et al. 2006). There is also evidence for induced expression and activity of the secretases in the brain of late-onset sporadic AD (Li et al. 2004), perhaps due to the presence of such inducing factors. Such findings suggest that an inflammatory environment can not only have a direct neuro/synaptotoxic effect but also induce APP processing and amyloid generation. Most likely, BACE1 expression in the brains of hypercholesterolemic mice that we observed is the result of cholesterol-induced inflammation and oxidative stress.
An obvious consequence of an upregulation of BACE1 is increased APP processing. However, although our Western blot data do indicate the generation of CTFs, the predominant form accumulating is CTFα and not CTFβ. Our observation of increased APP processing in the LDLR−/− mice contrasts with a recent study by (Elder et al. 2007) who found that a high plasma cholesterol level did not affect brain APP processing. The reason for this discrepancy is unclear but likely relates to different reactivities of the antibodies used for the Western analysis of APP. Increased APP processing activity we observed could be due to cholesterol’s direct influence on the activities of the APP processing enzymes (reviewed in (Sambamurti et al. 2004). Alternatively, cholesterol may affect both β- and γ-secretase activities indirectly via regulation of isoprenoid synthesis (Cole et al. 2005; Zhou et al. 2008). Although it is observed that the brains of AD do not contain increased levels of cholesterol (Lutjohann and von Bergmann 2003), there is enough evidence to indicate functional interactions between cholesterol and APP metabolism including recent studies showing that APPlnd/Swe mice treated with an ACAT inhibitor display decreased Aβ levels (Hutter-Paier et al. 2004) and that APP metabolism itself regulates cholesterol homeostasis via regulation of the expression of LRP1 (Liu et al. 2007). These interactions may play out along altered intra/subcellular distribution, transport and metabolism of cholesterol rather than alterations of the cholesterol content in the central compartment per se. In fact, the studies by (Elder et al. 2007) showed no increase in brain cholesterol levels in the hypercholesterolemic LDLR−/− mice. An alternative explanation for plasma cholesterol affecting brain function may be found in its metabolism to oxysterol derivatives that are able to pass the BBB. According to (Bjorkhem 2006), the flux of 27-OH cholesterol, an oxysterol that correlates with plasma cholesterol levels, into the brain may represent the missing link between hypercholesterolemia and AD. They have found evidence for elevated plasma and brain levels of this oxysterol in AD, potentially affecting amyloidogenesis. Future studies with our model would include an analysis of the levels of oxysterols including 27-hydroxy- and 24-hydroxy cholesterol, a ‘brain-specific’ oxysterol that, via its diffusion out of the brain, functions to maintain cholesterol homeostasis within the brain (Bjorkhem et al. 1998; Bjorkhem et al. 2006). It will also be interesting to investigate effects of cholesterol and a high dietary fat or lipid regimen separately since the latter is also known to significantly impact upon cognition (Grant 1999; Cooper 2003; Winocur and Greenwood 2005).
In conclusion, the present study has demonstrated the adverse effects of high cholesterol on memory performance in a non-FAD mouse model. Although our results demonstrate an important in vivo role for elevated cholesterol levels in the induction of APP processing potentially contributing to AD-like dementia, it is more likely that increased neuroinflammatory changes observed might play a primary role in cognitive dysfunction in response to hypercholesterolemia, a possibility amenable to a direct validation with the use of specific anti-inflammatory agents in future studies.
ACKNOWLEDGMENT
We thank Ms Kari Werness and Jacob Pugh for carrying out the behavioral testing and Ms Hong Zhu for help with immunohistological analysis. The studies were supported by grant # R01NS051575 from NINDS.
Abbreviations
- AD
Alzheimer’s disease
- Aβ
Abeta
- APP
amyloid precursor protein
- BACE1
beta-site APP cleaving enzyme 1
- LDLR
low density lipoprotein receptor
- WM
working memory
REFERENCES
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiol Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimonte HA, Denenberg VH. Sex differences in vicarious trial-and-error behavior during radial arm maze learning. Physiol Behav. 2000;68:495–499. doi: 10.1016/s0031-9384(99)00201-2. [DOI] [PubMed] [Google Scholar]
- Bimonte HA, Hyde LA, Hoplight BJ, Denenberg VH. In two species, females exhibit superior working memory and inferior reference memory on the water radial-arm maze. Physiol Behav. 2000;70:311–317. doi: 10.1016/s0031-9384(00)00259-6. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I. Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain. J Intern Med. 2006;260:493–508. doi: 10.1111/j.1365-2796.2006.01725.x. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Heverin M, Leoni V, Meaney S, Diczfalusy U. Oxysterols and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:43–49. doi: 10.1111/j.1600-0404.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- Bjorkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G, Wahren J. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res. 1998;39:1594–1600. [PubMed] [Google Scholar]
- Bodovitz S, Klein WL. Cholesterol modulates alpha-secretase cleavage of amyloid precursor protein. J Biol Chem. 1996;271:4436–4440. doi: 10.1074/jbc.271.8.4436. [DOI] [PubMed] [Google Scholar]
- Buga GM, Frank JS, Mottino GA, Hendizadeh M, Hakhamian A, Tillisch JH, Reddy ST, Navab M, Anantharamaiah GM, Ignarro LJ, Fogelman AM. D-4F decreases brain arteriole inflammation and improves cognitive performance in LDL receptor-null mice on a Western diet. J Lipid Res. 2006;47:2148–2160. doi: 10.1194/jlr.M600214-JLR200. [DOI] [PubMed] [Google Scholar]
- Cao D, Fukuchi K, Wan H, Kim H, Li L. Lack of LDL receptor aggravates learning deficits and amyloid deposits in Alzheimer transgenic mice. Neurobiol Aging. 2006;27:1632–1643. doi: 10.1016/j.neurobiolaging.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Casserly I, Topol E. Convergence of atherosclerosis and Alzheimer’s disease: inflammation, cholesterol, and misfolded proteins. Lancet. 2004;363:1139–1146. doi: 10.1016/S0140-6736(04)15900-X. [DOI] [PubMed] [Google Scholar]
- Cole SL, Grudzien A, Manhart IO, Kelly BL, Oakley H, Vassar R. Statins cause intracellular accumulation of amyloid precursor protein, beta-secretase-cleaved fragments, and amyloid beta-peptide via an isoprenoid-dependent mechanism. J Biol Chem. 2005;280:18755–18770. doi: 10.1074/jbc.M413895200. [DOI] [PubMed] [Google Scholar]
- Cooper JL. Dietary lipids in the aetiology of Alzheimer’s disease: implications for therapy. Drugs Aging. 2003;20:399–418. doi: 10.2165/00002512-200320060-00001. [DOI] [PubMed] [Google Scholar]
- Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder GA, Cho JY, English DF, Franciosi S, Schmeidler J, Sosa MA, Gasperi RD, Fisher EA, Mathews PM, Haroutunian V, Buxbaum JD. Elevated plasma cholesterol does not affect brain Abeta in mice lacking the low-density lipoprotein receptor. J Neurochem. 2007;102:1220–1231. doi: 10.1111/j.1471-4159.2007.04614.x. [DOI] [PubMed] [Google Scholar]
- Fan R, Xu F, Previti ML, Davis J, Grande AM, Robinson JK, Van Nostrand WE. Minocycline reduces microglial activation and improves behavioral deficits in a transgenic model of cerebral microvascular amyloid. J Neurosci. 2007;27:3057–3063. doi: 10.1523/JNEUROSCI.4371-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frears ER, Stephens DJ, Walters CE, Davies H, Austen BM. The role of cholesterol in the biosynthesis of beta-amyloid. Neuroreport. 1999;10:1699–1705. doi: 10.1097/00001756-199906030-00014. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Larsen B, Schrag M, Herman MM. High cholesterol content in neurons increases BACE, beta-amyloid, and phosphorylated tau levels in rabbit hippocampus. Exp Neurol. 2006a;200:460–467. doi: 10.1016/j.expneurol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Ghribi O, Golovko MY, Larsen B, Schrag M, Murphy EJ. Deposition of iron and beta-amyloid plaques is associated with cortical cellular damage in rabbits fed with long-term cholesterol-enriched diets. J Neurochem. 2006b;99:438–449. doi: 10.1111/j.1471-4159.2006.04079.x. [DOI] [PubMed] [Google Scholar]
- Grammas P, Samany PG, Thirumangalakudi L. Thrombin and inflammatory proteins are elevated in Alzheimer’s disease microvessels: implications for disease pathogenesis. J Alzheimers Dis. 2006;9:51–58. doi: 10.3233/jad-2006-9105. [DOI] [PubMed] [Google Scholar]
- Grant WB. Dietary links to Alzheimer’s disease: 1999 update. J Alzheimers Dis. 1999;1:197–201. doi: 10.3233/jad-1999-14-501. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Heneka MT, O’Banion MK. Inflammatory processes in Alzheimer’s disease. J Neuroimmunol. 2007;184:69–91. doi: 10.1016/j.jneuroim.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Hunter CL, Bimonte HA, Granholm AC. Behavioral comparison of 4 and 6 month-old Ts65Dn mice: age-related impairments in working and reference memory. Behav Brain Res. 2003;138:121–131. doi: 10.1016/s0166-4328(02)00275-9. [DOI] [PubMed] [Google Scholar]
- Hutter-Paier B, Huttunen HJ, Puglielli L, Eckman CB, Kim DY, Hofmeister A, Moir RD, Domnitz SB, Frosch MP, Windisch M, Kovacs DM. The ACAT inhibitor CP-113,818 markedly reduces amyloid pathology in a mouse model of Alzheimer’s disease. Neuron. 2004;44:227–238. doi: 10.1016/j.neuron.2004.08.043. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Hoplight BJ, Denenberg VH. Water version of the radial-arm maze: learning in three inbred strains of mice. Brain Res. 1998;785:236–244. doi: 10.1016/s0006-8993(97)01417-0. [DOI] [PubMed] [Google Scholar]
- Hyde LA, Sherman GF, Denenberg VH. Non-spatial water radial-arm maze learning in mice. Brain Res. 2000;863:151–159. doi: 10.1016/s0006-8993(00)02113-2. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Brown MS, Goldstein JL, Gerard RD, Hammer RE, Herz J. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92:883–893. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE, Okaichi H, Steward O, Goldschmidt RB. On the role of hippocampal connections in the performance of place and cue tasks: comparisons with damage to hippocampus. Behav Neurosci. 1984;98:946–954. doi: 10.1037//0735-7044.98.6.946. [DOI] [PubMed] [Google Scholar]
- Katzov H, Chalmers K, Palmgren J, Andreasen N, Johansson B, Cairns NJ, Gatz M, Wilcock GK, Love S, Pedersen NL, Brookes AJ, Blennow K, Kehoe PG, Prince JA. Genetic variants of ABCA1 modify Alzheimer disease risk and quantitative traits related to beta-amyloid metabolism. Hum Mutat. 2004;23:358–367. doi: 10.1002/humu.20012. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, Green KN, LaFerla FM. Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer’s disease. J Neurosci. 2005;25:8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin-Allerhand JA, Lominska CE, Smith JD. Increased amyloid-levels in APPSWE transgenic mice treated chronically with a physiological high-fat high-cholesterol diet. J Nutr Health Aging. 2002;6:315–319. [PubMed] [Google Scholar]
- Li L, Cao D, Garber DW, Kim H, Fukuchi K. Association of aortic atherosclerosis with cerebral beta-amyloidosis and learning deficits in a mouse model of Alzheimer’s disease. Am J Pathol. 2003;163:2155–2164. doi: 10.1016/s0002-9440(10)63572-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H, Wong P, Price D, Shen Y. Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci U S A. 2004;101:3632–3637. doi: 10.1073/pnas.0205689101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Zerbinatti CV, Zhang J, Hoe HS, Wang B, Cole SL, Herz J, Muglia L, Bu G. Amyloid precursor protein regulates brain apolipoprotein E and cholesterol metabolism through lipoprotein receptor LRP1. Neuron. 2007;56:66–78. doi: 10.1016/j.neuron.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjohann D, von Bergmann K. 24S-hydroxycholesterol: a marker of brain cholesterol metabolism. Pharmacopsychiatry. 2003;36(Suppl 2):S102–106. doi: 10.1055/s-2003-43053. [DOI] [PubMed] [Google Scholar]
- Mulder M, Jansen PJ, Janssen BJ, van de Berg WD, van der Boom H, Havekes LM, de Kloet RE, Ramaekers FC, Blokland A. Low-density lipoprotein receptor-knockout mice display impaired spatial memory associated with a decreased synaptic density in the hippocampus. Neurobiol Dis. 2004;16:212–219. doi: 10.1016/j.nbd.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Perry VH, Newman TA, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Pinnix I, Council JE, Roseberry B, Onstead L, Mallender W, Sucic J, Sambamurti K. Convertases other than furin cleave beta-secretase to its mature form. Faseb J. 2001a;15:1810–1812. doi: 10.1096/fj.00-0891fje. [DOI] [PubMed] [Google Scholar]
- Pinnix I, Musunuru U, Tun H, Sridharan A, Golde T, Eckman C, Ziani-Cherif C, Onstead L, Sambamurti K. A novel gamma -secretase assay based on detection of the putative C-terminal fragment-gamma of amyloid beta protein precursor. J Biol Chem. 2001b;276:481–487. doi: 10.1074/jbc.M005968200. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Tanzi RE, Kovacs DM. Alzheimer’s disease: the cholesterol connection. Nat Neurosci. 2003;6:345–351. doi: 10.1038/nn0403-345. [DOI] [PubMed] [Google Scholar]
- Refolo LM, Malester B, LaFrancois J, Bryant-Thomas T, Wang R, Tint GS, Sambamurti K, Duff K, Pappolla MA. Hypercholesterolemia accelerates the Alzheimer’s amyloid pathology in a transgenic mouse model. Neurobiol Dis. 2000;7:321–331. doi: 10.1006/nbdi.2000.0304. [DOI] [PubMed] [Google Scholar]
- Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387–400. doi: 10.1146/annurev.med.47.1.387. [DOI] [PubMed] [Google Scholar]
- Sambamurti K, Granholm AC, Kindy MS, Bhat NR, Greig NH, Lahiri DK, Mintzer JE. Cholesterol and Alzheimer’s disease: clinical and experimental models suggest interactions of different genetic, dietary and environmental risk factors. Curr Drug Targets. 2004;5:517–528. doi: 10.2174/1389450043345335. [DOI] [PubMed] [Google Scholar]
- Sastre M, Dewachter I, Rossner S, Bogdanovic N, Rosen E, Borghgraef P, Evert BO, Dumitrescu-Ozimek L, Thal DR, Landreth G, Walter J, Klockgether T, van Leuven F, Heneka MT. Nonsteroidal anti-inflammatory drugs repress beta-secretase gene promoter activity by the activation of PPARgamma. Proc Natl Acad Sci U S A. 2006;103:443–448. doi: 10.1073/pnas.0503839103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Keller P, De Strooper B, Beyreuther K, Dotti CG, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc Natl Acad Sci U S A. 1998;95:6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparks DL, Kuo YM, Roher A, Martin T, Lukas RJ. Alterations of Alzheimer’s disease in the cholesterol-fed rabbit, including vascular inflammation. Preliminary observations. Ann N Y Acad Sci. 2000;903:335–344. doi: 10.1111/j.1749-6632.2000.tb06384.x. [DOI] [PubMed] [Google Scholar]
- Sparks DL, Scheff SW, Hunsaker JC, 3rd, Liu H, Landers T, Gross DR. Induction of Alzheimer-like beta-amyloid immunoreactivity in the brains of rabbits with dietary cholesterol. Exp Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med. 2002;8:1211–1217. doi: 10.1038/nm1102-1211. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sparks DL. Activation of microglia in the brains of humans with heart disease and hypercholesterolemic rabbits. J Mol Med. 1997;75:130–138. doi: 10.1007/s001090050097. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Qing H, Zhou W, Dobie F, Cai F, Staufenbiel M, Huang LE, Song W. Hypoxia facilitates Alzheimer’s disease pathogenesis by up-regulating BACE1 gene expression. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0606298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagno E, Parola M, Bardini P, Piccini A, Borghi R, Guglielmotto M, Santoro G, Davit A, Danni O, Smith MA, Perry G, Tabaton M. Beta-site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J Neurochem. 2005;92:628–636. doi: 10.1111/j.1471-4159.2004.02895.x. [DOI] [PubMed] [Google Scholar]
- Winocur G, Greenwood CE. Studies of the effects of high fat diets on cognitive function in a rat model. Neurobiol Aging. 2005;26(Suppl 1):46–49. doi: 10.1016/j.neurobiolaging.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Kellman W, Ruosseau P, Celesia GG, Siegel G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- Wolozin B, Manger J, Bryant R, Cordy J, Green RC, McKee A. Re-assessing the relationship between cholesterol, statins and Alzheimer’s disease. Acta Neurol Scand Suppl. 2006;185:63–70. doi: 10.1111/j.1600-0404.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Xue QS, Sparks DL, Streit WJ. Microglial activation in the hippocampus of hypercholesterolemic rabbits occurs independent of increased amyloid production. J Neuroinflammation. 2007;4:20. doi: 10.1186/1742-2094-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Suram A, Venugopal C, Prakasam A, Lin S, Su Y, Li B, Paul SM, Sambamurti K. Geranylgeranyl pyrophosphate stimulates gamma-secretase to increase the generation of Abeta and APP-CTFgamma. Faseb J. 2008;22:47–54. doi: 10.1096/fj.07-8175com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]