Abstract
The electrodes of a cochlear implant are located far from the surviving neurons of the spiral ganglion, which results in decreased precision of neural activation compared to the normal ear. If the neurons could be induced to extend neurites toward the implant, it might be possible to stimulate more discrete subpopulations of neurons, and to increase the resolution of the device. However, a major barrier to neurite growth toward a cochlear implant is the fluid filling the scala tympani, which separates the neurons from the electrodes. The goal of this study was to evaluate the growth of cochlear neurites in three-dimensional extracellular matrix molecule gels, and to increase biocompatibility by using fibroblasts stably transfected to produce neurotrophin-3 and brain-derived neurotrophic factor. Spiral ganglion explants from neonatal rats were evaluated in cultures. They were exposed to soluble neurotrophins, cells transfected to secrete neurotrophins, and/or collagen gels. We found that cochlear neurites grew readily on collagen surfaces and in three-dimensional collagen gels. Co-culture with cells producing neurotrophin-3 resulted in increased numbers of neurites, and neurites that were longer than when explants were cultured with control fibroblasts stably transfected with green fluorescent protein. Brain-derived neurotrophic factor-producing cells resulted in a more dramatic increase in the number of neurites, but there was no significant effect on neurite length. It is suggested that extracellular matrix molecule gels and cells transfected to produce neurotrophins offer an opportunity to attract spiral ganglion neurites toward a cochlear implant.
Keywords: neural regeneration, peripheral nerve injury, cochlear implant, inner ear, neuron, neurite guidance, neurotrophin, extracellular matrix, collagen gel, grants-supported paper, neuroregeneration
Research Highlights
(1) The neurites of cochlear neurons grew readily from spiral ganglion explants through collagen gels in culture.
(2) Co-culture with fibroblasts producing neurotrophin-3 stimulated the growth of neurites from cochlear neurons, and enhanced their survival, although the fibroblasts were not targeted.
(3) Cells producing brain-derived neurotrophic factor enhanced spiral ganglion neuron survival, but not neurite extension, although the cells were not targeted.
(4) A combination of collagen gels and neurotrophin-secreting cells could enhance spiral ganglion neuron survival and draw neurites toward a cochlear implant.
INTRODUCTION
Cochlear implants, electrical prosthetic devices that stimulate inner ear neurons of individuals who have lost their cochlear sensory cells, restore usable hearing to deaf patients[1,2,3]. Profound hearing loss is associated with withdrawal of dendrites from the sensory epithelium and progressive degeneration of spiral ganglion neurons[4,5]. Therefore, neuronal survival may be a limiting factor in cochlear implant use, especially since there is evidence that the best results are obtained in patients with the highest number of surviving spiral ganglion neurons[6]. Cochlear implant electrodes are placed in the fluid-filled scala tympani of the cochlea, at a significant distance from the spiral ganglion and even from the spiral ganglion dendrites[7]. Stimulation via a cochlear implant electrode pair is therefore likely to activate large numbers of neurons concurrently[8,9,10]. This may decrease the resolution and dynamic range of information transmitted in patients with cochlear implants[8]. The low precision of electrical neural activation, compared to the precise activation that occurs in the normal cochlea, may explain why increasing the number of electrodes on a cochlear implant beyond 8–10 does not improve functionality[11]. However, if cochlear neurons could be induced to extend neurites toward a cochlear implant, it might be possible to stimulate more discrete subpopulations, and to increase the resolution of the device[12,13].
Spiral ganglion neurites grow toward and terminate upon sensory cells even in culture, perhaps because the cells produce neurotrophins[14], which have been shown to stimulate neurite extension from spiral ganglion neurons in vitro[15,16,17,18,19,20,21]. Neurotrophins might thus be used to direct spiral ganglion neurite growth toward a cochlear implant, but with fluid separating the neurons from the electrodes there is no substrate upon which the neurites might grow[22].
The growth of neurites from other types of neurons has been shown to be viable in three-dimensional collagen matrices, which is not surprising since collagen is one of the major components of normal extracellular matrix[23]. Studies suggest that collagen matrices provide a suitable and stable substrate for neurite growth, and may also suppress neuronal apoptosis. Neurons can also develop appropriate polarity and lengthy neurites in collagen gels[24], suggesting the potential for use in neuronal regeneration[25].
Both neurotrophin-3 and brain-derived neurotrophic factor have been shown to improve spiral ganglion neuron survival by up to 4–5-fold, and to affect development in vivo and in vitro[19,21,26,27,28,29,30]. Gene deletion models have implicated brain-derived neurotrophic factor as a major survival factor for vestibular neurons, and for type II spiral ganglion neurons that are responsible for outer hair cell innervation[31,32]. Similarly, neurotrophin-3 has been shown to promote the survival of type I neurons, responsible for inner hair cell innervation[31,33,34].
The goal of this project was to evaluate spiral ganglion neurite growth in a three-dimensional extracellular matrix gel, and the response of spiral ganglion explants to fibroblasts producing neurotrophin-3 or brain-derived neurotrophic factor. Such fibroblasts could serve as a permanent source of neurotrophic factors to direct the growth of spiral ganglion neurites toward a cochlear implant and improve the survival of spiral ganglion neurons. The three-dimensional extracellular matrix gel could serve as a bridge across the fluid-filled scala tympani, which contains a typical extracellular fluid[35], compatible with neuronal survival or spiral ganglion neurites to traverse as they grow toward the cochlear implant.
RESULTS
Growth of spiral ganglion neurites on two-dimensional collagen surfaces
Spiral ganglion explants grown on a two-dimensional collagen surface exhibited a pattern of neurite growth similar to that which we have previously described for growth on fibronectin or laminin[8,15,36,37]. Neurites grew in a radial array from the explant, sometimes exiting the explant in fascicles, but soon separating and then seldom crossing each other (Figure 1). The neurites tended to grow upon non-neuronal cells that also grew out of the explant[8], as can be seen in the figure.
Figure 1.
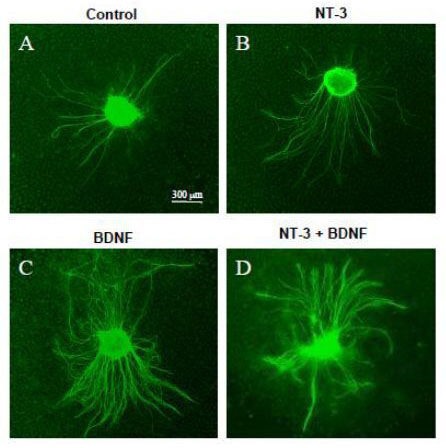
Appearance of spiral ganglion explants grown on a two-dimensional collagen surface, immunostained for neurofilament 200 (FITC).
(A) Untreated control explant; (B) explant exposed to 25 ng/mL exogenous neurotrophin-3 (NT-3); (C) explant exposed to 25 ng/mL exogenous brain-derived neurotrophic factor (BDNF); (D) explant exposed to both 25 ng/mL NT-3 and 25 ng/mL BDNF. In the presence of exogenous NT-3 or BDNF, explants exhibited more neurites than those exposed to control media. The images were obtained on an Olympus IX70 inverted fluorescent microscope.
Growth of neurites on a two-dimensional collagen surface in the presence of exogenous, soluble neurotrophins is illustrated in Figures 1–3. In some respects, the results followed the trend of previously published data regarding the effects of neurotrophins on spiral ganglion neurites grown on other extracellular matrix molecules such as fibronectin or laminin[38,39]. Explants exposed to exogenous neurotrophin-3 (Figure 1B) produced significantly more neurites (Figure 2; P < 0.05) than were seen on control explants (Figure 1A) cultured in the absence of neurotrophins. However, unlike prior reports on laminin or fibronectin, length of neurites grown on collagen with neurotrophin-3 was not significantly longer than that of neurites grown without neurotrophin support (Figure 3; P > 0.05). Consistent with prior data obtained on other extracellular matrix substrates, culture with exogenous brain-derived neurotrophic factor on collagen (Figure 1C) produced significantly more neurites than were observed on control explants (Figure 2; P < 0.001), and also more than observed with neurotrophin-3 (P < 0.001). As observed previously with other extracellular matrix substrates, there was no effect of brain-derived neurotrophic factor on neurite length (Figure 3; P > 0.05). As expected, both neurotrophins were combined (Figure 1D) and significantly enhanced neurite number and length (Figure 1D, Figures 2, 3; P < 0.01 or P < 0.05) when compared to untreated, control explants.
Figure 3.
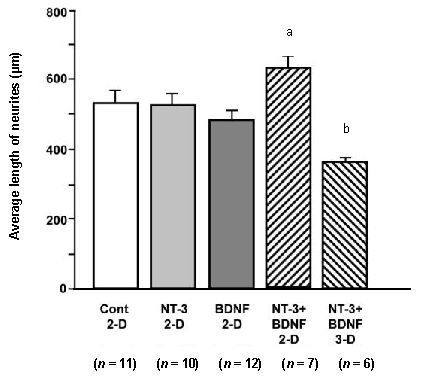
Average length of neurites on spiral ganglion explants exposed to exogenous neurotrophins in solution, either on a two-dimensional (2-D) surface or in a three-dimensional (3-D) collagen gel.
Experimental groups are presented on the X axis. On 2-D collagen, individual neurotrophins had no effect on neurite length. However, neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF) combined significantly enhanced neurite length (aP < 0.05). Also, growth in a 3-D collagen gel significantly inhibited neurite length (bP < 0.01) when compared to any other condition. Data are presented as mean ± SD. The number of explants in each group is provided in parentheses. Data were analyzed by analysis of variance (ANOVA) and Fisher's Protected Least Significant Difference (PLSD) post-hoc test. Cont: Control.
Figure 2.
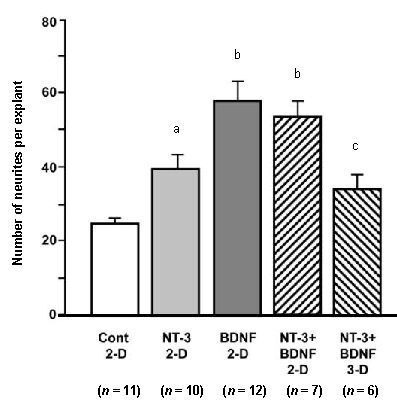
Average number of neurites on spiral ganglion explants exposed to exogenous neurotrophins in solution, either on a two-dimensional (2-D) surface or in a three-dimensional (3-D) collagen gel.
Experimental groups are presented on the X axis. On 2-D collagen, neurotrophin-3 (NT-3; aP < 0.05), brain-derived neurotrophic factor (BDNF; bP < 0.001) or the two neurotrophins combined (bP < 0.001) significantly increased neurite number when compared to media without neurotrophins (Cont). In contrast, in a 3-D gel even with both neurotrophins present, the number of neurites counted was not significantly different from that observed on explants grown on 2-D collagen without neurotrophins. The number of neurites per explant in 3-D gels was also significantly less than that observed on explants grown on 2-D surfaces with both neurotrophins (cP < 0.05). Data are presented as mean ± SD. The number of explants in each group is provided in parentheses. Data were analyzed by analysis of variance (ANOVA) and Fisher's Protected Least Significant Difference (PLSD) post-hoc test.
Growth of spiral ganglion neurites on two-dimensional collagen surfaces with neurotrophin-secreting cells
When co-cultured with control fibroblasts producing green fluorescent protein, the neurites extending from spiral ganglion explants were relatively sparse, and the neurites were thin and unbranched (Figures 4, 5).
Figure 4.
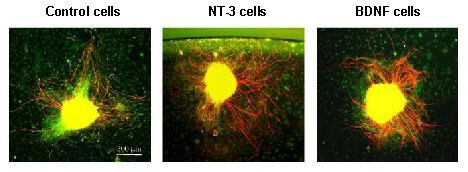
Response of spiral ganglion neurites to cellular sources of neurotrophins.
Explants were immunostained for neurofilament 200 (Texas Red). Non-neuronal cells were visualized with phalloidin (FITC) to label cellular actin to identify any potential relationships between non-neuronal cells and neurites, such as targeting. On a two-dimensional collagen substrate, spiral ganglion explants co-cultured with brain-derived neurotrophic factor (BDNF) secreting cells exhibit enhanced neurite number when compared to explants maintained with control, non-secreting fibroblasts. Neurotrophin-3 (NT-3) secreting cells enhanced neurite length. The images were obtained on an Olympus IX70 inverted fluorescent microscope.
Figure 5.
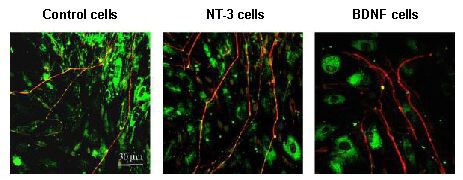
Confocal images of spiral ganglion neurites co-cultured with neurotrophin-producing cells.
As above, neurites were immunostained for neurofilament 200 (Texas Red) and FITC phalloidin was used to visualize actin in non-neuronal cells. On a two-dimensional collagen substrate, spiral ganglion neurites did not terminate on fibroblasts, regardless of whether or not neurotrophins were secreted. However, the neurites appeared thicker in the presence of neurotrophin-producing fibroblasts. The images were obtained on a Zeiss 510 confocal microscope. NT-3: Neurotrophin-3; BDNF: brain-derived neurotrophic factor.
Co-culturing with cells producing neurotrophin-3 resulted in neurites that were longer (P < 0.001) than those of explants co-cultured with the non-neurotrophin-producing cells (Figure 6). However, the number of neurites was not significantly greater (P > 0.05) (Figure 7). The neurites also appeared to be thicker and to exhibit more terminal branches (Figures 4 and 5). When explants were co-cultured with brain-derived neurotrophic factor-producing cells, neurites were not significantly longer in comparison to explants co-cultured with non-neurotrophin-producing cells (P > 0.05) (Figure 6), but there were significantly more neurites (P < 0.001) (Figure 7). As with neurotrophin-3, the neurites tended to be thicker and exhibit more branches (Figures 4, 5). The spiral ganglion neurites exhibited no evidence of termination on fibroblasts, whether or not they produced neurotrophins, nor did neurites appear to grow preferentially on neurotrophin-producing cells when compared to control fibroblasts.
Figure 6.
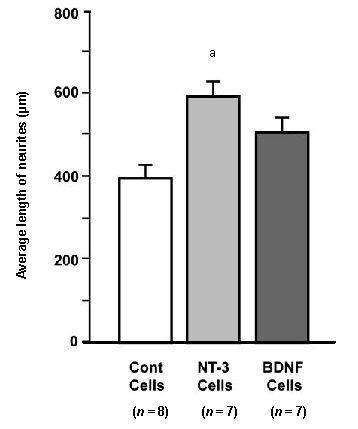
Average length of neurites on spiral ganglion explants co-cultured with neurotrophin-secreting or control (Cont) fibroblasts.
Neurotrophin-3 (NT-3) secreting cells significantly enhanced neurite length (aP < 0.001), while brain-derived neurotrophic factor (BDNF)-secreting cells did not (P > 0.05). The number of explants in each group is provided in parentheses. Data are presented as mean ± SD and analyzed by analysis of variance (ANOVA) and Fisher's Protected Least Significant Difference (PLSD) post-hoc test.
Figure 7.
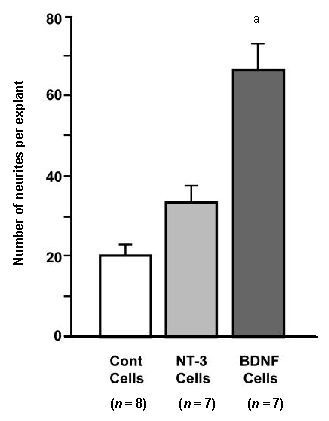
Average number of neurites from spiral ganglion explants co-cultured with neurotrophin-secreting cells.
Neurite numbers were greatly enhanced by brain-derived neurotrophic factor (BDNF)-secreting cells (aP < 0.001), but not by neurotrophin-3 (NT-3)-secreting cells (P > 0.05). The number of explants in each group is provided in parentheses. Data are presented as mean ± SD and analyzed by analysis of variance (ANOVA) and Fisher's Protected Least Significant Difference (PLSD) post-hoc test.
Growth of spiral ganglion neurites in three-dimensional collagen gels
Spiral ganglion neurites stimulated with both brain-derived neurotrophic factor and neurotrophin-3 grew readily in three-dimensional collagen gels (Figure 8). A large number of neurites were observed on the explants. They extended primarily laterally from the explants, but also moved in the vertical dimension, both up and down from the explant. Because of this, the neurites could only be completely visualized by altering the plane of focus vertically around the explant. The number of neurites counted in gels was significantly less than on two-dimensional collagen (P < 0.05) (Figure 2). However, it should be noted that the three-dimensional gel counts could have been an underestimate of true neurite number, since the values were obtained from a single digital image for each explant, and not all neurites were visible and in focus in a single focal plane. With respect to neurite length, when compared to growth on a two-dimensional collagen surface, neurites in a three-dimensional collagen gel were significantly shorter (P < 0.01) (Figure 3). This did not appear to be an artifact caused by neurites exiting the plane of focus, since only neurites that could be traced to their ends were evaluated.
Figure 8.
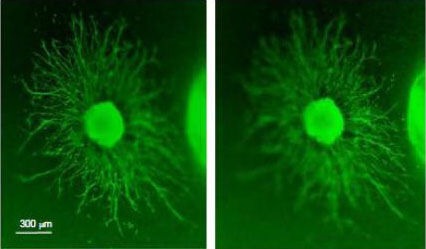
Growth of spiral ganglion neurites in three-dimensional (3-D) plane.
The explant was immunolabeled for neurofilament 200 (FITC). In a collagen gel, neurites grow from a spiral ganglion explant exposed to both neurotrophin-3 and brain-derived neurotrophic factor both in the horizontal and vertical dimensions. The left and right images show two focal planes, in order to illustrate the three-dimensional nature of neurite extension through the gel. The mean length of neurites was shorter than that observed on explants grown on a two-dimensional collagen substrate (Figure 3). The images were obtained on an Olympus IX70 inverted fluorescent microscope.
DISCUSSION
The growth of spiral ganglion neurites on two-dimensional collagen surfaces was similar in many ways to that observed on other extracellular matrix molecules, such as laminin or fibronectin. That is, neurites tended to grow radially from explants, often in fascicles initially but separating within about 100 μm of the explant and then rarely crossing. Branching of neurites was relatively infrequent. Spiral ganglion neurites on two-dimensional collagen responded to soluble, exogenous neurotrophins with increased neurite numbers, especially for brain-derived neurotrophic factor. However, the effects of exogenous neurotrophins on neurite length were distinct. Neurotrophin-3, which usually produces significant increases in spiral ganglion neurite length on other extracellular matrix surfaces, had no effect on collagen. One potential explanation might be a stimulatory effect of two-dimensional collagen alone on spiral ganglion neurite length. The lengths that we observed with collagen alone were greater than 500 μm. This is as long as neurites that we have observed on fibronectin or laminin with neurotrophin-3 treatment[36,38]. Thus, neurite extension could have been saturated even in the control explants. However, the combination of neurotrophin-3 and brain-derived neurotrophic factor did enhance neurite length, indicating that the response was not saturated. An alternative explanation is differential cell signaling. Differences between spiral ganglion fiber responses to neurotrophins on collagen versus those observed on laminin or fibronectin suggest that collagen generates different intracellular signals. This could reflect the stimulation of different integrins. Receptors that consist of a1, a2 or a3 in combination with b1 are the primary receptors for collagen. In contrast, many more integrins respond to laminin and fibronectin[40]. Perhaps the combined intracellular signaling cascades activated by collagen and neurotrophin receptors are quite different from those activated by laminin or fibronectin and neurotrophins[18,39,41,42,43].
The growth of neurites on a collagen substrate was not limited to two dimensions. Neurites grew vigorously in collagen gels, and extended in the vertical as well as the horizontal direction. However, growth was significantly different than on a two-dimensional surface. The fact that neurites appeared to be fewer and were shorter in three-dimensional gels than on a two-dimensional surface likely reflects greater difficulties experienced by neurites in maneuvering through the complex architecture of the gel matrix as compared to a flat surface. Alternatively, access to neurotrophins and nutrients, or removal of metabolic waste, could have been impeded by the same factor. However, these conclusions must be qualified by the limitations of our study. First, we did not culture spiral ganglion explants in three-dimensional gels without neurotrophins. If the number and length of neurites in this condition were lower than that seen on two-dimensional collagen without neurotrophins, the degree of stimulation observed on three-dimensional gels might have been equivalent. Second, we evaluated the explants in three-dimensional gels from two-dimensional images. It may be that three-dimensional reconstructions of explants would have revealed more neurites that were not visible in two-dimensional angles or longer neurites if three-dimensional angles were included. The latter seems unlikely, since neurites that were in focus throughout their lengths in our images also appeared shorter than those on two-dimensional collagen.
As noted above, spiral ganglion neurites responded to neurotrophin stimulation in a manner generally similar to that seen in other studies. This included the observed response of neurites to cells that secreted neurotrophin-3 or brain-derived neurotrophic factor. On the other hand, the neurites did not display significant targeting of the cells, either in terms of approach or termination, as has been observed with fibroblast growth factor-secreting cells[44] or fibroblast growth factor coated beads[45]. This may reflect the rapid diffusion of neurotrophins away from the cells, so that neurites responded to the factors in solution. We have shown that strong neurotrophin gradients maintained by strong fluid flow in microchannels do direct spiral ganglion neurite growth but uniform solutions do not[22,46]. This would not be the case with fibroblast growth factor, which binds readily to extracellular matrix molecules[47,48] and might form a strong concentration gradient around the cells. Of course, it is possible that a different rate of production of neurotrophins might have had a different result. We did not measure the concentration of neurotrophins produced in the culture media by the cells. However, it was clearly above the threshold concentration of each neurotrophin which is required to evoke a neurite response (1–2 ng/mL)[19,21,49], given the observed effects of each neurotrophin-secreting cell type.
It should be noted that out results are relevant primarily for type I spiral ganglion neurons, since these neurons make up 95% of the spiral ganglion[50,51]. We have shown that the neurotrophin dependence of type II neurons is different from that of type I neurons[52]. However, type I neurons are by far the dominant transmitters of auditory information to the brain[53] and thus are the neurons relevant to cochlear implant function[54].
Our results have positive implications for the guidance of spiral ganglion neurites toward a cochlear implant. For example, the growth of neurites in a three-dimensional collagen gel suggests that this or a similar matrix could be used to bridge the fluid gap between the modiolus and the electrodes of an implant. The number of neurites could be influenced by neurotrophin stimulation, since brain-derived neurotrophic factor significantly enhanced the number of neurites that extended from spiral ganglion explants. Similarly, neurotrophin-3 could be added to enhance the length of the extending neurites. The use of cells that stably secrete neurotrophins has the advantage that the factors would be produced for the life of the cells.
Biocompatible scaffolds could readily be applied to the surface of a cochlear implant, since they are much smaller in diameter than the fluid space, the scala tympani, into which they are inserted. While a collagen gel as employed in this study is one possibility, it should be noted that there are many other potential substrates that have been used in other applications, such as nerve repair[55] and tissue reconstruction[56]. Gel scaffolds with engineered microchannels for neurite growth[57,58] could also be employed.
However, our results also suggest limitations to this approach. In order for neurites to interact successfully with a cochlear implant, they would ideally need to exhibit directional growth, and perhaps to terminate on the electrodes. The neurites in our study did not exhibit directional responses to cellular neurotrophin sources, nor did they exhibit termination. Other stimuli might be needed to produce directional and termination signals. For example, repulsive signaling by EphA4[43], micropatterned molecules[22,37] or microchannels[46] could produce directional growth, while fibroblast growth factor-1 could induce termination[44,45].
In conclusion, overall, our results suggest that transduced, non-neuronal cells may be effective as long-term sources of neurotrophic support and for stimulation of neurite extension from cochlear neurons.
In combination, three-dimensional collagen gels and cells transfected to produce neurotrophins offer an opportunity to induce growth of spiral ganglion neurites into the fluid spaces of the cochlear scalae, and thus closer to a cochlear implant. However, additional sources of cellular signals that would induce directional growth of neurites to the implant and their termination on the device remain to be identified.
MATERIALS AND METHODS
Design
An in vitro, controlled experiment.
Time and setting
The experiments described were performed between July 2005 and August 2012 at the Division of Otolaryngology, San Diego Veterans Administration Medical Center and University of California, San Diego, CA, USA.
Materials
All procedures were approved by the local animal committee (Veterans Administration Medical Center, San Diego, CA, USA) in accordance with the guidelines laid down by the National Institute of Health regarding the care and use of animals for experimental procedures. Postnatal day 3–5 Sprague-Dawley rats were used to obtain spiral ganglion explants.
Methods
Animal dissection and tissue culture
Sprague-Dawley rats were sacrificed as described by Van De Water and Ruben with slight modifications[59]. Under sterile conditions, the animal skulls were opened mid-sagitally following cervical transection. After identification of the temporal bone under a dissecting microscope, the membranous labyrinth was exposed by peeling off the bony and cartilaginous cochlear capsule. Following removal of the stria vasularis and the organ of Corti, the spiral ganglion was separated from the modiolus and cut into eight to ten approximately equal portions to serve as explants for culture.
Soluble neurotrophinc factors and brain-derived neurotrophic factor, neurotrophin-3, and green flourescent protein-producing fibroblast cultures
Brain-derived neurotrophic factor (Upstate, Charlottesville, VA, USA), neurotrophin-3 (Upstate) were applied to spiral ganglion explant cultures as soluble factors.
In addition, rat fibroblasts that had been stably transfected with neurotrophin-3, fibroblasts stably transfected with brain-derived neurotrophic factor, or fibroblasts stably transfected with green fluorescent protein, kindly provided by Professor Mark Tuszynski of UCSD[60], were cultured in cell media consisting of 500 mL Dulbecco’s Modified Eagle’s medium (DMEM) with 4.5 g/L of glucose, 10% fetal bovine serum, 1% of stock Pen-strep/L-glutamine mixture, and 1% of stock G418, grown initially in T25 flasks, and then expanded up to T150 flasks and stored at 37°C, 5% CO2 and 95% humidity for harvesting. When fibroblast cell lines were needed, they were lifted from flask surfaces with trypsin. Trypsin activity was then halted by the addition of DMEM. The solution was collected in a 15 mL conical tube and then centrifuged at 1 100 r/min for 3-5 minutes. The precipitate was then separated from the supernatant by decantation, and the pellet was resuspended at the desired cell density for use in two-dimensional culture plates.
Preparation of two-dimensional collagen tissue culture plates
To establish a two-dimensional collagen surface, tissue culture plates (24-well, Falcon, Chino, CA, USA) were incubated with rat tail collagen type 1 at 5 μg/cm2 (BD Biosciences, Bedford, MA, USA) in PBS at 4°C overnight. After coating and two washes with 10 × volume PBS, each well was filled with poly-L-lysine at 1 mg/mL in DMEM for 1 hour at 37°C and then washed using PBS. Spiral ganglion explants (see above) were placed in individual wells containing 170 μL of attachment media containing DMEM with 10% fetal bovine serum, 25 mmol/L Hepes buffer, and 300 U/mL penicillin for 24 hours. This was then replaced with 250 μL of growth medium consisting of DMEM, 25 mmol/L Hepes buffer, 300 U/mL penicillin, 6 mg/mL glucose, and 1% N-2 supplement. For soluble neurotrophin experiments (Figures 1–3), 25 ng/mL neurotrophin-3, 25 ng/mL brain-derived neurotrophic factor or both neurotrophins (each at 25 ng/mL) were added. For experiments with neurotrophin-secreting cells (Figures 4–7), media were mixed in a 15 mL conical tube and then used to resuspend one of the fibroblast pellets generated as described above. The cell suspension was then evenly distributed at 250 μL/well, and a spiral ganglion explant was then placed in each well. This co-culture was then incubated at 37°C, 5% CO2 and 95% humidity for 72 hours.
Preparation of three-dimensional collagen gels
Rat tail collagen type I (BD Biosciences), sterile 10 × PBS, and sterile 1 mol/L NaOH, and a 25 mL conical tube from Fisher were placed on ice. While in a hood, using sterile technique, rat tail collagen was diluted from 3.28 mg/mL to 0.4 mg/mL. 20 mL of DMEM, 0.750 mL of Hepes, 0.165 mL of glucose, 0.150 mL of penicillin, 0.300 mL of N-2 supplement, 0.075 mL of neurotrophin-3 and 0.024 mL of brain-derived neurotrophic factor were first added to the tube, followed by 0.071 9 mL of NaOH. Finally, 0.085 mL of collagen was added to the tube. The solution was mixed by inversion. 500 μL of solution was then pipetted into each well of 24 well Falcon tissue culture plates. This was then kept on ice during the dissection process to prevent gelation, after which a spiral ganglion explant was suspended centrally in each well. The plate was then immediately incubated at 37°C, 5% CO2 and 95% humidity for 72 hours.
Fixation and immunohistochemistry
After 72 hours in culture, the spiral ganglion explants were fixed with 4% paraformaldehyde for 20 minutes at room temperature and then washed with PBS twice. Following permeabilization of the cells with 10% fetal bovine serum/1% Triton X-100 in PBS, the specimens were incubated with 15 μL/mL donkey serum (Sigma, St. Louis, MO, USA) in PBS for 10 minutes to prevent non-specific antibody binding. In order to detect 200 kDa neurofilament, the explants were then incubated with a rabbit polyclonal anti-NF200 antibody (Sigma) at a 1:500 dilution in PBS with 15 μL/mL fetal bovine serum at 4°C for 24 hours. The specimens were labeled with a FITC-or Texas red-conjugated secondary donkey anti-rabbit antibody (Jackson ImmunoResearch, West Grove, PA, USA) for 30 minutes at room temperature. Fibroblasts and other non-neuronal cells that migrated from the explants were visualized with FITC-conjugated phalloidin (Sigma) to label beta-actin. Cultures were visualized on an inverted fluorescence microscope (Olympus IX70, Melville, NY, USA), or for greater details on a confocal miscrocope (Zeiss 510, Thornwood, NY, USA). For three-dimensional cultures in gels, images were obtained in the focal plane that allowed the maximum number of neurites to be observed and analyzed. More than one focal plane was imaged for some explants to illustrate neurite extension in a three-dimensional manner.
Data analysis
Neurite lengths and numbers were evaluated as previously described[38,39]. Briefly, explants were digitally imaged at a standard magnification. The length of extending spiral ganglion neurites was determined by tracing all neurites on each spiral ganglion explant and measuring the length from the edge of the explant to the tip of the neurite using National Institutes of Health Image software (National Institutes of Health, Bethesda, MD, USA). This number was averaged for each explant, and then across all of the explants for a given treatment condition. The number of neurites was determined by counting all traced fibers from each explant. Counts were taken beyond the point of neurite fasciculation that is typically observed close to the explant (Figure 1).
Data were analyzed by analysis of variance (ANOVA) and by Fisher’s Protected Least Significant Difference (PLSD) tests with Bonferroni correction for multiple post-hoc tests, using Statview 5.0 software (Statview, Cary, NC, USA). Probabilities less than 0.05 were accepted as statistically significant.
Acknowledgments:
Fibroblasts stably transfected with neurotrophin-3 or brain-derived neurotrophic factor were generously supplied by Dr. Mark Tuszynski from Department of Neurosciences of the San Diego Veterans Administration Medical Center and the University of California, San Diego, CA, USA.
Footnotes
Joanna Xie, Studying for doctorate.
Funding: This study was supported by grants from the Research Service of the United States Veterans Administration (to Allen Frederic Ryan and Stephen Fausti); the National Institute of Health/National Institute on Deafness and Other Communication Disorders (to Allen Frederic Ryan); the National Institute of Health Summer Research Program (to Joanna Xie); the Deafness Research Foundation (to Lina Mullen); and the National Organization for Hearing Research (to Lina Mullen).
Conflicts of interest: None declared.
Ethical approval: All animal experimental procedures were approved by the Institutional Animal Care and Use Committee of the San Diego Veterans Administration Medical Center (La Jolla, CA, USA), and followed the National Institutes of Health Guidelines for the Ethical Treatment of Animal Subjects.
(Reviewed by Cesca F, Rybak L, van de Water T)
(Edited by Li CH, Song LP)
REFERENCES
- [1].Syms CA, 3rd, House WF. Surgical rehabilitation of deafness. Otolaryngol Clin North Am. 1997;30(5):777–782. [PubMed] [Google Scholar]
- [2].Eshraghi AA, Nazarian R, Telischi FF, et al. The cochlear implant: historical aspects and future prospects. Anat Rec (Hoboken) 2012;295(11):1967–1980. doi: 10.1002/ar.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Leigh J, Dettman S, Dowell R, et al. Communication development in children who receive a cochlear implant by 12 months of age. Otol Neurotol. 2013;34(3):443–450. doi: 10.1097/MAO.0b013e3182814d2c. [DOI] [PubMed] [Google Scholar]
- [4].Schuknecht HF. 2nd ed. Philadelphia: Lea & Febiger; 1993. Pathology of the Ear. [Google Scholar]
- [5].Glueckert R, Pfaller K, Kinnefors A, et al. The human spiral ganglion: new insights into ultrastructure, survival rate and implications for cochlear implants. Audiol Neurotol. 2005;10(5):258–273. doi: 10.1159/000086000. [DOI] [PubMed] [Google Scholar]
- [6].Khan AM, Whiten DM, Nadol JB, Jr, et al. Histopathology of human cochlear implants: correlation of psychophysical and anatomical measures. Hear Res. 2005;205(1-2):83–93. doi: 10.1016/j.heares.2005.03.003. [DOI] [PubMed] [Google Scholar]
- [7].Postnov A, Zarowski A, De Clerck N, et al. High resolution micro-CT scanning as an innovative tool for evaluation of the surgical positioning of cochlear implant electrodes. Acta Otolaryngol. 2006;126(5):467–474. doi: 10.1080/00016480500437377. [DOI] [PubMed] [Google Scholar]
- [8].Brors D, Aletsee C, Schwager K, et al. Interaction of spiral ganglion neuron processes with alloplastic materials in vitro(1) Hear Res. 2002;167(1-2):110–121. doi: 10.1016/s0378-5955(02)00355-6. [DOI] [PubMed] [Google Scholar]
- [9].Shannon RV. Multichannel electrical stimulation of the auditory nerve in man. II. Channel interaction. Hear Res. 1983;12(1):1–16. doi: 10.1016/0378-5955(83)90115-6. [DOI] [PubMed] [Google Scholar]
- [10].Izzo AD, Suh E, Pathria J, Walsh JT, et al. Selectivity of neural stimulation in the auditory system: a comparison of optic and electric stimuli. J Biomed Opt. 2007;12(2):021008. doi: 10.1117/1.2714296. [DOI] [PubMed] [Google Scholar]
- [11].Shannon RV, Zeng FG, Kamath V, et al. Speech recognition with primarily temporal cues. Science. 1995;270(5234):303–304. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- [12].Marzella PL, Clark GM. Growth factors, auditory neurones and cochlear implants: a review. Acta Otolaryngol. 1999;119(4):407–412. doi: 10.1080/00016489950180919. [DOI] [PubMed] [Google Scholar]
- [13].Euteneuer S, Hansen S, Ryan AF. The role of the spiral ganglion neurons in cochlear implants. Today and in future regenerative inner ear treatment. HNO. 2008;56(4):457–460. doi: 10.1007/s00106-008-1709-y. [DOI] [PubMed] [Google Scholar]
- [14].Pirvola U, Ylikoski J, Palgi J, et al. Brain-derived neurotrophic factor and neurotrophin 3 mRNAs in the peripheral target fields of developing inner ear ganglia. Proc Natl Acad Sci U S A. 1992;89(20):9915–9919. doi: 10.1073/pnas.89.20.9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Aletsee C, Brors D, Palacios S, et al. The effects of laminin-1 on spiral ganglion neurons are dependent on the MEK/ERK signaling pathway and are partially independent of Ras. Hear Res. 2002;164(1-2):1–11. doi: 10.1016/s0378-5955(01)00364-1. [DOI] [PubMed] [Google Scholar]
- [16].Greenwood D, Jagger DJ, Huang LC, et al. P2X receptor signaling inhibits BDNF-mediated spiral ganglion neuron development in the neonatal rat cochlea. Development. 2007;134:1407–1417. doi: 10.1242/dev.002279. [DOI] [PubMed] [Google Scholar]
- [17].Brors D, Hansen S, Mlynski R, et al. Spiral ganglion outgrowth and hearing development in p75NTR-deficient mice. Audiol Neurotol. 2008;13:388–395. doi: 10.1159/000148202. [DOI] [PubMed] [Google Scholar]
- [18].Mullen L, Pak K, Chavez E, et al. Ras/P38 and PI3K/Akt, but not Mek/Erk, signaling mediate BDNF-induced neurite formation on neonatal cochlear spiral ganglion explants. Brain Res. 2012;1430:25–34. doi: 10.1016/j.brainres.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jin Y, Kondo K, Ushio M, et al. Developmental changes in the responsiveness of rat spiral ganglion neurons to neurotrophic factors in dissociated culture: differential responses for survival, neuritogenesis and neuronal morphology. Cell Tissue Res. 2013;351(1):15–27. doi: 10.1007/s00441-012-1526-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Euteneuer S, Yang KH, Chavez E, et al. Glial cell line-derived neurotrophic factor (GDNF) induces neuritogenesis in the cochlear spiral ganglion via neural cell adhesion molecule (NCAM) Mol Cell Neurosci. 2013;54C:30–43. doi: 10.1016/j.mcn.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kondo K, Pak K, Chavez E, et al. Developmental changes in responsiveness of rat spiral ganglion neurons to neurotrophins: differential regulation of survival and neuritogenesis in explant culture. Int J Neurosci. doi: 10.3109/00207454.2013.764497. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ryan AF, Wittig J, Evans A, et al. Environmental micro-patterning for the study of spiral ganglion neurite guidance. Audiol Neurootol. 2006;11(2):134–143. doi: 10.1159/000090686. [DOI] [PubMed] [Google Scholar]
- [23].Bergsteinsdottir K, Hashimoto Y, Brennan A, et al. The effect of three dimensional collagen type I preparation on the structural organization of guinea pig enteric ganglia in culture. Exp Cell Res. 1993;209(1):64–75. doi: 10.1006/excr.1993.1286. [DOI] [PubMed] [Google Scholar]
- [24].O’Connor SM, Stenger DA, Shaffer KM, et al. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci Lett. 2001;304(3):189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- [25].O’Shaughnessy TJ, Lin HJ, Ma W. Functional synapse formation among rat cortical neurons grown on three-dimensional collagen gels. Neurosci Lett. 2003;340(3):169–172. doi: 10.1016/s0304-3940(03)00083-1. [DOI] [PubMed] [Google Scholar]
- [26].Marzella PL, Clark GM, Shepherd RK, et al. Synergy between TGF-beta 3 and NT-3 to promote the survival of spiral ganglia neurones in vitro. Neurosci Lett. 1998;240(2):77–80. doi: 10.1016/s0304-3940(97)00928-2. [DOI] [PubMed] [Google Scholar]
- [27].Avila MA, Varela-Nieto I, Romero G, et al. Brain-derived neurotrophic factor and neurotrophin-3 support the survival and neuritogenesis response of developing cochleovestibular ganglion neurons. Dev Biol. 1993;159(1):266–275. doi: 10.1006/dbio.1993.1239. [DOI] [PubMed] [Google Scholar]
- [28].Fukui H, Wong HT, Beyer LA, et al. BDNF gene therapy induces auditory nerve survival and fiber sprouting in deaf Pou4f3 mutant mice. Sci Rep. 2012;2:838. doi: 10.1038/srep00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Staecker H, Liu W, Hartnick C, et al. NT-3 combined with CNTF promotes survival of neurons in modiolus-spiral ganglion explants. Neuroreport. 1995;6(11):1533–1537. doi: 10.1097/00001756-199507310-00017. [DOI] [PubMed] [Google Scholar]
- [30].Staecker H, Kopke R, Malgrange B, et al. NT-3 and/or BDNF therapy prevents loss of auditory neurons following loss of hair cells. Neuroreport. 1996;7(4):889–894. doi: 10.1097/00001756-199603220-00011. [DOI] [PubMed] [Google Scholar]
- [31].Ernfors P, Kucera J, Lee KF, et al. Studies on the physiological role of brain-derived neurotrophic factor and neurotrophin-3 in knockout mice. Int J Dev Biol. 1995;39(5):799–807. [PubMed] [Google Scholar]
- [32].Bianchi LM, Conover JC, Fritzsch B, et al. Degeneration of vestibular neurons in late embryogenesis of both heterozygous and homozygous BDNF null mutant mice. Development. 1996;122(6):1965–1973. doi: 10.1242/dev.122.6.1965. [DOI] [PubMed] [Google Scholar]
- [33].Ernfors P, Lee KF, Jaenisch R. Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature. 1994;368(6467):147–150. doi: 10.1038/368147a0. [DOI] [PubMed] [Google Scholar]
- [34].Fritzsch B, Silos-Santiago II, Bianchi LM, et al. Effects of neurotrophin and neurotrophin receptor disruption on the afferent inner ear innervation. Semin Cell Dev Biol. 1997;8(3):277–284. doi: 10.1006/scdb.1997.0144. [DOI] [PubMed] [Google Scholar]
- [35].Ryan AF, Woolf NK. Energy dispersive x-ray analysis of inner ear fluids and tissues during the ontogeny of cochlear function. Scan Electron Microsc. 1983;(Pt 1):201–7. [PubMed] [Google Scholar]
- [36].Aletsee C, Mullen L, Kim D, et al. The disintegrin kistrin inhibits neurite extension from spiral ganglion explants cultured on laminin. Audiol Neurootol. 2001;6(2):57–65. doi: 10.1159/000046811. [DOI] [PubMed] [Google Scholar]
- [37].Evans AR, Euteneuer S, Chavez E, et al. Laminin and fibronectin modulate inner ear spiral ganglion neurite outgrowth in an in vitro alternate choice assay. Dev Neurobiol. 2007;67(13):1721–1730. doi: 10.1002/dneu.20540. [DOI] [PubMed] [Google Scholar]
- [38].Aletsee C, Beros A, Mullen L, et al. Ras/MEK but not p38 signaling mediates NT-3-induced neurite extension from spiral ganglion neurons. J Assoc Res Otolaryngol. 2001;2(4):377–387. doi: 10.1007/s10162001000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brors D, Bodmer D, Pak K, et al. EphA4 provides repulsive signals to developing cochlear ganglion neurites mediated through ephrin-B2 and -B3. J Comp Neurol. 2003;462(1):90–100. doi: 10.1002/cne.10707. [DOI] [PubMed] [Google Scholar]
- [40].Van der Flier A, Sonnenberg A. Function and interactions of integrins. Cell Tissue Res. 2001;305(3):285–298. doi: 10.1007/s004410100417. [DOI] [PubMed] [Google Scholar]
- [41].Aletsee C, Brors D, Mlynski R, et al. Wortmannin, a specific inhibitor of phosphatidylinositol-3-kinase influences neurotrophin-induced spiral ganglion neurite growth. Laryngorhinootologie. 2002;81(3):189–195. doi: 10.1055/s-2002-25039. [DOI] [PubMed] [Google Scholar]
- [42].Bodmer D, Gloddek B, Ryan AF, et al. Inhibition of the c-Jun N-terminal kinase signaling pathway influences neurite outgrowth of spiral ganglion neurons in vitro. Laryngoscope. 2002;112(11):2057–2061. doi: 10.1097/00005537-200211000-00028. [DOI] [PubMed] [Google Scholar]
- [43].Brors D, Aletsee C, Dazert S, et al. Clostridium difficile toxin B, an inhibitor of the small GTPases Rho, Rac and Cdc42, influences spiral ganglion neurite outgrowth. Acta Otolaryngol. 2003;123(1):20–25. doi: 10.1080/003655402000028055. [DOI] [PubMed] [Google Scholar]
- [44].Dazert S, Kim D, Luo L, et al. Focal delivery of fibroblast growth factor-1 by transfected cells induces spiral ganglion neurite targeting in vitro. J Cell Physiol. 1998;177(1):123–129. doi: 10.1002/(SICI)1097-4652(199810)177:1<123::AID-JCP13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- [45].Aletsee C, Brors D, Mlynski R, et al. Branching of spiral ganglion neurites is induced by focal application of fibroblast growth factor-1. Laryngoscope. 2003;113(5):791–796. doi: 10.1097/00005537-200305000-00005. [DOI] [PubMed] [Google Scholar]
- [46].Wittig JH, Jr, Ryan AF, Asbeck PM. A reusable microfluidic plate with alternate-choice architecture for assessing growth preference in tissue culture. J Neurosci Methods. 2005;144(1):79–89. doi: 10.1016/j.jneumeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- [47].Bashkin P, Doctrow S, Klagsbrun M, et al. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry. 1989;28(4):1737–1743. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- [48].Kan M, Shi EG. Fibronectin, not laminin, mediates heparin-dependent heparin-binding growth factor type I binding to substrata and stimulation of endothelial cell growth. In Vitro Cell Dev Biol. 1990;26(12):1151–1516. doi: 10.1007/BF02623692. [DOI] [PubMed] [Google Scholar]
- [49].Mou K, Hunsberger CL, Cleary JM, et al. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386(4):529–539. [PubMed] [Google Scholar]
- [50].Ryan AF, Schwartz IR. Preferential amino acid uptake identifies Type II spiral ganglion neurons in the gerbil. Hear Res. 1983;9(2):173–184. doi: 10.1016/0378-5955(83)90026-6. [DOI] [PubMed] [Google Scholar]
- [51].Spoendlin H. Neural connections of the outer haircell system. Acta Otolaryngol. 1979;87(3-4):381–387. doi: 10.3109/00016487909126437. [DOI] [PubMed] [Google Scholar]
- [52].Barclay M, Ryan AF, Housley GD. Type I vs type II spiral ganglion neurons exhibit differential survival and neuritogenesis during cochlear development. Neural Dev. doi: 10.1186/1749-8104-6-33. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Jagger DJ, Housley GD. Membrane properties of type II spiral ganglion neurons identified in a neonatal rat cochlear slice. J Physiol. 2003;552(Pt 2):525–533. doi: 10.1113/jphysiol.2003.052589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117(3 Pt 1):220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- [55].Macaya D, Spector M. Injectable hydrogel materials for spinal cord regeneration: a review. Biomed Mater. 2012;7(1):012001. doi: 10.1088/1748-6041/7/1/012001. [DOI] [PubMed] [Google Scholar]
- [56].Chung S, King MW. Design concepts and strategies for tissue engineering scaffolds. Biotechnol Appl Biochem. 2011;58(6):423–438. doi: 10.1002/bab.60. [DOI] [PubMed] [Google Scholar]
- [57].Lynam D, Bednark B, Peterson C, et al. Precision microchannel scaffolds for central and peripheral nervous system repair. J Mater Sci Mater Med. 2011;22(9):2119–2130. doi: 10.1007/s10856-011-4387-3. [DOI] [PubMed] [Google Scholar]
- [58].Gros T, Sakamoto JS, Blesch A, et al. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials. 2010;31(26):6719–6729. doi: 10.1016/j.biomaterials.2010.04.035. [DOI] [PubMed] [Google Scholar]
- [59].Van de Water TR, Ruben RJ. Organ culture of the mammalian inner ear. Acta Otolaryngol. 1971;71(4):303–312. doi: 10.3109/00016487109125368. [DOI] [PubMed] [Google Scholar]
- [60].Nakahara Y, Gage FH, Tuszynski MH. Grafts of fibroblasts genetically modified to secrete NGF, BDNF, NT-3, or basic FGF elicit differential responses in the adult spinal cord. Cell Transplant. 1996;5(2):191–204. doi: 10.1177/096368979600500209. [DOI] [PubMed] [Google Scholar]


