Abstract
Leptin is secreted by adipocytes, the placenta, and the stomach. It not only controls appetite through leptin receptors in the hypothalamus, it also regulates immunity. In the current study, we produced leptin-deficient MRL/Mp-Faslpr mice to investigate the potential role of leptin in autoimmunity. C57BL/6J-ob/ob mice were backcrossed with MRL/Mp-Faslpr mice, which develop human systemic lupus erythematosus (SLE)-like lesions. The effects of leptin deficiency on various SLE-like manifestations were investigated in MRL/Mp-Faslpr mice. The regulatory T cell population in the spleen was analyzed by flow cytometry, and the effects of leptin on regulatory T cells and Th17 cells were evaluated in vitro. Compared with leptin-producing MRL/Mp-Faslpr mice, leptin-deficient MRL/Mp-Faslpr mice showed less marked splenomegaly and a particularly low population of CD3+CD4−CD8−B220+ T cells (lpr cells). Their serum concentrations of Abs to dsDNA were lower, and renal histological changes at age 20 wk were ameliorated. Regulatory T cells were increased in the spleens of leptin-deficient MRL/Mp-Faslpr mice. Leptin suppressed regulatory T cells and enhanced Th17 cells in vitro. In conclusion, blockade of leptin signaling may be of therapeutic benefit in patients with SLE and other autoimmune diseases.
Introduction
Leptin is a product of the obese gene that is mainly secreted by adipocytes (1), with serum leptin concentrations proportional to body mass index. Leptin binds to leptin receptors within the ventromedial hypothalamus (2), where it inhibits the production of neuropeptide, a stimulator of food intake (3), thus decreasing food intake, increasing energy expenditure, and reducing body weight (2).
Nutritional status and immune function are closely related (4). Food deprivation leads to impaired immune responses and an increased incidence of infectious diseases, although the involved mechanisms have not been determined. Adipose tissue plays an important role in energy homeostasis through the storage of triglycerides; however, it was recently shown to secrete several cytokine-like molecules, including leptin, TNF-α, and plasminogen activator inhibitor-1 (5), suggesting that adipose tissue is involved in the regulation of the immune and hematopoietic systems.
Leptin receptors are expressed in peripheral tissues, including the kidneys, lungs, and adrenal glands (6), and several in vitro studies confirmed that leptin acts directly on leptin receptors (7, 8). At least six splice variants of the leptin receptor are known, from Ob-Ra to Ob-Rf. One of these six variants, Ob-Rb, has a long intracellular domain homologous to gp130, a subunit of the IL-6 family of cytokine receptors (9).
Ob-Rb is expressed in fetal liver hematopoietic precursor cells, bone marrow, and peripheral T cells (10, 11), and leptin receptors are expressed in both CD34+ and CD34− cells in adult human bone marrow, suggesting that leptin regulates body weight, as well as modulates the immune system. Indeed, leptin was shown to increase the proliferation of multilineage progenitor hematopoietic stem cells (11), to enhance alloproliferative MLRs, and to enhance cellular immune function in fasted mice (12). In addition, leptin may act as a growth factor for myeloid leukemia (13) and lung cancer (14) cells. Taken together, these findings suggest that leptin serves as a link between nutritional status and immune function.
The murine leptin and leptin-receptor mutants, ob/ob and db/db, respectively, serve as animal models of obesity; these mice develop marked obesity, diabetes, reduced activity, reduced metabolic rate, and infertility due to deficiencies in leptin signaling (15). In contrast to their nontransgenic littermates, leptin-transgenic mice with elevated plasma leptin concentrations lack brown and white adipose tissue, show reduced food intake, and are markedly leaner (16). Diminished cell-mediated immunity and decreased lymphocyte counts were reported in ob/ob and db/db mice (17, 18). In a previous study, (19) we demonstrated that leptin replacement reverses lymphoid atrophy associated with acute starvation and steroid injections in mice and that leptin inhibits lymphocyte apoptosis by upregulating bcl-xL gene expression, enabling the recovery of immune suppression in malnourished mice.
To assess the role of leptin in the development of murine lupus, heterozygous leptin-deficient mice (C57BL/6J-ob/ob) were backcrossed onto the MRL/Mp-Faslpr background. MRL/Mp-Faslpr mice spontaneously develop lesions similar to those observed in human systemic lupus erythematosus (SLE) and are characterized by the production of autoantibodies against self-Ags, hypocomplementemia, and proliferative glomerulonephritis (20). These mice lack Fas protein, which is necessary for apoptosis, and show lymphoproliferation, with accumulation of CD3+CD4−CD8−B220+ T cells (21). Using these mice, we investigated the roles of leptin signaling in lymphoid proliferation, the production of Abs to dsDNA, and renal impairment.
Materials and Methods
Mice and reagents
Female MRL/Mp-Faslpr mice (6 wk old) and male C57BL/6J-ob/+ mice (6 wk old) were purchased from CLEA (Tokyo, Japan). Mice were maintained in a specific pathogen–free facility, under a 12-h light, 12-h dark cycle at 22°C. Recombinant mouse leptin was purchased from R&D Systems (Minneapolis, MN). Experiments and animal care were performed in accordance with the guidelines for animal experimentation of Kanazawa Medical University. Fluorescent-conjugated mAbs and peroxidase-conjugated anti-mouse IgG were purchased from Becton-Dickinson (Franklin Lakes, NJ). PMA, ionomycin, and brefeldin A were purchased from Sigma-Aldrich (St. Louis, MO). Anti-dsDNA Abs and rheumatoid factor (RF) were measured using ELISA kits purchased from Shibayagi (Gunma, Japan). Serum IL-17 concentrations were measured using ELISA kits from R&D Systems.
Backcrossing of C57BL/6J-ob/+ mice with MRL/Mp-Faslpr mice
Female MRL/Mp-Faslpr mice were bred with male C57BL/6J-ob/+ mice to produce F1 offspring heterozygous for lpr (mutant Fas gene) and for ob (mutant leptin gene). These mice were backcrossed with MRL/Mp-Faslpr mice 10 times to change their genetic background from C57BL/6J to MRL/Mp. The resulting mice, termed MRL/Mp-Faslpr-ob/ob mice, were analyzed for the presence of wild-type and mutant Fas and leptin by PCR of tail DNA.
The specific PCR primers for wild-type and mutant Fas and leptin were described (22, 23). Wild-type genomic Fas DNA was amplified using the primers FASI2FX (5′-ACA GCA TAG ATT CCA TTT GCT GCT-3′) and FASI2 REV (5′-TGA GTA ATG GGC TCA GTG CAG CA-3′), yielding a 178-bp product. Mutant Fas gene was amplified using FASI2FX and FASZ8XTR (5′-CAA ATT TTA TTG TTG CGA CAC CA-3′), yielding a 228-bp PCR product. Wild-type genomic leptin DNA was amplified using the primers OB-ComF (5′-CCC TGC CAC TTG CTA AAG CAC C-3′), and OB-WR2 (5′-CCA GCA GAT GGA GGA GGT CTC G-3′), yielding a 230-bp PCR product; and mutant leptin DNA was amplified using OB-ComF and OB-MR2 (5′-CCA GCA GAT GGA GGA GGT CTC A-3′), yielding a 230-bp PCR product.
Serum samples
Mice were bled by retro-orbital puncture under anesthesia, and serum samples were stored at −20°C. Anti-dsDNA Abs, total IgG, RF, and IL-17 were analyzed using ELISA kits, according to the manufacturers’ protocols. To assess renal function, serum creatinine concentrations were measured.
Flow cytometry
All mice were sacrificed at 20 wk of age. Splenocytes (1 × 105) were stained with allophycocyanin-conjugated anti-CD4 mAb, PE-conjugated anti-CD8 mAb, PerCP-conjugated anti-CD3 mAb, FITC-conjugated anti-B220 mAb, and PE-conjugated anti-IgM mAb and analyzed on a FACSCalibur flow cytometer (Becton Dickinson), as described (24).
Proteinuria, histology, and morphometric analysis
Urinary albumin concentrations were measured using mouse albumin ELISA kits (Exocell, Philadelphia, PA) and normalized to urinary creatinine concentrations. For light microscopy, sagittal kidney sections were fixed in 10% formalin neutral buffer solution and embedded in paraffin. Sections (1 μm) were stained with periodic acid-Schiff stain. Glomerular cell number and mesangial area were quantitatively measured with a computer-aided manipulator (KS400; Carl Zeiss Vision, Munich, Germany) by counting the nuclei and analyzing the periodic acid-Schiff–positive area within the glomerular tuft (25). More than 10 glomerular sections, randomly selected in each mouse by scanning from the outer cortex, were examined by investigators blinded to the origin of the slides, after which mean values were calculated. To detect IgG deposits, 5-μm sections were deparaffinized, rehydrated, and incubated with peroxidase-conjugated anti-mouse IgG. Staining was visualized using the chromogenic substrate 3-3′ diaminobenzidine.
Intracellular staining
Naive CD4+CD62L+ T cells were isolated from MRL/Mp-Faslpr mice using CD4+CD62L+ T cell isolation kits (Miltenyi Biotec, Bergisch Gladbach, Germany). The purified T cells were cultured in 24-well plates coated with anti-CD3 and anti-CD28 Abs for 72 h in medium containing 5 ng/ml TGF-β, 20 ng/ml IL-6, and 20 ng/ml IL-23, in the presence or absence of leptin. To detect Foxp3, IL-17, and IFN-γ, the cells were incubated for 4 h at 37°C with 50 ng/ml PMA, 750 ng/ml ionomycin, and 20 μg/ml brefeldin A and stained intracellularly with a mouse Foxp3 buffer set (Becton Dickinson), according to the manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± SD and were compared using the Student t test. Unless otherwise specified, all results are representative of at least five independent experiments. A p value < 0.05 was defined as statistically significant.
Results
MRL/Mp-Faslpr-ob/ob mice
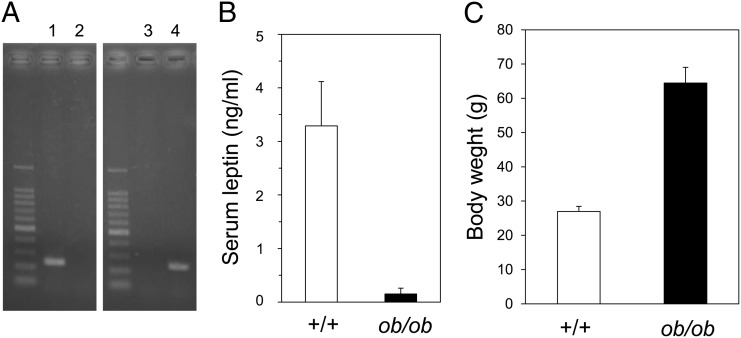
C57BL/6J-ob/+ mice were backcrossed with MRL/Mp-Faslpr mice 10 times to produce leptin-deficient MRL/Mp-Faslpr mice, a strain designated as MRL/Mp-Faslpr-ob/ob. The presence of the wild-type or mutant leptin gene was assessed by PCR amplification of tail DNA (Fig. 1A). Serum leptin was almost undetectable in the MRL/Mp-Faslpr-ob/ob mice (Fig. 1B). Mutant Fas gene (lpr) was also assayed by PCR amplification of tail DNA (data not shown). Body weight gain was much greater in MRL/Mp-Faslpr-ob/ob mice than in control littermates capable of producing leptin (Fig. 1C).
FIGURE 1.
Backcrossing of leptin-deficient mice with MRL/Mp-Faslpr mice. (A) MRL/Mp-Faslpr mice were crossed with C57BL/6J-ob/+ mice to produce offspring heterozygous for ob (mutant leptin gene). After 10 backcrosses with MRL/Mp-Faslpr mice, the mice were intercrossed, and progeny were analyzed for wild-type and mutant leptin genes by PCR amplification of tail DNA. Lanes 1 and 2 show DNA from wild-type (+/+) mice, and lanes 3 and 4 show DNA from leptin mutant (ob/ob) mice. Wild-type primers were used in lanes 1 and 3, and mutant type primers were used in lanes 2 and 4. (B) Serum leptin concentrations in MRL/Mp-Faslpr-ob/ob mice and control littermates, as measured by ELISA. Data are mean ± SEM (n = 10/group). (C) Body weights of 20-wk-old MRL/Mp-Faslpr-ob/ob mice and control littermates. Data are mean ± SEM (n = 10/group).
Splenomegaly and cell populations in MRL/Mp-Faslpr-ob/ob mice
MRL/Mp-Faslpr-ob/ob mice and control littermates were sacrificed at 20 wk of age. Their splenic weights and the overall numbers of splenic lymphocytes, T cells, and B cells were compared. The spleens of MRL/Mp-Faslpr-ob/ob mice weighed significantly less than did those of their control littermates (Table I).MRL/Mp-Faslpr mice showed marked splenomegaly, whereas leptin-deficient mice had smaller spleens. Spleen cell counts were significantly lower in MRL/Mp-Faslpr-ob/ob mice than in their control littermates (Table II). Flow cytometric analysis of splenic lymphocyte subsets showed fewer T and B cells in MRL/Mp-Faslpr-ob/ob mice than in control mice. Although the proportions of CD4+, CD8+, and CD4+CD8+ (double-positive) T cells were similar in leptin-deficient and control mice, the former had a greater proportion of B220+IgM+ cells and a smaller proportion of CD3+CD4−CD8−B220+ cells (lpr cells).
Table I. Spleen weights of 20-wk-old MRL/Mp-Faslpr-ob/ob mice and control littermates.
| Mice | Spleen Weight (g) |
|---|---|
| Control littermates | 0.979 ± 0.396 |
| MRL/Mp-Faslpr-ob/ob | 0.343 ± 0.103a |
Data are mean ± SEM (n = 10/group).
p < 0.05 versus control littermates.
Table II. Spleen cell counts and subsets in 20-wk-old MRL/Mp-Faslpr-ob/ob mice and control littermates.
| Control Littermates (× 106 [%]) | MRL/Mp-Faslpr-ob/ob (× 106 [%]) | |
|---|---|---|
| Total | 1127 ± 460 | 531 ± 250a |
| CD3+CD4+ | 220 ± 96.6 (19.5) | 114 ± 58.6 (21.4)a |
| CD3+CD8+ | 88.6 ± 45.6 (7.86) | 38.4 ± 26.7 (7.22)a |
| CD3+CD4+CD8+ | 13.5 ± 9.19 (1.19) | 6.76 ± 5.61 (1.27)a |
| CD3+CD4−CD8−B220+ | 455 ± 298 (40.3) | 132 ± 90.6 (24.9)a |
| B220+IgM+ | 203 ± 143 (18.0) | 152 ± 90.3 (28.7) |
Data are mean ± SEM (n = 10/group).
p < 0.05 versus control littermates.
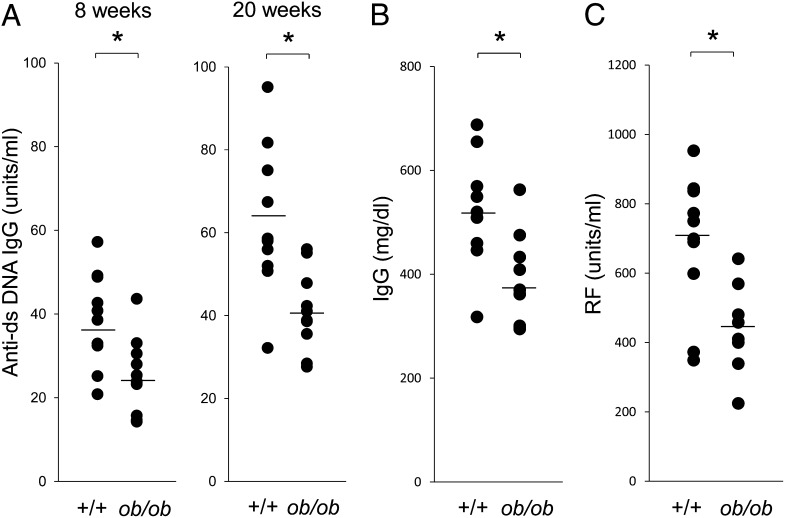
Anti-DNA Abs, total IgG, and RF
The serum concentrations of anti-dsDNA Abs were lower in MRL/Mp-Faslpr-ob/ob mice than in their control littermates (Fig. 2A). Total IgG concentration and RF were also lower in MRL/Mp-Faslpr-ob/ob mice than in the controls (Fig. 2B, 2C). These findings indicated that deficiencies in leptin signaling suppress abnormalities in the immune system and reduce the severity of SLE lesions in MRL/Mp-Faslpr mice.
FIGURE 2.
Suppression of SLE lesions in MRL/Mp-Faslpr mice by deficient leptin signaling. (A) Serum anti-dsDNA Ab concentrations were measured by ELISA. Ab levels were significantly lower in 8- and 20-wk-old MRL/Mp-Faslpr-ob/ob mice than in age-matched control littermates. (B) Serum IgG was measured by ELISA. Total IgG levels were significantly lower in 20-wk-old MRL/Mp-Faslpr-ob/ob mice than in age-matched control littermates. (C) Serum RF was measured by ELISA. RF levels were significantly lower in 20-wk-old MRL/Mp-Faslpr-ob/ob mice than in age-matched control littermates. All data are mean ± SEM (n = 10/group). *p < 0.05.
Renal function and histopathology
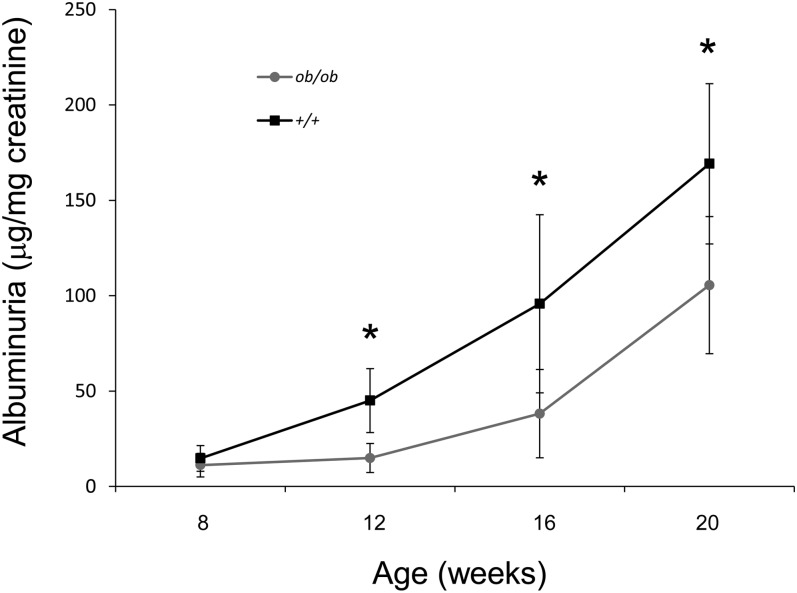
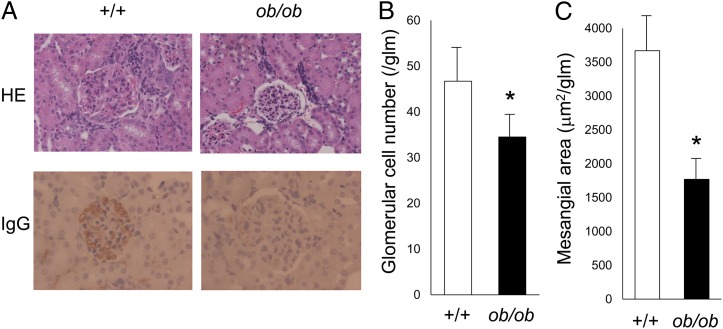
Although the control mice had increased proteinuria by 20 wk of age, urinary protein levels in MRL/Mp-Faslpr-ob/ob mice remained significantly lower throughout the observation period (Fig. 3). To clarify the role of leptin in the development of lupus nephritis, we examined renal histological changes in 20-wk-old MRL/Mp-Faslpr-ob/ob mice and control mice. Control mice showed marked glomerular changes, including glomerular hypercellularity and mesangial expansion, with occasional glomerular crescents and global sclerosis (Fig. 4A). In addition, marked mesangial hypercellularity, proliferation of mesangial matrix, and tubulointerstitial inflammation, as characterized by interstitial leukocyte infiltration and tubular atrophy, were observed. In contrast, MRL/Mp-Faslpr-ob/ob mice exhibited only mild glomerular and tubulointerstitial changes (Fig. 4A). Although control mice showed dense mesangial and capillary deposition of IgG, significantly reduced IgG deposition was observed in MRL/Mp-Faslpr-ob/ob mice (Fig. 4A). Quantitative analysis, using a computer-aided manipulator (KS400; Carl Zeiss Vision), showed that the number of glomerular cells (Fig. 4B) and the sizes of mesangial areas (Fig. 4C) were markedly lower in MRL/Mp-Faslpr-ob/ob mice than in control littermates. These findings indicated that histological changes were milder in MRL/Mp-Faslpr mice with leptin-signaling deficiencies. In contrast, no significant differences in serum creatinine concentrations were observed between 20-wk-old leptin signal–deficient and control mice (data not shown). Skin and joints did not differ significantly in the two strains.
FIGURE 3.
Decreased proteinuria in MRL/Mp-Faslpr mice by deficient leptin signaling. Albuminuria was measured every 4 wk. MRL/Mp-Faslpr-ob/ob mice had significantly reduced albuminuria compared with control littermates. Data are mean ± SEM (n = 10/group). *p < 0.05.
FIGURE 4.
Histological examination of the kidneys of mice with and without deficiencies in leptin signaling. (A) Representative light microscopy images of kidney tissue from 20-wk-old MRL/Mp-Faslpr-ob/ob mice and control littermates. Original magnification ×400. The sections were stained with H&E (upper panels) and Ab to IgG (lower panels). (B) Quantitative analysis of parameters of glomerulonephritis. Glomerular cell number was significantly lower in MRL/Mp-Faslpr-ob/ob mice than in control littermates. Data are mean ± SEM (n = 10/group). (C) Mesangial area within glomerular tufts was significantly lower in MRL/Mp-Faslpr-ob/ob mice than in control littermates. Data are mean ± SEM (n = 10/group). *p < 0.05.
Regulatory T cells in MRL/Mp-Faslpr-ob/ob mice
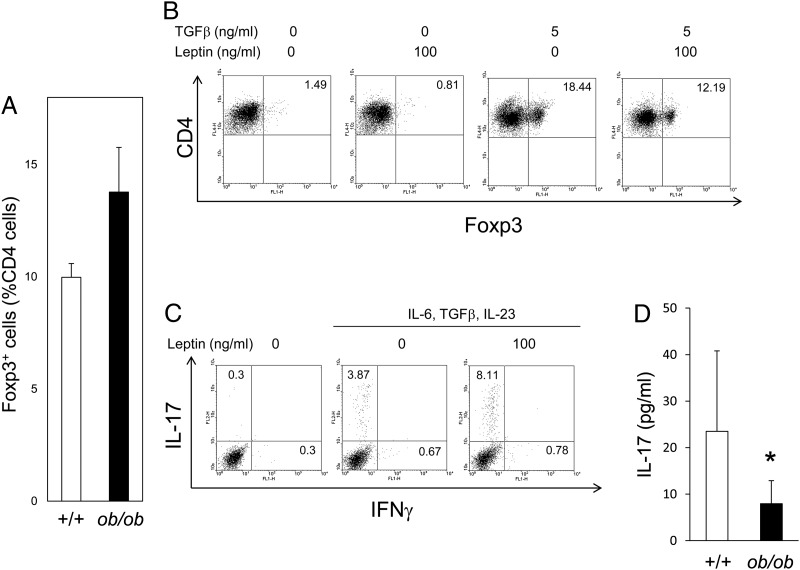
Recent reports suggested that leptin can act as a negative signal for regulatory T cells. Flow cytometry showed that the number of regulatory T cells was substantially higher in the spleens of MRL/Mp-Faslpr-ob/ob mice than in control littermates, suggesting that leptin deficiency enhances regulatory T cell production and improves SLE lesions in MRL/Mp-Faslpr mice (Fig. 5A).
FIGURE 5.
Suppression of regulatory T cells and enhancement of Th17 cells by leptin. (A) The percentage of Foxp3+ regulatory T cells relative to CD4+ T cells was higher in 20-wk-old MRL/Mp-Faslpr-ob/ob mice than in control littermates, as assessed by flow cytometry. Data are mean ± SEM of five independent experiments. (B) Leptin inhibits regulatory T cell expansion in vitro. Purified naive CD4+ T cells from the spleens of MRL/Mp-Faslpr mice were cultured with TGF-β in the presence or absence of leptin for 72 h. Flow cytometric data are representative of five independent experiments. (C) Purified naive CD4+ T cells from the spleens of MRL/Mp-Faslpr mice were cultured in the presence or absence of leptin (100 ng/ml), plus IL-6 (20 ng/ml), TGF-β (5 ng/ml), or IL-23 (20 ng/ml), for 72 h. Flow cytometric data are representative of five independent experiments. (D) Serum concentrations of IL-17 measured by ELISA. IL-17 concentrations were significantly lower in 20-wk-old MRL/Mp-Faslpr-ob/ob mice than in control littermates. Data are mean ± SEM (n = 10/group). *p <0.05.
Leptin suppresses regulatory T cell generation and enhances Th17 cell production from naive T cells in vitro
We purified naive CD4+ T cells from the spleens of MRL/Mp-Faslpr mice and cultured them for 72 h in the presence or absence of TGF-β or leptin. Culture with TGF-β increased the numbers of Foxp3+ regulatory T cells, whereas culture with leptin suppressed regulatory T cell generation and proliferation (Fig. 5B).
Because recent reports suggested that Th17 cells play a role in the pathogenesis of SLE, we examined whether leptin induces Th17 cell production in vitro. Although culture of naive CD4+ T cells with IL-6, TGF-β, and IL-23 induced their differentiation into Th17 cells, the number of these cells was increased >2-fold in the presence, rather than in the absence, of leptin (Fig. 5C). The involvement of leptin in Th17 differentiation in MRL/Mp-Faslpr mice in vivo was assessed by measuring serum concentrations of IL-17. We found that IL-17 concentrations were lower in MRL/Mp-Faslpr-ob/ob mice than in control littermates (Fig. 5C). These results indicated that leptin directly reduces the number of regulatory T cells and increases the number of Th17 cells, providing a mechanism by which leptin deficiency ameliorates SLE lesions in MRL/Mp-Faslpr mice.
Discussion
Although originally found to modulate body weight, leptin recently was recognized as an immune regulator. Ob-Rb is expressed in T and B cells, and leptin has direct effects on lymphocytes. Administration of leptin to fasted mice reversed the impairment of T cell function (12). We previously demonstrated that leptin inhibits stress-induced apoptosis of T cells by binding to leptin receptors and upregulating the expression of Bcl-xL (21). Moreover, leptin stimulated T cell proliferation in vitro, promoted Th1 responses (12), and protected against corticosteroid-induced apoptosis (21). In vitro, leptin was shown to stimulate the production of proinflammatory cytokines by monocytes and macrophages (26).
The pathogenesis of human SLE has been associated with several immunological abnormalities, including the breakdown of tolerance to self-Ags, resulting in the production of autoantibodies that react with multiple self-Ags. Ag-driven, T cell–dependent selective B cell proliferation was observed in lupus-prone MRL/Mp-Faslpr mice, indicating that both T and B cells are necessary for the development of lupus (27). However, the mechanisms by which autoreactive T cells persist remain unknown.
Normally, autoreactive T cells are eliminated by functional inactivation (anergy) and activation-induced cell death, or apoptosis, through death receptor (Fas) signaling. The antiapoptotic effects of leptin may result in the generation of autoreactive T cells, thus leading to the development of autoimmune diseases. Blockade of leptin signaling may facilitate the apoptosis of autoreactive lymphocytes and ameliorate autoimmune diseases.
Higher serum leptin concentrations were observed in patients with SLE (28), rheumatoid arthritis (29), and Behçet’s disease (30) than in healthy controls. Therefore, leptin may play a role in the pathogenesis of inflammatory rheumatic diseases and weight changes associated with disease activity.
We observed decreased numbers of lymphocytes in leptin-mutant MRL/Mp-Faslpr mice, a finding consistent with studies on the antiapoptotic effects of leptin. These mice showed a marked reduction in CD3+CD4−CD8−B220+ T cells (lpr cells), suggesting that leptin signal blockade may reverse the immunological abnormalities observed in MRL/Mp-Faslpr mice. Interestingly, our results are consistent with previous findings, showing that double-negative T cells are expanded in SLE and produce IL-17, contributing to the pathogenesis of kidney damage in patients with SLE (31, 32). Moreover, leptin-deficient mice have reduced numbers of circulating and bone marrow B cells.
We also observed that the concentrations of Abs to dsDNA were reduced, and the development of glomerulonephritis decreased in 20-wk-old leptin-mutant MRL/Mp-Faslpr mice compared with their controls, suggesting the importance of leptin in the pathogenesis of SLE-like lesions in MRL/Mp-Faslpr mice. However, the survival of the two strains did not differ significantly, despite the suppression of lupus-like lesions in MRL/Mp-Faslpr-ob/ob mice, perhaps because the latter are extremely fatty and overweight. However, the survival of these mice may be prolonged by food restriction to avoid extreme overweight.
Leptin has a broad range of immunological properties. In addition to being antiapoptotic, leptin favors Th1 over Th2 immune responses, modulates phagocyte function (16), and activates NK function (33). Defective leptin signaling may increase the number of regulatory T cells. Fasting-induced hypoleptinemia in (NZB × NZW)F1 mice induced the proliferation of regulatory T cells, an effect reversed by leptin supplementation (34). Moreover, leptin-deficient low-density lipoprotein receptor–knockout (ldlr–) mice showed enhanced Foxp3 expression and reduced atherosclerotic lesion formation (35). The mechanism by which leptin negatively affects regulatory T cells has not been determined, although our results, showing increased numbers of regulatory T cells in MRL/Mp-Faslpr-ob/ob mice, are consistent with previous findings (36).
Leptin was reported to promote Th17 cell responses (37) and to exacerbate collagen-induced arthritis (38). We found that leptin enhanced the in vitro generation of Th17 cells from naive CD4+ T cells of MRL/Mp-Faslpr mice. SLE patients were recently reported to have increased numbers of IL-17–producing CD4+ T cells in their peripheral blood, with cell number correlating with disease activity (39, 40). Furthermore, the levels of expression of CCR4 and CCR6, receptor molecules associated with Th17 cells, were higher in SLE patients than in healthy controls (41). In agreement with findings in human SLE, IL-17–producing T cells are increased in MRL/Mp-Faslpr mice (32). Although the mechanisms underlying Th17 cell increases in SLE, as well as the role of IL-17 in the pathogenesis of SLE, remain unknown, Th17 cells are likely to be involved in the pathogenesis of SLE. Therefore, further investigations of the role of leptin in the development of autoimmune diseases are warranted.
In summary, we showed that leptin signal–deficient MRL/Mp-Faslpr mice are strongly protected from the development of SLE lesions. Although the mechanisms by which this occurs remain to be determined, our findings suggest that blockade of leptin signaling confers therapeutic benefits in the treatment of autoimmune diseases.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan.
- RF
- rheumatoid factor
- SLE
- systemic lupus erythematosus.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. 1994. Positional cloning of the mouse obese gene and its human homologue. Nature 372: 425–432. [DOI] [PubMed] [Google Scholar]
- 2.Satoh N., Ogawa Y., Katsuura G., Tsuji T., Masuzaki H., Hiraoka J., Okazaki T., Tamaki M., Hayase M., Yoshimasa Y., et al. 1997. Pathophysiological significance of the obese gene product, leptin, in ventromedial hypothalamus (VMH)-lesioned rats: evidence for loss of its satiety effect in VMH-lesioned rats. Endocrinology 138: 947–954. [DOI] [PubMed] [Google Scholar]
- 3.Stephens T. W., Basinski M., Bristow P. K., Bue-Valleskey J. M., Burgett S. G., Craft L., Hale J., Hoffmann J., Hsiung H. M., Kriauciunas A., et al. 1995. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377: 530–532. [DOI] [PubMed] [Google Scholar]
- 4.Chandra R. K. 1997. Nutrition and the immune system: an introduction. Am. J. Clin. Nutr. 66: 460S–463S. [DOI] [PubMed] [Google Scholar]
- 5.Shimomura I., Funahashi T., Takahashi M., Maeda K., Kotani K., Nakamura T., Yamashita S., Miura M., Fukuda Y., Takemura K., et al. 1996. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat. Med. 2: 800–803. [DOI] [PubMed] [Google Scholar]
- 6.Hoggard N., Mercer J. G., Rayner D. V., Moar K., Trayhurn P., Williams L. M. 1997. Localization of leptin receptor mRNA splice variants in murine peripheral tissues by RT-PCR and in situ hybridization. Biochem. Biophys. Res. Commun. 232: 383–387. [DOI] [PubMed] [Google Scholar]
- 7.Sivitz W. I., Walsh S. A., Morgan D. A., Thomas M. J., Haynes W. G. 1997. Effects of leptin on insulin sensitivity in normal rats. Endocrinology 138: 3395–3401. [DOI] [PubMed] [Google Scholar]
- 8.Bai Y., Zhang S., Kim K. S., Lee J. K., Kim K. H. 1996. Obese gene expression alters the ability of 30A5 preadipocytes to respond to lipogenic hormones. J. Biol. Chem. 271: 13939–13942. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita T., Murakami T., Otani S., Kuwajima M., Shima K. 1998. Leptin receptor signal transduction: OBRa and OBRb of fa type. Biochem. Biophys. Res. Commun. 246: 752–759. [DOI] [PubMed] [Google Scholar]
- 10.Cioffi J. A., Shafer A. W., Zupancic T. J., Smith-Gbur J., Mikhail A., Platika D., Snodgrass H. R. 1996. Novel B219/OB receptor isoforms: possible role of leptin in hematopoiesis and reproduction. Nat. Med. 2: 585–589. [DOI] [PubMed] [Google Scholar]
- 11.Bennett B. D., Solar G. P., Yuan J. Q., Mathias J., Thomas G. R., Matthews W. 1996. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 6: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 12.Lord G. M., Matarese G., Howard J. K., Baker R. J., Bloom S. R., Lechler R. I. 1998. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature 394: 897–901. [DOI] [PubMed] [Google Scholar]
- 13.Konopleva M., Mikhail A., Estrov Z., Zhao S., Harris D., Sanchez-Williams G., Kornblau S. M., Dong J., Kliche K. O., Jiang S., et al. 1999. Expression and function of leptin receptor isoforms in myeloid leukemia and myelodysplastic syndromes: proliferative and anti-apoptotic activities. Blood 93: 1668–1676. [PubMed] [Google Scholar]
- 14.Tsuchiya T., Shimizu H., Horie T., Mori M. 1999. Expression of leptin receptor in lung: leptin as a growth factor. Eur. J. Pharmacol. 365: 273–279. [DOI] [PubMed] [Google Scholar]
- 15.Coleman D. L. 1978. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14: 141–148. [DOI] [PubMed] [Google Scholar]
- 16.Ogawa Y., Masuzaki H., Hosoda K., Aizawa-Abe M., Suga J., Suda M., Ebihara K., Iwai H., Matsuoka N., Satoh N., et al. 1999. Increased glucose metabolism and insulin sensitivity in transgenic skinny mice overexpressing leptin. Diabetes 48: 1822–1829. [DOI] [PubMed] [Google Scholar]
- 17.Chandra R. K. 1980. Cell-mediated immunity in genetically obese C57BL/6J ob/ob) mice. Am. J. Clin. Nutr. 33: 13–16. [DOI] [PubMed] [Google Scholar]
- 18.Mandel M. A., Mahmoud A. A. 1978. Impairment of cell-mediated immunity in mutation diabetic mice (db/db). J. Immunol. 120: 1375–1377. [PubMed] [Google Scholar]
- 19.Fujita Y., Murakami M., Ogawa Y., Masuzaki H., Tanaka M., Ozaki S., Nakao K., Mimori T. 2002. Leptin inhibits stress-induced apoptosis of T lymphocytes. Clin. Exp. Immunol. 128: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fujii T., Okada M., Mimori T., Craft J. 2002. The transmembrane form of TNF-alpha drives autoantibody production in the absence of CD154: studies using MRL/Mp-Fas(lpr) mice. Clin. Exp. Immunol. 130: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujii T., Okada M., Fujita Y., Sato T., Tanaka M., Usui T., Umehara H., Mimori T. 2009. Vaccination with autoreactive CD4(+)Th1 clones in lupus-prone MRL/Mp-Fas(lpr/lpr) mice. J. Autoimmun. 33: 125–134. [DOI] [PubMed] [Google Scholar]
- 22.Ma J., Xu J., Madaio M. P., Peng Q., Zhang J., Grewal I. S., Flavell R. A., Craft J. 1996. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J. Immunol. 157: 417–426. [PubMed] [Google Scholar]
- 23.Namae M., Mori Y., Yasuda K., Kadowaki T., Kanazawa Y., Komeda K. 1998. New method for genotyping the mouse Lep(ob) mutation, using a polymerase chain reaction assay. Lab. Anim. Sci. 48: 103–104. [PubMed] [Google Scholar]
- 24.Murakami M., Tsubata T., Okamoto M., Shimizu A., Kumagai S., Imura H., Honjo T. 1992. Antigen-induced apoptotic death of Ly-1 B cells responsible for autoimmune disease in transgenic mice. Nature 357: 77–80. [DOI] [PubMed] [Google Scholar]
- 25.Suganami T., Mukoyama M., Sugawara A., Mori K., Nagae T., Kasahara M., Yahata K., Makino H., Fujinaga Y., Ogawa Y., et al. 2001. Overexpression of brain natriuretic peptide in mice ameliorates immune-mediated renal injury. J. Am. Soc. Nephrol. 12: 2652–2663. [DOI] [PubMed] [Google Scholar]
- 26.Zarkesh-Esfahani H., Pockley G., Metcalfe R. A., Bidlingmaier M., Wu Z., Ajami A., Weetman A. P., Strasburger C. J., Ross R. J. 2001. High-dose leptin activates human leukocytes via receptor expression on monocytes. J. Immunol. 167: 4593–4599. [DOI] [PubMed] [Google Scholar]
- 27.Seaman W. E., Wofsy D., Greenspan J. S., Ledbetter J. A. 1983. Treatment of autoimmune MRL/Ipr mice with monoclonal antibody to Thy-1.2: a single injection has sustained effects on lymphoproliferation and renal disease. J. Immunol. 130: 1713–1718. [PubMed] [Google Scholar]
- 28.Garcia-Gonzalez A., Gonzalez-Lopez L., Valera-Gonzalez I. C., Cardona-Muñoz E. G., Salazar-Paramo M., González-Ortiz M., Martínez-Abundis E., Gamez-Nava J. I. 2002. Serum leptin levels in women with systemic lupus erythematosus. Rheumatol. Int. 22: 138–141. [DOI] [PubMed] [Google Scholar]
- 29.Anders H. J., Rihl M., Heufelder A., Loch O., Schattenkirchner M. 1999. Leptin serum levels are not correlated with disease activity in patients with rheumatoid arthritis. Metabolism 48: 745–748. [DOI] [PubMed] [Google Scholar]
- 30.Evereklioglu C., Inalöz H. S., Kirtak N., Doganay S., Bülbül M., Ozerol E., Er H., Ozbek E. 2002. Serum leptin concentration is increased in patients with Behçet’s syndrome and is correlated with disease activity. Br. J. Dermatol. 147: 331–336. [DOI] [PubMed] [Google Scholar]
- 31.Crispín J. C., Oukka M., Bayliss G., Cohen R. A., Van Beek C. A., Stillman I. E., Kyttaris V. C., Juang Y. T., Tsokos G. C. 2008. Expanded double negative T cells in patients with systemic lupus erythematosus produce IL-17 and infiltrate the kidneys. J. Immunol. 181: 8761–8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Z., Kyttaris V. C., Tsokos G. C. 2009. The role of IL-23/IL-17 axis in lupus nephritis. J. Immunol. 183: 3160–3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tian Z., Sun R., Wei H., Gao B. 2002. Impaired natural killer (NK) cell activity in leptin receptor deficient mice: leptin as a critical regulator in NK cell development and activation. Biochem. Biophys. Res. Commun. 298: 297–302. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Yu Y., Matarese G., La Cava A. 2012. Cutting edge: fasting-induced hypoleptinemia expands functional regulatory T cells in systemic lupus erythematosus. J. Immunol. 188: 2070–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taleb S., Herbin O., Ait-Oufella H., Verreth W., Gourdy P., Barateau V., Merval R., Esposito B., Clément K., Holvoet P., et al. 2007. Defective leptin/leptin receptor signaling improves regulatory T cell immune response and protects mice from atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27: 2691–2698. [DOI] [PubMed] [Google Scholar]
- 36.De Rosa V., Procaccini C., Calì G., Pirozzi G., Fontana S., Zappacosta S., La Cava A., Matarese G. 2007. A key role of leptin in the control of regulatory T cell proliferation. Immunity 26: 241–255. [DOI] [PubMed] [Google Scholar]
- 37.Wang S., Baidoo S. E., Liu Y., Zhu C., Tian J., Ma J., Tong J., Chen J., Tang X., Xu H., Lu L. 2013. T cell-derived leptin contributes to increased frequency of T helper type 17 cells in female patients with Hashimoto’s thyroiditis. Clin. Exp. Immunol. 171: 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng J., Liu Y., Yang M., Wang S., Zhang M., Wang X., Ko K. H., Hua Z., Sun L., Cao X., Lu L. 2012. Leptin exacerbates collagen-induced arthritis via enhancement of Th17 cell response. Arthritis Rheum. 64: 3564–3573. [DOI] [PubMed] [Google Scholar]
- 39.Chen X. Q., Yu Y. C., Deng H. H., Sun J. Z., Dai Z., Wu Y. W., Yang M. 2010. Plasma IL-17A is increased in new-onset SLE patients and associated with disease activity. J. Clin. Immunol. 30: 221–225. [DOI] [PubMed] [Google Scholar]
- 40.Wong C. K., Lit L. C., Tam L. S., Li E. K., Wong P. T., Lam C. W. 2008. Hyperproduction of IL-23 and IL-17 in patients with systemic lupus erythematosus: implications for Th17-mediated inflammation in auto-immunity. Clin. Immunol. 127: 385–393. [DOI] [PubMed] [Google Scholar]
- 41.Shah K., Lee W. W., Lee S. H., Kim S. H., Kang S. W., Craft J., Kang I. 2010. Dysregulated balance of Th17 and Th1 cells in systemic lupus erythematosus. Arthritis Res. Ther. 12: R53. [DOI] [PMC free article] [PubMed] [Google Scholar]