Abstract
Developmental changes in responsiveness of rat spiral ganglion neurons (SGNs) to neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF) were examined using an explant culture system. Spiral ganglion (SG) explants at embryonic Day 18 (E18), postnatal Day 0 (P0), P5, P10 and P20 were cultured with the addition of either NT-3 or BDNF at various concentrations (0.1–100 ng/ml) and analyzed the dose-response characteristics of three parameters: SGN survival, the number of neurites emanating from the explants and the length of neurite extension. In E18 cultures, SGN survival and neurite number were enhanced more strongly by NT-3 than by the BDNF. As the explants became more mature, the effects of NT-3 decreased, whereas those of BDNF increased, peaking at P0. Although the intrinsic capacity of SGNs to produce and extend neurites declined considerably by P20, they still retained the capacity to respond to both NT-3 and BDNF. These temporal patterns in responsiveness of SGNs to neurotrophins correspond well to the expression pattern of the two neurotrophins in cochlear sensory epithelium in vivo and also correlate with the time course of developmental events in SGNs such as cell death and the establishment of mature hair cell innervation patterns.
Keywords: NT-3, BDNF, age-related, neurite growth, neurite extension
Introduction
Spiral ganglion neurons (SGNs) are primary afferent bipolar neurons, with a peripheral dendrite receiving synaptic input from hair cells in organ of Corti, and a central axon projecting to the cochlear nucleus. During development, immature SGNs derived from the otic placode extend neurites toward the presumptive sensory epithelium and brainstem, then establish functional connections [1,2]. Initially, overproduced SGNs are then eliminated by programmed cell death [3-5]. The surviving neurons undergo further maturational processes including remodeling of the dendritic projec tion to hair cells [6-10].
Studies of the developing auditory system have shown that the neurotrophic factors neurotrophin-3 (NT-3) and brain-derived neurotrophic factor (BDNF) are essential for the development of innervation in the inner ear [see 2,11,12 for reviews], including the response properties of SGNs [e.g. 13,14]. In various peripheral nervous systems, it is known that neuronal responses to neurotrophins can be age dependent. For example, trigeminal neuron survival dependence switches from BDNF and NT-3 to nerve growth factor (NGF) during the early stages of target field innervation [15]. Similarly, some dorsal root ganglion (DRG) neurons depend on NGF in the embryonic stage and switch to GDNF in early postnatal life [16]. These data indicate that neurotrophic factors can support different events in different developmental stages. Although some studies have addressed this issue in avian auditory and vestibular end organs [17,18], the extent to which neurotrophin effects in the mammalian inner ear vary across age remains unclear. To address this, we performed a systematic study of the effects of NT-3 and BDNF on rat SGNs, from the late embryonic stage through maturation, in explant cultures.
To address this, we performed a systematic study of the effects of NT-3 and BDNF on rat SGNs, from the late embryonic stage through maturation, in explant cultures.
Materials and methods
Animal dissection and explant culture
All animal procedures were approved by the local animal subjects committee (San Diego VA Medical Center) in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Explant cultures of SGNs were prepared from 95 Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) at embryonic Day 18 (E18; the day the positive vaginal plug was considered as E0), postnatal Day 0 (P0, the day of the birth), P5, P10 and P20. The embryonic animals were obtained from timed pregnant rats anesthetized with an intraperitoneal injection of rodent cocktail (ketamine 12.5 mg/ml, xylazine 1.26 mg/ml, acepromazine 0.25 mg/ml; 0.4 ml/100g body weight). After decapitation of the pups and embryos, the mandible was removed and skulls were opened midsagitally. The brain was removed and the temporal bones were harvested and transferred to Petri dishes containing sterile phosphate buffered saline (PBS; pH 7.4). The membranous labyrinth was exposed by peeling off the bony or cartilaginous cochlear capsule. The spiral ligament, stria vascularis and organ of Corti were removed and then the spiral ganglion (SG) was separated from the modiolus using fine forceps. The SG of P10 and P20 were covered with a thin bony layer. Since the bone inhibits the SG from efficient attachment to the bottom of the well, special attention was paid to removing bony fragments as completely as possible in this procedure. The procedure used for dissection allowed for harvesting of the entire SG, from base to apex. The ganglion was then divided into eight explants of approximately equal size, which ensured that here was no sampling bias toward any part of the ganglion.
Each SG explant was placed on the center of one well of a 48-well tissue culture plate that had been precoated overnight at 4 °C with 10 μg/ml fibronectin from human plasma (Sigma, St. Louis, MO, USA) and with 20 μg/ml poly-L-lysine (Sigma, St. Louis, MO, USA) for 1 hour at 37 °C. To promote attachment of the tissue to the bottom of the wells, the explants were incubated overnight at 37 °C with 5% CO2, in 80 μl of primary growth media consisting of Dulbecco’s modified Eagle’s medium (DMEM; Sigma, St. Louis, MO, USA), 10% fetal bovine serum (FBS) (Invitrogen Life Technologies, Grand Island, NY, USA), 10-mM HEPES buffer (Invitrogen Life Technologies, Grand Island, NY, USA) and 300 U/ml penicillin (Sigma, St. Louis, MO, USA). The explants were then cultured in 200 μl of maintenance medium consisting of DMEM, 10 μl/ml N2 supplement (Invitrogen Life Technologies, Grand Island, NY, USA), 10-mM HEPES buffer, 300 U/ml penicillin and glucose (to achieve a final concentration of 6 g/l; Sigma, St. Louis, MO, USA) for an additional 72 hours. To compare the effects of neurotrophins, the cultures of each age were divided into 15 groups consisting of 8-12 explants each, and supplemented with either recombinant human NT-3 (EMD Biosciences, La Jolla, CA, USA) or recombinant human BDNF (Upstate Biotechnology, Lake Placid, NY, USA) at concentrations ranging from 0.1 to 100 ng/ml, with one group cultured without neurotrophin to serve as an untreated control, for a total of 75 groups across ages.
Immunohistochemistry
Immunostaining was performed using the avidin–biotin complex (ABC) method (Vectastain Elite kit; Vector Laboratories, Burlingame, CA, USA). SG explants were fixed by immersion in 4% paraformaldehyde in 0.1-M phosphate buffer for 30 minutes at room temperature (RT). After washes with PBS, endogeneous peroxidase activity was blocked by treatment with 0.3% hydrogen peroxide in methanol for 30 minutes at RT. The tissues were then incubated with PBS containing 1% glycine and blocking solution (PBS containing 4% FBS and 0.2% Triton X-100) for 30 minutes each at RT to reduce nonspecific antibody binding. The explants were then incubated with anti-neurofilament (NF) 200 mouse monoclonal antibody (1:500 in blocking solution; Sigma, St. Louis, MO, USA) or anti-active caspase-3 rabbit polyclonal antibody (1:1000 in blocking solution; Abcam, Cambridge, MA, USA) at 4 °C overnight. After three washes in PBS, the tissues were then incubated with biotinylated horse anti-mouse IgG or goat anti-rabbit IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA) corresponding to the primary antibody for 1 hour at RT. After three washes in PBS, the tissues were then reacted with ABC solution (Vector Laboratories, Burlingame, CA, USA) for 30 minutes at RT, following the manufacturers’ instruction. Immunoreactivity was visualized by diaminobenzidine (DAB; Vector Laboratories, Burlingame, CA, USA) reaction. After washing in distilled water, the tissues were coated with crystal mount (Fisher Scientific, Tustin, CA, USA) and air dried for several days. The primary antibody was omitted from the procedure as a negative control, which showed no labeling.
RNA isolation and polymerase chain reaction (PCR)
The SG tissue from E18, P0, P5, P10 and P20 animals were harvested in the same manner, as described above for explant culture, and immediately immersed and kept in RNA later (Qiagen, Valencia, CA, USA). Total RNA was isolated using a guanidine thiocyanate/phenol/ chloroform-based extraction procedure (TRIzol Reagent; Invitrogen Life Technologies, Grand Island, NY, USA) according to the company’s instructions. Reverse transcription was performed using Superscript First-Strand cDNA synthesis system for reverse transcription-polymerase chain reaction (RT-PCR) (Invitrogen Life Technologies, Grand Island, NY, USA) using oligo (dT) primers. Subsequent PCR amplification was performed using the Platinum Taq DNA Polymerase kit (Invitrogen Life Technologies, Grand Island, NY, USA) in a 50-μl solution, with 3 μl of the reverse-transcribed complementary DNA (cDNA), 1 × PCR buffer, 1.0-U Taq DNA polymerase, 200-nM dNTPs, 1.5-mM MgCl2, 4% DMSO and 200-nM primers. Primer sequences for TrkB were sense: 5′-AGT CCA GAC ACT CAG GAT TTG TAC-3′; antisense: 5′-CTC CGT GTG ATT GGT AAC ATG-3′. Primer sequences for TrkC were sense: 5′-CAA CCA TGG CAT CAC TAC ACC-3′; antisense: 5′-TAA GAG GCT TGG AAT GTC CG-3′. Primer sequences for p75 were sense: 5′-GCA TAA GCC TGA AGC CAA CAC G-3′; antisense: 5′-CCC ACT CAT TCC AAC AGC AAG C-3′. Primer sequences for beta-actin were sense: 5′-GCT CGT CGT CGA CAA CGG CTC-3′; antisense: 5′-CAA ACA TGA TCT GGG TCA TCT TCT C-3′. The predicted fragment lengths of the PCR products were 522 base pairs (bp) for TrkB, 365 bp for TrkC, 583 bp for p75 and 353 bp for actin, respectively. The PCR reaction was performed on cDNA for 35 cycles at 94 °C for 15 seconds, 56 °C for 30 seconds and 72 °C for 1 minute with a final extension at 72 °C for 5 minutes. The omission of reverse transcriptase during reverse transcription served as a negative control to confirm the absence of genomic DNA contamination. Eight microliters of the reaction product were analyzed by agarose gel electrophoresis with ethidium bromide staining.
Image presentation and data analysis
Images of the immunostained explants were captured by a Spot digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) attached to an inverted microscope (Olympus IX70) under brightfield illumination. Digital images were then assembled into panels using Adobe Photoshop 6.0 software (Adobe Systems Incorporated, San Jose, CA, USA). The images were not modified except for the minor adjustment of the brightness and contrast.
We used three parameters for evaluation of the effects of neurotrophins on SGNs: number of surviving neurons in each explant, number of neurites emanating from each explant and the length of neurite extension. We also evaluated the neurites visually for differences in other aspects of growth behavior.
To evaluate the SGN survival and the neurite number, each explant after immunostaining was examined under an inverted microscope. The number of surviving neurons and the number of neurites were counted manually by adjusting the focus, if necessary, to assess the full depth of the explant. Our criteria of identifying surviving neurons were based on their immunostained morphology: (1) positivity for NF200 and (2) visible nucleus. The immunostained E18, P0 and P5 explants were sufficiently transparent that individual neurons could easily be identified and counted (Figure 1). For P10 and P20 explants, however, the tissue after immunostaining was not as transparent, and it was more difficult to count the surviving SGNs reliably. Therefore, we excluded these developmental ages from the analysis of SGN survival. Neurite number was determined by counting neurites emanating from each explant at its edge. Therefore, branching in more peripheral areas was not taken into account. Since we could not distinguish central axons from peripheral dendrites, we refer to them simply as neurites.
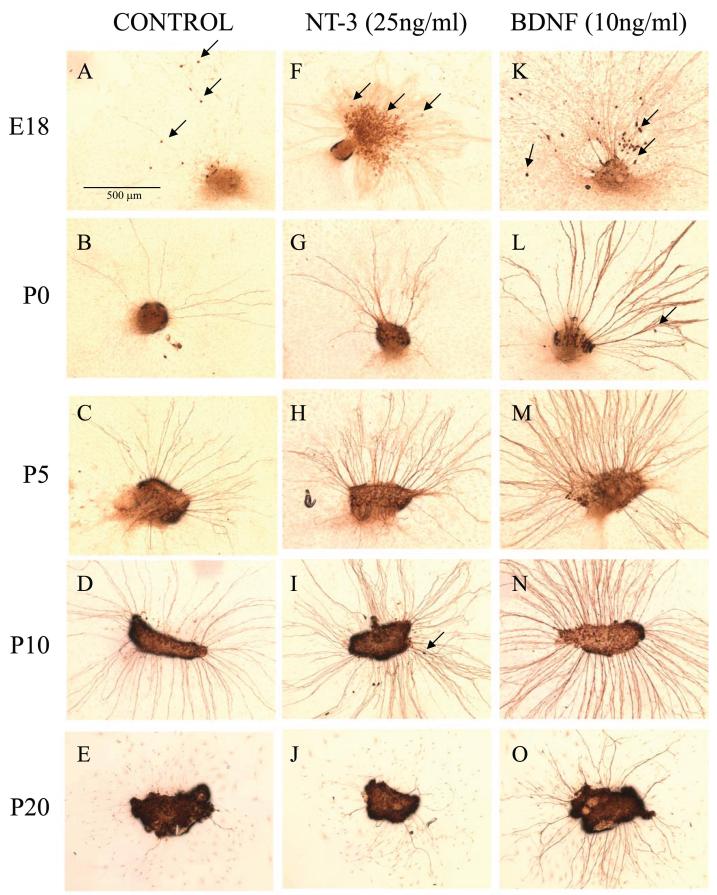
Figure 1.
Representative photomicrographs of SG explants from E18 to P20 cochlea. Each explant was cultured for 3 days in media without neurotrophin (A-E) or supplemented with either 25 ng/ml of NT-3 (F-J) or 10 ng/ml of BDNF (K-O), the lowest maximally effective dose of each factor. Explants were then fixed and immunostained with anti-NF200 antibody. In E18 cultures, large number of neurons migrated outside of the explants (arrows in A, F and K), whereas only a few such migrating neurons were observed at other ages (arrows in I and L). Neurites were thinnest at E18, thickest from P0-P10. Thickness was variable at P20. Neurite number and length declined significantly at P20. Responses to NT-3 and BDNF varied depending upon the ages of the culture, with NT-3 responses greatest at E18 (F) and BDNF dominant at later ages (L-O). Scale bar = 0.5 mm.
The magnitude of apoptosis in the culture was evaluated similarly by manually counting immunolabeled cells for active caspase-3 in each explant. This evaluation was performed only for E18, P0 and P5 cultures and could not be performed on P10 and P20 cultures because of the intransparency of older explants.
For the evaluation of neurite length extending from the explant, we used the five longest neurites from each explant for analysis. Using Spot advanced software (Diagnostic Instruments, Sterling Heights, MI, USA), each neurite was traced by drawing a line along the neurite, resulting in a series of short, linear segments. The lengths of these segments were then summed and calculated by the program. The selected neurites from all cultures in the same condition were pooled for analysis. When the explant had fewer than five neurites, all of the neurites were included in the analysis.
Statistical analysis
The data for each experimental condition were compiled from at least four independent experiments, each of which contained two to three explant cultures for each given age and concentration of neurotrophin. Results were presented as the mean ± standard error of all samples. For neurite length, the average value of all the neurite length selected for analysis in each condition was designated as the maximum neurite length (MNL). Statistical analyses for survival, neurite number and MNL were performed using one-way analysis of variance (ANOVA), followed by Dunnet post hoc test with correction for multiple tests, using Statview 5.0 software to compare each neurotrophin subgroup with the corresponding untreated control group. Differences associated with p values < 0.05 were considered as statistically significant.
Results
General morphology of the rat SGN explants in vitro
Figure 1 illustrates the effects of NT-3 or BDNF on the appearance of SG explants at various ages. Generally, neurites extended from SG explants in a radial direction, as previously described [19,20]. In E18 explants, some neurons migrated from the explants (Figure 1A, F, K). Older SGNs exhibited little migratory capacity and remained inside the original explant (Figure 1B-E, G-J, L-O). We also observed an age-dependent difference in neurite thickness. E18 neurites appeared consistently thinner upon visualization (Figure 1A, F, K) when compared with P0, P5 and P10 neurites (Figure 1B-D, G-I, L-N). In contrast, P20 explants showed considerable variability in apparent neurite thickness (Figure 1E, J, O). These age-related morphological differences in thickness did not appear to be affected by either neurotrophin.
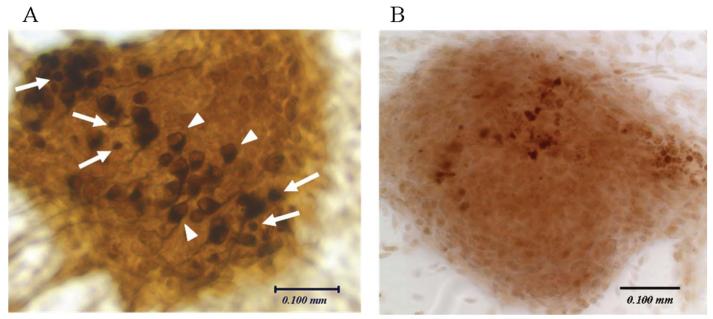
An unusually large number of neurons showed morphological signs of apoptosis in P0 explants (Figure 2A). That is they showed anti-NF200 immunoreactivity, but the cell bodies were smaller and exhibited pyknotic nuclei. The average number of such cells per explant in P0 control explants (22.9 ± 7.7) was significantly greater than in E18 (11.7 ± ± 4.4, p < 0.02) or P5 (2.3 ± 0.6, p < 0.01) explants.
Figure 2.
Degenerating neuronal cells in a P0 explant culture (A). The explant was maintained for 3 days in maintenance medium supplemented with BDNF (10 ng/ml), then fixed and immunostained with anti-NF200 antibody. Degenerating cells (white arrows) are small, exhibit condensed immunolabeling for NF200, lack neurites and do not show a clear contour of nucleus. Surviving SGNs (white arrowheads) show a clear, unstained nucleus and typically form neurites. Apoptosis in a P0 explant culture (B). The explant was immunostained with anti-active caspase-3 antibody. Scale bar = 0.1 mm.
Effect of neurotrophins on neuronal survival
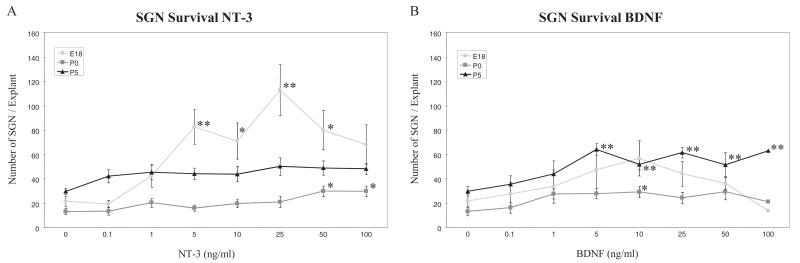
Survival of the SGN somata could readily be evaluated in E18, P0 and P5 explants. However, at older ages, explant density precluded reliable visualization of cell bodies. A dose-response analysis of SGN survival, assessed by normalized SGN cell body counts (Figure 3), revealed that NT-3 exhibited a strong effect on neuronal survival at only E18. In contrast, BDNF supported survival at all the ages.
Figure 3.
Dose-response effects of neurotrophins on SGN survival in E18, P0 and P5 explant cultures. Graphs show the average number of surviving SGNs observed after 3 days in maintenance medium with various concentrations of NT-3 (A) or BDNF (B). Error bars represent the standard error of the mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01) compared with control culture (Dunnett post hoc test).
Caspase-3 activation in the explant cultures
Since morphology suggested some apoptosis, we immunostained E18, P0 and P5 control explants with anti-active caspase-3 antibody and counted the number of labeled cells in each explant (Figure 2B). The number of active caspase-3-positive cells/explant observed across all turns was 15.7 ± 1.6 at E18, 58.2 ± 13.0 at P0 and 38.7 ± 10.2 at P5. The number of labeled cells at P0 was significantly larger than the other ages (p < 0.01), representing ~10% of all neurons present.
When P0 explants were treated with NT-3, significantly fewer cells (33.7 5.6) were caspase-positive (p < 0.03). However, although fewer cells were caspase-positive after BDNF treatment (38.8 ± 4.9), this difference was not statistically significant (p = 0.073). Neurotrophin treatment had no effect on caspase positivity for either E18 or P5 explants.
Effect of neurotrophins on neurite number
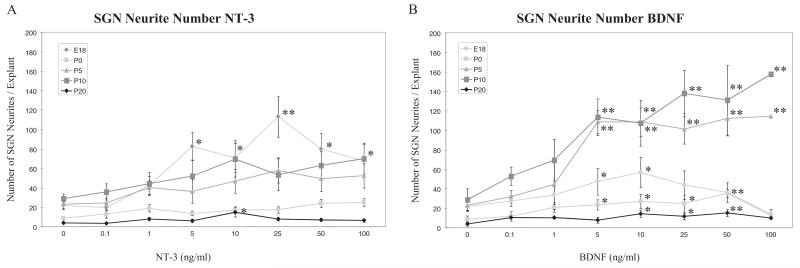
Quantitative analysis for the number of neurites extending from each explant also demonstrated clear age-dependent differences (Figure 4). In untreated explants (neurotrophin concentration 0), the fewest neurites were observed at P0 and P20, and the most at P5 and P10. NT-3 had a large and significant effect on neurite number only at E18, although a more modest increase was seen at one NT-3 dose at P20. In contrast, BDNF increased neurite number at all ages, although it exhibited an especially strong effect at P5 and P10.
Figure 4.
Dose-response effects of neurotrophins on the neurite number observed in SG explant cultures at E18, P0, P5, P10 and P20. Graphs show the average number of neurites emanating from each explant after 3 days in maintenance medium with various concentrations of NT-3 (A) or BDNF (B). Error bars represent the standard error of the mean. Asterisks indicate a significant difference (*p < 0.05, **P < 0.01) compared with the control culture (Dunnett post hoc test).
Effect of neurotrophins on neurite length
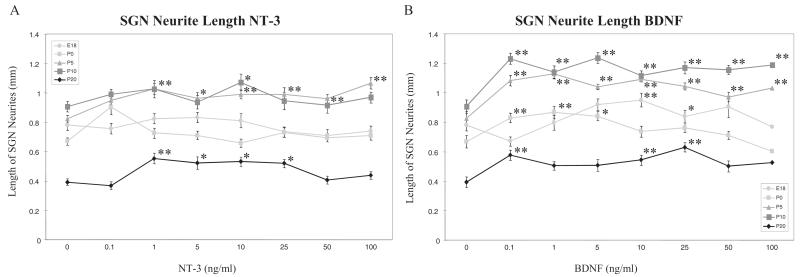
As shown in Figure 5, there was also a strong effect of age on neurite length. The shortest neurites were observed on untreated explants from P20 rats, and the longest at P5 and P10. These age differences were generally maintained during neurotrophin treatment. However, NT-3 treatment produced a modest increase in neurite length at P5, P10 and P20, and no effect at E18 or P0. BDNF treatment produced moderate increases in neurite length at all ages tested, with the greatest effect at P5, P10 and P20.
Figure 5.
Dose-response effects of neurotrophins on the neurite extention observed in SG explant cultures at P0, P5, P10 and P20. Graphs show the average length of longest neurites selected from each explant (5 neurites/explant) and pooled in the same culture condition. Each explant was cultured for 3 days in maintenance medium with various concentrations of NT-3 (A) or BDNF (B). Error bars represent the standard error of the mean. Asterisks indicate a significant difference (*p < 0.05, **p < 0.01) compared with the control culture (Dunnett post hoc test).
Expression of neurotrophin receptors in developing SGNs determined by RT-PCR
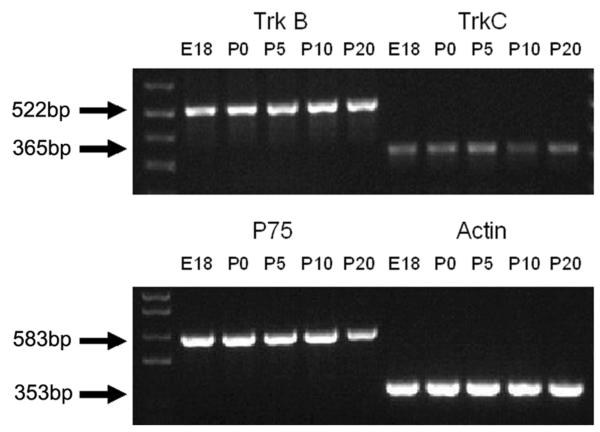
We assessed the expression of messenger RNA (mRNA) for TrkC, TrkB and p75, the receptor proteins for NT-3 and BDNF, in SG across age using RT-PCR. As shown in Figure 6, we observed robust PCR products for the mRNA of each receptor throughout the developmental period analyzed. Assuming that the mRNA is translated, these data suggest that changes in Trk or p75 receptors may be unlikely to mediate changes in the responsiveness of SG explants. However, it must be noted that the PCR products potentially represent expression by both neuronal and nonneuronal elements within the ganglion. Therefore, variation in expression between neurons and other cell types is certainly possible.
Figure 6.
Expression of mRNA for TrkB, TrkC and p75 receptors in SG during rat development. Ethidium bromide-stained agarose gel demonstrating RT-PCR products for TrkB (522 bp), TrkC (365 bp) and p75 (583 bp) throughout the developmental period examined (E18-P20). DNA ladder and the expression of mRNA for actin (internal control) are also shown.
Discussion
The aim of the present study was to investigate the age-dependence of SGN survival, neurite outgrowth and neurite extension regulation by NT-3 and BDNF. Our results demonstrate that both NT-3 and BDNF support these aspects of SGN development in a dose- and stage-dependent manner and that the response pattern to neurotrophins varies between each of these parameters. The findings suggest that BDNF and NT-3 mediate separate ontogenetic events at different developmental stages.
A switch in neurotrophin dependence for SGN survival
We found that SGNs change their dependence for survival from NT-3 at E18 to BDNF at older ages. Although this report appears to be the first longitudinal study to show such a shift in mammals, it is consistent with previous reports from individual developmental stages. Pirvola et al. [21] observed greater survival-promoting effect of NT-3 over BDNF on rat embryonic (E13) cochleovestibular ganglion neurons, whereas others [22-24] reported greater BDNF dependence for postnatal SGNs. Developmental changes in neurotrophin dependence occur in other parts of the peripheral nervous system as well: trigeminal ganglion neurons switch from NT-3 and BDNF to NGF [15], whereas a subpopulation of DRG neurons switch from NGF to GDNF [16].
Our data are also similar to the developmental changes in the chick cochlea described by Avila et al. [17]. In their report, the chick cochlear neurons in culture predominantly depend on NT-3 for their survival in the early embryonic period. The response is maximum at E7 and decreased thereafter, being negligible from E13 to hatching. In contrast, the effect of BDNF for survival is more delayed and peaked at E9-E11 and although diminishing from then onward, remains in a significant range until hatching. This is roughly comparable with the timing of neurotrophin dependence in the rat, suggesting that age-dependent support of primary auditory neuron survival by NT-3 followed by BDNF may be a common molecular mechanism shared by birds and mammals.
Changes in survival response of SGNs to neurotrophins during development could be due to the selective death of a subset of neurons that respond preferentially to NT-3. For example, based on knockout mouse data, it has been suggested that developing type I SGNs depend preferentially upon NT-3 for their survival, whereas type II neurons depend upon BDNF [25]. Alternatively, our observation could be due to changes in the neurotrophin responsiveness of individual neurons. We do not have evidence to reach a definite conclusion regarding this point. Because rat SGNs upregulate peripherin in culture [26], we could not distinguish between type I and type II neurons. However, Mou et al. [27] found that the survival of dissociated postnatal (P1-P10) type 1 and type II mouse SGNs was preferentially enhanced by BDNF when compared with NT-3, in agreement with our results at P5 and P10. With respect to other potential subtypes of SGNs, previous immunohistochemical examinations showed that all SGNs in both embryonic and postnatal mammals uniformly express both TrkB and TrkC receptors and that there appear to be no distinct subsets of neurons based on Trk expression [22,28]. This suggests that the overall population of SGNs may switch their dependence for survival from NT-3 to BDNF based on changes in the intracellular responses to TrkB and TrkC stimulation, rather than on receptor expression.
Effects of neurotrophin concentration
For each of the measures employed, a neurotrophin concentration effect was noted. This typically consisted of an increase in neuronal survival, neurite number or neurite length with increasing dose. In most cases, the response appeared to saturate at between 5 and 10 ng/ml. An exception was NT-3, where 25 ng/ml produced significantly greater SGN survival and neurite number than either lower or higher concentrations. The response saturation may reflect maximal utilization of all Trk receptors at relatively low concentrations. Another possibility is that neurotrophin receptors were downregulated at higher ligand concentrations, as has been observed in other systems (e.g. [29]). In this case, greater amounts of ligand may have been required to produce the same effect. In general, we did not observe systematic changes in threshold dose across age. When an effect was robust, it typically showed a similar minimal effective dose at all ages (e.g., Figure 4, BDNF; Figure 5, NT-3 and BDNF). Only when responses were minimal did we see variation in threshold dose. These data suggest that neurotrophin receptors are not expressed on SGNs gradually with age. Rather, age-dependent changes observed in some SGN responses may be related to changes in intracellular signaling in response to receptor activation.
A critical period of SGN death
Comparing the total number of rat SGNs/cochlea in situ (18 000-25 000; [4]) and the small numbers of surviving SGNs after 4 days in vitro in our culture system (20-120 neurons/explant in about 1/8 of the whole SG), considerable cell death occurred when SGNs were placed into explant cultures at any age. However, P0 cultures exhibited a significantly lower number of surviving SGNs, both in the presence or absence of neurotrophins than did either in E18 or P5 explants. P0 explants also exhibited significantly higher levels of caspase activity, indicating apoptosis. Interestingly, the dose response of SGNs to NT-3 for survival appeared to shift to higher concentrations, and the enhancement of survival at P0 was saturated at a lower magnitude (2.3-fold compared with control explants) than in E18 culture (more than fivefold compared with control explants; Figure 3A). At the same time, BDNF influence on survival remains low (Figure 3B). It is significant that the period of culture for P0 ends at the time equivalent to P4 in vivo. This corresponds to the peak of naturally occurring cell death in the rat SG in vivo [4]. Our findings in culture suggest that the in vivo increase in cell death may reflect a downregulation of SGN sensitivity to NT-3, without an increase in BDNF sensitivity, resulting in neuronal apoptosis.
The expression of p75 by SG explants suggests another potential pathway for the regulation of cell death and survival. Stimulation of the p75 receptor by the proforms of neurotrophins is well known to mediate apoptosis, including in the SG [30,31] and has also been implicated in regulation of neurite length [32]. Since we applied mature neurotrophins to our cultures, this could not have resulted directly from our experimental manipulations. Although neurotrophins are not expressed in the SG of neonatal or adult rats in vivo [33], the potential for autocrine neurotrophic effects in SGNs [34] should be considered. The low level of survival of SGNs that we observed in untreated SGNs suggests that there is not extensive production of neurotrophins in our cultures. However, Zha et al. [35] have reported that neonatal SGNs can express neurotrophins, at least in culture. Thus neurotrophin genes expressed in their proforms could potentially mediate apoptosis in vitro via an autocrine process. Stimulation of p75 by mature neurotrophins is also well known to modulate the response of Trk receptors to mature neurotrophins [30]. Thus, changes in the interaction of p75 and Trk receptors across age could also contribute to altered responses to neurotrophins. On the basis of our PCR results, the expression of p75 mRNA did not appear to vary across age. Assuming that this mRNA was translated, any age-related changes would not be based on differential expression but could reflect downstream signaling changes.
The effects of neurotrophins on neurite number
Quantitative assessment of explants indicated a larger number of neurites in the presence of NT-3 than BDNF at E18 (Figure 1F and K, Figure 4). In contrast, at P0 neither neurotrophin had a strong effect. However, the number of neurites emanating from P5 and P10 SG explants was much more strongly enhanced by BDNF than NT-3. Thus, the effects of neurotrophins on neurite number resemble those observed for survival in E18, P0 and P5 cultures.
Our neurite outgrowth index (the ratio of neurite number/number of SGNs in each explant) demonstrated that none of the neurotrophin subgroups, either for NT-3 or for BDNF, had significantly different ratios compared with the untreated control in P0 culture. In contrast, the BDNF subgroups at higher concentrations (10 ng/ml and 50 ng/ml) had significantly greater index values compared with the control group in P5 culture. This finding suggests that the modest increase in neurite number induced at P0 by neurotrophins reflects primarily an increase in surviving SGNs, whereas the effects on neurite number at P5 and P10 are mediated primarily by neuritogenesis.
Comparison of neurotrophic effects in vitro with the expression of neurotrophins and developmental events in vivo
The period of E18 culture (equivalent to E18-E21 in vivo), the earliest developmental stage we examined, corresponds to the period when the afferent fibers of SGNs reach the cochlear sensory epithelium [1,36]. By this stage, expression of both NT-3 and BDNF extends throughout the organ of Corti longitudinally [37], implying that both neurotrophins are available for all SGNs. NT-3 is more strongly expressed than BDNF [37-39] and distributed more widely in the sensory epithelium, since both hair cells and supporting cells express this neurotrophin, whereas BDNF is more restricted to hair cells [37,39]. This is consistent with our observation of greater enhancement of SGN survival by NT-3 than BDNF in E18 explants.
It should be noted, however, that there is a discrepancy between our result and the results of gene deletion studies [37]. Although mice null for the NT-3 gene show a considerably reduced number of SGNs in the basal cochlea (less than 20% of wild type) at birth [25,40], replacement of the NT-3-coding sequence with that for BDNF almost completely rescues the loss of basal turn SGNs (85%) by NT-3 absence [37,41]. Similar rescue in the number of SGNs has been demonstrated in mice for which the coding part of the BDNF gene was replaced with that of NT-3 [42]. These results suggest that NT-3 and BDNF can be functionally equivalent for the survival of SGNs prenatally [37,42]. The discrepancy between this finding and ours may be related to the mode of exposure of SGNs to neurotrophins: in the in vivo condition, neurotrophins are supplied to the SGNs basically through the targets of their neurites, and concentrations at these targets may be very high. In the in vitro condition, the entire SGN is exposed to the neurotrophins and concentration is uniform. The synergistic effects of neurotrophins with other survival factors in vivo must also be considered.
The period of P0 explant culture (equivalent to P0-P4 in vivo) corresponds not only to that of naturally occurring cell death [4,5] as mentioned above, but also to a relatively low level of responsiveness to NT-3 and BDNF with respect to survival, neurite number and neurite length. Interestingly, the expression of BDNF in the cochlear sensory epithelium, which disappears in the early postnatal period [10,39], reappears at P6-P7 in hair cells and supporting cells [10], which is temporally coincident with strong effects of BDNF, which we observed on survival, neurite number and neurite extension. SGNs may, therefore, tailor their responsiveness to coincide with developmental trends in neurotrophin availability.
P20 cultures, the oldest stage we examined, correspond to P20-P24 in vivo. Almost all of the major developmental events for SGNs are complete and hearing function has matured by this age [1]. Although the intrinsic capacity for neurite growth has considerably declined in this period, SGNs still retained the capacity to respond to both NT-3 and BDNF. This finding is consistent with the enhancement of survival and neurite regrowth of adult SGNs by neurotrophins in vivo [43-46]. NT-3 is highly expressed in inner hair cells and their supporting cells [36,39,47,48], so responsiveness to this neurotrophin is not surprising. However, BDNF expression is almost absent in the target field of adult SGNs [10,33,39]. Moreover, BDNF expression is observed in SGNs themselves [49] and may be acting in an autocrine manner [49].
Mechanisms for differential regulation by neurotrophins
Our RT-PCR results demonstrate that three kinds of neurotrophin receptors, TrkC, TrkB and p75, all of the neurotrophin receptors involved in the signaling of NT-3 and BDNF, are expressed in SG throughout the developmental period we examined. These data are in good agreement with previous immunohistochemical and in situ hybridization studies [9,22,28,33,37,47].
Our results appear in line with the results of transgenic mouse studies in which the coding part of the NT-3 gene is replaced by BDNF [37,50] and vice versa [42]. These studies suggest that although NT-3 and BDNF can be functionally equivalent for the survival of SGNs prenatally [37,42], they have distinct roles for the axon guidance and innervations in the cochlea [50]. These findings cannot be explained simply by the expression pattern of each neurotrophin receptor. As noted above, the biological responses to neurotrophins are presumably regulated by molecular cascades downstream of Trk/p75 signaling. It has been shown that multiple signal transduction pathways are involved in neurotrophin-mediated biological effects on neurons, which may contribute to age-dependent differential regulation of SGN biological responses to neurotrophins.
Conclusion
The present study demonstrates that neurotrophins regulate developing rat SGNs in an age-dependent manner. The temporal patterns of responsiveness of SGNs to NT-3 and BDNF presented here correspond well to the expression pattern of the two neurotrophins in cochlear sensory epithelium in vivo and also correlate with the time course of developmental events in the SG, such as neuronal cell death and the remodeling of afferent innervation. Our data, therefore, suggest multiple, age-specific roles for NT-3 and BDNF in the ontogeny of cochlear innervation.
Acknowledgments
This work was supported by the Research Service of the VA Merit grant 1108966, the NIH/NIDCD grant DC000139 and the Japan Foundation for Aging and Health, Promoting Projects of Researches on Sensory and Communicative Disorders.
Footnotes
Declaration of interest: The authors report no conflict of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Pujol R, Lavigne-Rebillard M, Lenoir M. Development of sensory and neural structures in the mammalian cochlea. Springer: Development of Auditory System; New York: 1998. pp. 146–92. [Google Scholar]
- 2.Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- 3.Ard MD, Morest DK. Cell death during development of the cochlear and vestibular ganglion of the chick. Int J Dev Neurosci. 1984;2:535–47. doi: 10.1016/0736-5748(84)90031-5. [DOI] [PubMed] [Google Scholar]
- 4.Rueda J, de la Sen C, Juiz JM, Merchan JA. Neuronal loss in the spiral ganglion of young rats. Acta Otolaryngol. 1987;104:417–21. doi: 10.3109/00016488709128269. [DOI] [PubMed] [Google Scholar]
- 5.Echteler SM, Magardino T, Rontal M. Spatiotemporal patterns of neuronal programmed cell death during postnatal development of the gerbil cochlea. Brain Res Dev Brain Res. 2005;157:192–200. doi: 10.1016/j.devbrainres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Lenoir M, Shnerson A, Pujol R. Cochlear receptor development in the rat with emphasis on synaptogenesis. Anat Embryol (Berl) 1980;60:253–62. doi: 10.1007/BF00305106. [DOI] [PubMed] [Google Scholar]
- 7.Hafidi A, Romand R. First appearance of type II neurons during ontogenesis in the spiral ganglion of the rat. An immunocytochemical study. Brain Res Dev Brain Res. 1989;48:143–9. doi: 10.1016/0165-3806(89)90098-9. [DOI] [PubMed] [Google Scholar]
- 8.Echteler SM. Developmental segregation in the afferent projections to mammalian auditory hair cells. Proc Natl Acad Sci USA. 1992;89:6324–7. doi: 10.1073/pnas.89.14.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knipper M, Zimmermann U, Rohbock K, et al. Expression of neurotrophin receptor trkB in rat cochlear hair cells at time of rearrangement of innervation. Cell Tissue Res. 1996;283:339–53. doi: 10.1007/s004410050545. [DOI] [PubMed] [Google Scholar]
- 10.Wiechers B, Gestwa G, Mack A, et al. A changing pattern of brain-derived neurotrophic factor expression correlates with the rearrangement of fibers during cochlear development of rats and mice. J Neurosci. 1999;19:3033–42. doi: 10.1523/JNEUROSCI.19-08-03033.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fritzsch B, Silos-Santiago I, Bianchi LM, Farinas I. The role of neurotrophic factors in regulating the development of inner ear innervation. Trends Neurosci. 1997;20:159–64. doi: 10.1016/s0166-2236(96)01007-7. [DOI] [PubMed] [Google Scholar]
- 12.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–78. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 13.Adamson CL, Reid MA, Davis RL. Opposite actions of brain-derived neurotrophic factor and neurotrophin-3 on firing features and ion channel composition of murine spiral ganglion neurons. J Neurosci. 2002;22:1385–96. doi: 10.1523/JNEUROSCI.22-04-01385.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun W, Salvi RJ. Brain derived neurotrophic factor and neurotrophic factor 3 modulate neurotransmitter receptor expressions on developing spiral ganglion neurons. Neuroscience. 2009;164:1854–66. doi: 10.1016/j.neuroscience.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 16.Molliver DC, Wright DE, Leitner ML, et al. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–61. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 17.Avila MA, Varela-Nieto I, Romero G, et al. Brain-derived neurotrophic factor and neurotrophin-3 support the survival and neuritogenesis response of developing cochleovestibular ganglion neurons. Dev Biol. 1993;159:266–75. doi: 10.1006/dbio.1993.1239. [DOI] [PubMed] [Google Scholar]
- 18.Hashino E, Dolnick RY, Cohan CS. Developing vestibular ganglion neurons switch trophic sensitivity from BDNF to GDNF after target innervation. J Neurobiol. 1999;38:414–27. doi: 10.1002/(sici)1097-4695(19990215)38:3<414::aid-neu9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 19.Dazert S, Kim D, Luo L, et al. Focal delivery of fibroblast growth factor-1 by transfected cells induces spiral ganglion neurite targeting in vitro. J Cell Physiol. 1998;177:123–9. doi: 10.1002/(SICI)1097-4652(199810)177:1<123::AID-JCP13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 20.Aletsee C, Beros A, Mullen L, et al. Ras/MEK but not p38 signaling mediates NT-3-induced neurite extension from spiral ganglion neurons. J Assoc Res Otolaryngol. 2001;2:377–87. doi: 10.1007/s10162001000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pirvola U, Arumae U, Moshnyakov M, et al. Coordinated expression and function of neurotrophins and their receptors in the rat inner ear during target innervation. Hear Res. 1994;75:131–44. doi: 10.1016/0378-5955(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 22.Zheng JL, Stewart RR, Gao WQ. Neurotrophin-4/5 enhances survival of cultured spiral ganglion neurons and protects them from cisplatin neurotoxicity. J Neurosci. 1995;15:5079–87. doi: 10.1523/JNEUROSCI.15-07-05079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegarty JL, Kay AR, Green SH. Trophic support of cultured spiral ganglion neurons by depolarization exceeds and is additive with that by neurotrophins or cAMP and requires elevation of [Ca2+]i within a set range. J Neurosci. 1997;17:1959–70. doi: 10.1523/JNEUROSCI.17-06-01959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marzella PL, Gillespie LN, Clark GM, et al. The neurotrophins act synergistically with LIF and members of the TGF-beta superfamily to promote the survival of spiral ganglia neurons in vitro. Hear Res. 1999;138:73–80. doi: 10.1016/s0378-5955(99)00152-5. [DOI] [PubMed] [Google Scholar]
- 25.Ernfors P, Van De Water T, Loring J, Jaenisch R. Complementary roles of BDNF and NT-3 in vestibular and auditory development. Neuron. 1995;14:1153–64. doi: 10.1016/0896-6273(95)90263-5. [DOI] [PubMed] [Google Scholar]
- 26.Lallemend F, Vandenbosch R, Hadjab S, et al. New insights into peripherin expression in cochlear neurons. Neuroscience. 2007;150:212–22. doi: 10.1016/j.neuroscience.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 27.Mou K, Adamson CL, Davis RL. Time-dependence and cell-type specificity of synergistic neurotrophin actions on spiral ganglion neurons. J Comp Neurol. 1998;402:129–39. [PubMed] [Google Scholar]
- 28.Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–39. [PubMed] [Google Scholar]
- 29.Sommerfeld MT, Schweigreiter R, Barde YA, Hoppe E. Down-regulation of the neurotrophin receptor TrkB following lig- and binding. Evidence for an involvement of the proteasome and differential regulation of TrkA and TrkB. J Biol Chem. 2000;275:8982–90. doi: 10.1074/jbc.275.12.8982. [DOI] [PubMed] [Google Scholar]
- 30.Volosin M, Song W, Almeida RD, et al. Interaction of survival and death signaling in basal forebrain neurons: roles of neurotrophins and proneurotrophins. J Neurosci. 2006;26:7756–66. doi: 10.1523/JNEUROSCI.1560-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauris J, Gustafsen C, Christensen EI, et al. Proneurotrophin-3 may induce sortilin-dependent death in inner ear neurons. Eur J Neurosci. 2011;33:622–31. doi: 10.1111/j.1460-9568.2010.07556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Lim Y, Li F, et al. ProBDNF collapses neurite outgrowth of primary neurons by activating RhoA. PLoS One. 2012;7:e35883. doi: 10.1371/journal.pone.0035883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ylikoski J, Pirvola U, Moshnyakov M, et al. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- 34.Ramekers D, Versnel H, Grolman W, Klis SF. Neurotrophins and their role in the cochlea. Hear Res. 2012;288:19–33. doi: 10.1016/j.heares.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Zha XM, Bishop JF, Hansen MR, et al. BDNF synthesis in spiral ganglion neurons is constitutive and CREB-dependent. Hear Res. 2001;156:53–68. doi: 10.1016/s0378-5955(01)00267-2. [DOI] [PubMed] [Google Scholar]
- 36.Angulo A, Merchan JA, Merchan MA. Morphology of the rat cochlear primary afferents during prenatal development: a Cajal’s reduced silver and rapid Golgi study. J Anat. 1990;168:241–55. [PMC free article] [PubMed] [Google Scholar]
- 37.Farinas I, Jones KR, Tessarollo L, et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J Neurosci. 2001;21:6170–80. doi: 10.1523/JNEUROSCI.21-16-06170.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schecterson LC, Bothwell M. Neurotrophin and neurotrophin receptor mRNA expression in developing inner ear. Hear Res. 1994;73:92–100. doi: 10.1016/0378-5955(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 39.Wheeler EF, Bothwell M, Schecterson LC, von Bartheld CS. Expression of BDNF and NT-3 mRNA in hair cells of the organ of Corti: quantitative analysis in developing rats. Hear Res. 1994;73:46–56. doi: 10.1016/0378-5955(94)90281-x. [DOI] [PubMed] [Google Scholar]
- 40.Farinas I, Jones KR, Backus C, et al. Severe sensory and sympathetic deficits in mice lacking neurotrophin-3. Nature. 1994;369:658–61. doi: 10.1038/369658a0. [DOI] [PubMed] [Google Scholar]
- 41.Coppola V, Kucera J, Palko ME, et al. Dissection of NT3 functions in vivo by gene replacement strategy. Development. 2001;128:4315–27. doi: 10.1242/dev.128.21.4315. [DOI] [PubMed] [Google Scholar]
- 42.Agerman K, Hjerling-Leffler J, Blanchard MP, et al. BDNF gene replacement reveals multiple mechanisms for establishing neurotrophin specificity during sensory nervous system development. Development. 2003;130:1479–91. doi: 10.1242/dev.00378. [DOI] [PubMed] [Google Scholar]
- 43.Wise AK, Richardson R, Hardman J, et al. Resprouting and survival of guinea pig cochlear neurons in response to the administration of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3. J Comp Neurol. 2005;27:147–65. doi: 10.1002/cne.20563. [DOI] [PubMed] [Google Scholar]
- 44.Miller JM, Le Prell CG, Prieskorn DM, et al. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: effects of brain-derived neurotrophic factor and fibroblast growth factor. J Neurosci Res. 2007;85:1959–69. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- 45.Vieira M, Christensen BL, Wheeler BC, et al. Survival and stimulation of neurite outgrowth in a serum-free culture of spiral ganglion neurons from adult mice. Hear Res. 2007;230:17–23. doi: 10.1016/j.heares.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 46.Glueckert R, Bitsche M, Miller JM, et al. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol. 2008;507:1602–21. doi: 10.1002/cne.21619. [DOI] [PubMed] [Google Scholar]
- 47.Oestreicher E, Knipper M, Arnold A, et al. Neurotrophin 3 potentiates glutamatergic responses of IHC afferents in the cochlea in vivo. Eur J Neurosci. 2000;12:1584–90. doi: 10.1046/j.1460-9568.2000.00049.x. [DOI] [PubMed] [Google Scholar]
- 48.Stankovic K, Rio C, Xia A, et al. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–61. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schimmang T, Tan J, Müller M, et al. Lack of BDNF and TrkB signalling in the postnatal cochlea leads to a spatial reshaping of innervation along the tonotopic axis and hearing loss. Development. 2003;130:4741–50. doi: 10.1242/dev.00676. [DOI] [PubMed] [Google Scholar]
- 50.Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–84. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]