Gaining a systems-level understanding of complex cellular processes will require new analytic approaches that account for the effects of perturbations on a large number of functional parameters with high resolution and high throughput. A recent study by Collinet et al.1 in Nature provides an instructive example of how this might be achieved. Focusing on endocytosis, the authors combine multiparametric imaging with a genome-wide RNA interference (RNAi) screen in HeLa cells to analyze many parameters of the endocytic system in unprecedented detail.
Endocytosis allows eukaryotic cells to remove signaling receptors from their surfaces and to take up extracellular molecules. Internalized cargo are shuttled through a maze of intracellular sorting and transport stations until they reach their destinations. Primary endocytic vesicles fuse with early endosomes, from where cargo is either recycled back to the plasma membrane or sorted into the endolysosomal pathway for degradation. Clathrin-mediated endocytosis is a major endocytic route used by transferrin, growth-factor receptors and pathogenic viruses during infectious entry. Although clathrin-dependent uptake is the best-studied endocytic pathway, a ystems-level understanding of the dynamic and interconnected endocytic pathways remains elusive.
Earlier large-scale, imaging-based RNAi approaches have probed the endocytic system using transferrin or viruses as endocytic cargo to identify novel regulators2–5. Owing to the inherent noise in RNAi screens, these studies sought to obtain a small number of validated hits rather than to define the function of every tested gene. Typically, the high-throughput nature of such approaches required relatively low-resolution images and therefore allowed the evaluation of only a small number of parameters.
In contrast, Collinet et al.1 aimed to determine the role of all genes in the endocytic system with high accuracy. They began by pulsing HeLa cells with two ligands that enter cells by clathrin-mediated endocytosis—fluorescently tagged transferrin and epidermal growth factor (Fig. 1). Once endocytosed, these ligands and their receptors follow distinct routes inside the cell: transferrin and transferrin receptor recycle back to the plasma membrane, whereas epidermal growth factor and its receptor enter the degradation pathway. For RNAi perturbations, the authors used three genome-wide libraries, or 7–8 small interfering RNAs (siRNAs) or endoribonuclease-prepared siRNAs per gene, yielding ~161,000 knockdown conditions in total. High-resolution images of fixed cells were acquired by automated spinning disc confocal microscopy, allowing visualization of subcellular structures and intracellular cargo distribution.
Figure 1.
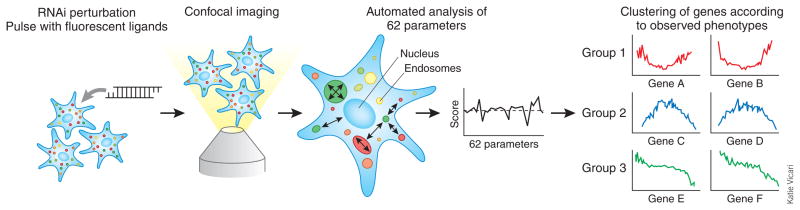
Workflow of the high-content siRNA screen developed by Collinet et al.1. HeLa cells are treated with an siRNA or endoribonuclease-prepared siRNA from one of three genome-wide libraries, followed by a pulse of two fluorescently labeled endocytic cargos. The cells are then fixed, and images are recorded using automated confocal microscopy. A custom image analysis software measures 62 different parameters (such as endosome size, cargo content, and distance from each other and from the nucleus) in each of the images. The parameters are used to assemble a phenotypic profile for each of the targeted genes. Genes that show similar effects on all of the parameters are predicted to be involved in similar endocytic processes.
During their life cycle, endosomes typically travel from the cell periphery toward the cell center while changing shape and the extent of their tubular extensions in accordance with ongoing sorting processes. In an effort to comprehensively describe this system, Collinet et al.1 extracted 62 parameters from the high-resolution images. These included the total amount of internalized cargo as well as parameters that define endosomal shape, number and distribution. Using these parameters, they generated phenotypic profiles for all genes and then analyzed the profiles to identify 4,609 genes whose knockdown significantly altered the state of the endocytic system for either one or both of the endocytic ligands. These hits were clustered into 14 groups according to their phenotypic profiles (Fig. 1).
As expected, established players in endocytic trafficking were well represented. But the screen also identified genes not previously associated with endocytic trafficking, such as those encoding components of the transforming growth factor beta, Wnt and Notch signaling pathways, and many genes of unknown function. Among the various classes of genes identified, those that regulate endocytosis of transferrin and epidermal growth factor differently are of special interest. Although both ligands enter cells by a clathrin-dependent mechanism, there is evidence that they use distinct populations of vesicles6. Collinet et al.1 now provide a catalog of genes whose products selectively regulate endocytosis of one or the other ligand, further demonstrating the plasticity of clathrin-mediated endocytosis.
Future studies could investigate the potential therapeutic relevance of these results. For example, uncontrolled cell growth caused by defects in receptor internalization might be corrected by specifically stimulating the degradation of these receptors. In the context of infectious disease, it may be possible to selectively block infection by pathogenic viruses that rely on clathrin-mediated endocytosis. Ideally, such strategies would target the disease-related subtype of clathrin-mediated endocytosis while allowing the cell to take up nutrients and remain healthy.
Previous large-scale siRNA screens studying similar or other mammalian systems often produced hit lists with relatively poor overlap. Divergent screening strategies may partly account for this effect, but off-target effects of individual siRNAs and variability in cell-culture systems remain a major concern. True validation of the current dataset will ultimately come from detailed follow-up studies that establish protein function of individual hits at a mechanistic level.
Nevertheless, the work of Collinet et al.1 provides a road map of how to generate a comprehensive genetic data set of the mammalian endocytic system and other cellular processes. Their screening data are readily accessible online (http://gwsdisplayer.mpi-cbg.de/), allowing interrogations of single genes or groups of genes. By combining this data set with complementary multiparametric genome-wide data on other endocytic processes, it should be possible to construct a comprehensive endocytic database. Ultimately, this database, if standardized in format and quality, could be combined with analogous data on other cellular processes such as mitosis7 or the secretory pathway to create a repository for mammalian loss-of-function screening data similar to existing resources for sequence, proteomics and microarray data. Such databases have proven very useful in other model organisms (http://www.flyrnai.org/, http://www.wormbase.org/).
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Collinet C, et al. Nature. 2010;464:243–249. doi: 10.1038/nature08779. [DOI] [PubMed] [Google Scholar]
- 2.Galvez T, et al. Genome Biol. 2007;8:R142. doi: 10.1186/gb-2007-8-7-r142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pelkmans L, et al. Nature. 2005;436:78–86. doi: 10.1038/nature03571. [DOI] [PubMed] [Google Scholar]
- 4.Karlas A, et al. Nature. 2010;463:818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 5.Konig R, et al. Nature. 2010;463:813–817. doi: 10.1038/nature08699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leonard D, et al. J Cell Sci. 2008;121:3445–3458. doi: 10.1242/jcs.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neumann B, et al. Nature. 2010;464:721–727. doi: 10.1038/nature08869. [DOI] [PMC free article] [PubMed] [Google Scholar]


