Abstract
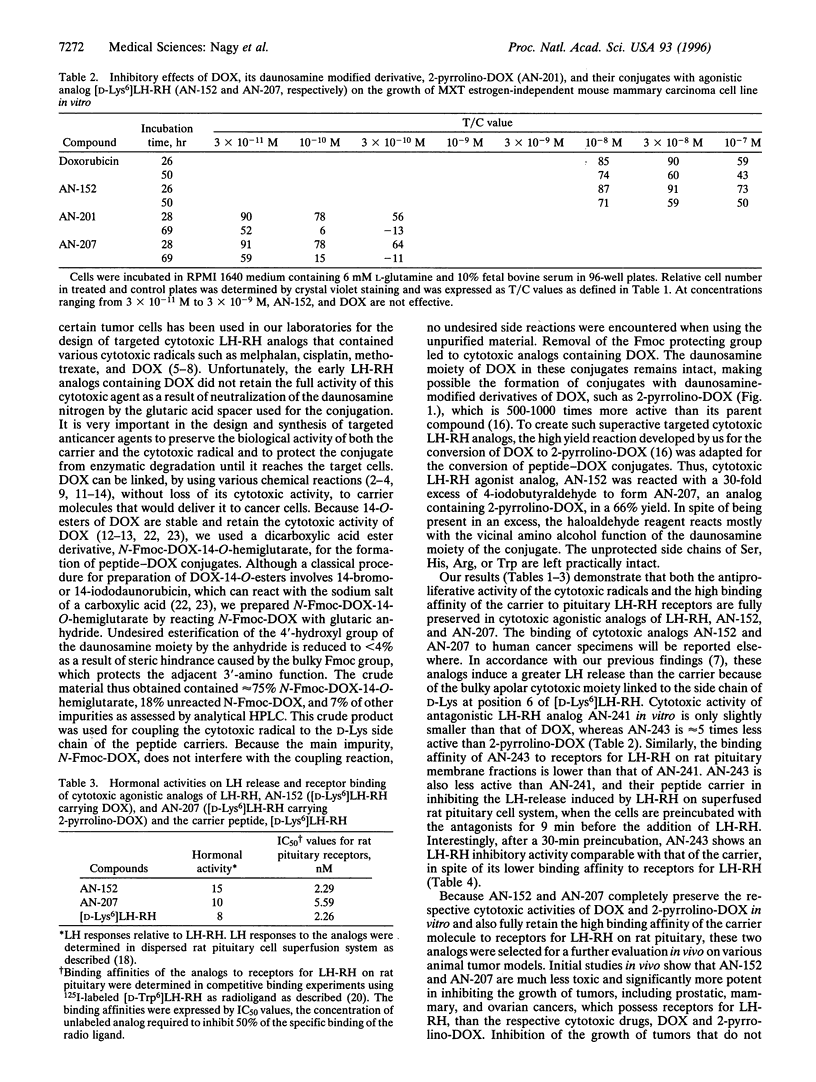
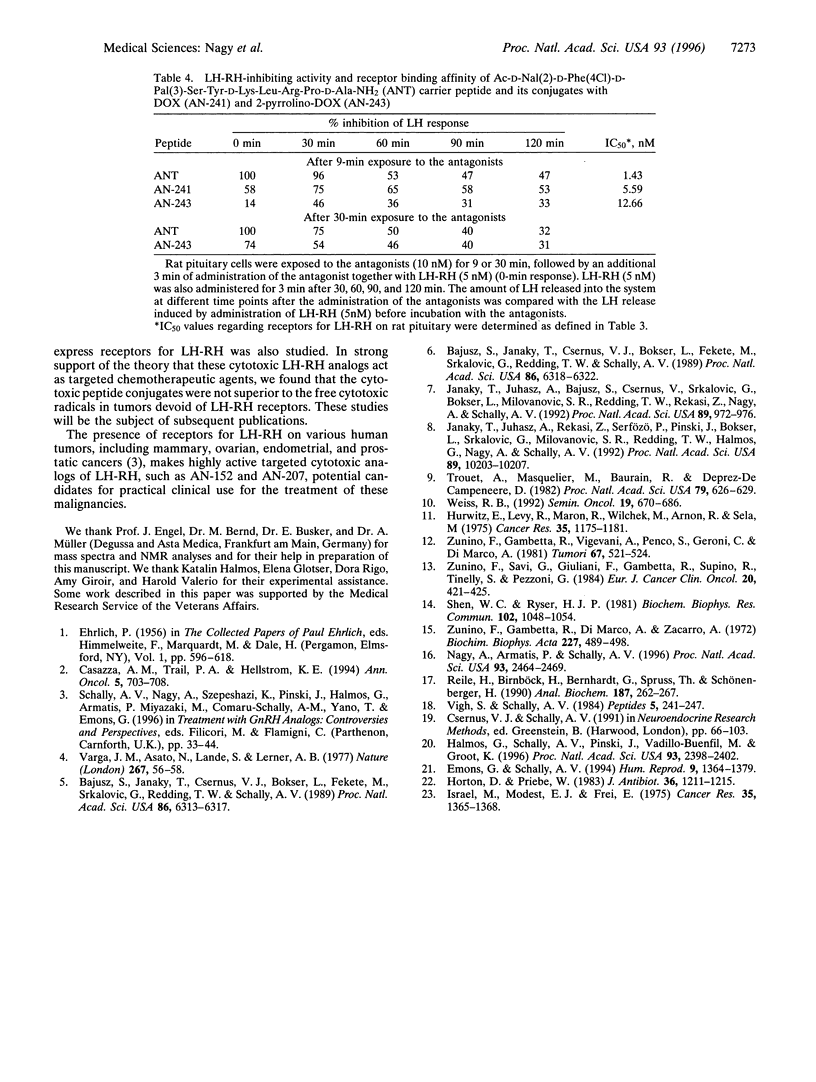
Doxorubicin (DOX) and its daunosamine-modified derivative, 2-pyrrolino-DOX, which is 500-1000 times more active than DOX, were incorporated into agonistic and antagonistic analogs of luteinizing hormone-releasing hormone (LH-RH). The conjugation of DOX with LH-RH analogs was performed by using N-(9-fluorenylmethoxycarbonyl)-DOX-14-O-hemiglutarate, a dicarboxylic acid ester derivative of DOX. Coupling this derivative covalently to the epsilon-amino group of the D-Lys side chain of agonist [D-Lys6]LH-RH or antagonistic analog AC-D-Nal(2)-D-Phe(4Cl)-D-Pal(3)-Ser-Tyr-D-Lys-Leu-Arg-Pro-D-Ala-NH 2 [where Nal(2) = 3-(2-naphthyl)alanine, Pal(3) = 3-(3-pyridyl)alanine, and Phe(4CI) = 4-chlorophenylalanine] was followed by the removal of the 9-fluorenylmethoxycarbonyl protective group to yield cytotoxic derivatives of LH-RH analogs containing DOX. From these DOX containing LH-RH hybrids, intensely potent analogs with daunosamine-modified derivatives of DOX can be readily formed. Thus, cytotoxic LH-RH agonist containing DOX (AN-152) can be converted in a 66% yield by a reaction with a 30-fold excess of 4-iodobutyraldehyde in N,N-dimethylformamide into a derivative having 2-pyrrolino-DOX (AN-207). Hybrid molecules AN-152 and AN-207 fully preserve the cytotoxic activity of their radicals, DOX or 2-pyrrolino-DOX, respectively, in vitro, and also retain the high binding affinity of the peptide hormone portion of the conjugates to rat pituitary receptors for LH-RH. These highly potent cytotoxic analogs of LH-RH were designed as targeted anti-cancer agents for the treatment of various tumors that possess receptors for the carrier peptide. Initial in vivo studies show that the hybrid molecules are much less toxic than the respective cytotoxic radicals incorporated and significantly more active in inhibiting tumor growth.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent analogues of luteinizing hormone-releasing hormone containing D-phenylalanine nitrogen mustard in position 6. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6318–6322. doi: 10.1073/pnas.86.16.6318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajusz S., Janaky T., Csernus V. J., Bokser L., Fekete M., Srkalovic G., Redding T. W., Schally A. V. Highly potent metallopeptide analogues of luteinizing hormone-releasing hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6313–6317. doi: 10.1073/pnas.86.16.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casazza A. M., Trail P. A., Hellström K. E. Drug immunotargeting for carcinomas: a reality at last? Ann Oncol. 1994 Oct;5(8):703–708. doi: 10.1093/oxfordjournals.annonc.a058974. [DOI] [PubMed] [Google Scholar]
- Emons G., Schally A. V. The use of luteinizing hormone releasing hormone agonists and antagonists in gynaecological cancers. Hum Reprod. 1994 Jul;9(7):1364–1379. doi: 10.1093/oxfordjournals.humrep.a138714. [DOI] [PubMed] [Google Scholar]
- Halmos G., Schally A. V., Pinski J., Vadillo-Buenfil M., Groot K. Down-regulation of pituitary receptors for luteinizing hormone-releasing hormone (LH-RH) in rats by LH-RH antagonist Cetrorelix. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2398–2402. doi: 10.1073/pnas.93.6.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton D., Priebe W. 14-esters of 7-O-(3,4-di-O-acetyl-2,6-dideoxy-alpha-L-lyxo-hexopyranosyl)adriamycinone: synthesis and antitumor activity. J Antibiot (Tokyo) 1983 Sep;36(9):1211–1215. doi: 10.7164/antibiotics.36.1211. [DOI] [PubMed] [Google Scholar]
- Hurwitz E., Levy R., Maron R., Wilchek M., Arnon R., Sela M. The covalent binding of daunomycin and adriamycin to antibodies, with retention of both drug and antibody activities. Cancer Res. 1975 May;35(5):1175–1181. [PubMed] [Google Scholar]
- Israel M., Modest E. J., Frei E., 3rd N-trifluoroacetyladriamycin-14-valerate, an analog with greater experimental antitumor activity and less toxicity than adriamycin. Cancer Res. 1975 May;35(5):1365–1368. [PubMed] [Google Scholar]
- Janáky T., Juhász A., Bajusz S., Csernus V., Srkalovic G., Bokser L., Milovanovic S., Redding T. W., Rékási Z., Nagy A. Analogues of luteinizing hormone-releasing hormone containing cytotoxic groups. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):972–976. doi: 10.1073/pnas.89.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janáky T., Juhász A., Rékási Z., Serfözö P., Pinski J., Bokser L., Srkalovic G., Milovanovic S., Redding T. W., Halmos G. Short-chain analogs of luteinizing hormone-releasing hormone containing cytotoxic moieties. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10203–10207. doi: 10.1073/pnas.89.21.10203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Armatis P., Schally A. V. High yield conversion of doxorubicin to 2-pyrrolinodoxorubicin, an analog 500-1000 times more potent: structure-activity relationship of daunosamine-modified derivatives of doxorubicin. Proc Natl Acad Sci U S A. 1996 Mar 19;93(6):2464–2469. doi: 10.1073/pnas.93.6.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reile H., Birnböck H., Bernhardt G., Spruss T., Schönenberger H. Computerized determination of growth kinetic curves and doubling times from cells in microculture. Anal Biochem. 1990 Jun;187(2):262–267. doi: 10.1016/0003-2697(90)90454-h. [DOI] [PubMed] [Google Scholar]
- Shen W. C., Ryser H. J. cis-Aconityl spacer between daunomycin and macromolecular carriers: a model of pH-sensitive linkage releasing drug from a lysosomotropic conjugate. Biochem Biophys Res Commun. 1981 Oct 15;102(3):1048–1054. doi: 10.1016/0006-291x(81)91644-2. [DOI] [PubMed] [Google Scholar]
- Trouet A., Masquelier M., Baurain R., Deprez-De Campeneere D. A covalent linkage between daunorubicin and proteins that is stable in serum and reversible by lysosomal hydrolases, as required for a lysosomotropic drug-carrier conjugate: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1982 Jan;79(2):626–629. doi: 10.1073/pnas.79.2.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J. M., Asato N., Lande S., Lerner A. B. Melanotropin-daunomycin conjugate shows receptor-mediated cytotoxicity in cultured murine melanoma cells. Nature. 1977 May 5;267(5606):56–58. doi: 10.1038/267056a0. [DOI] [PubMed] [Google Scholar]
- Vigh S., Schally A. V. Interaction between hypothalamic peptides in a superfused pituitary cell system. Peptides. 1984;5 (Suppl 1):241–247. doi: 10.1016/0196-9781(84)90282-1. [DOI] [PubMed] [Google Scholar]
- Weiss R. B. The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992 Dec;19(6):670–686. [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Di Marco A., Zaccara A. Interaction of daunomycin and its derivatives with DNA. Biochim Biophys Acta. 1972 Sep 14;277(3):489–498. doi: 10.1016/0005-2787(72)90092-5. [DOI] [PubMed] [Google Scholar]
- Zunino F., Gambetta R., Vigevani A., Penco S., Geroni C., Di Marco A. Biologic activity of daunorubicin linked to proteins via the methylketone side chain. Tumori. 1981 Dec 31;67(6):521–524. doi: 10.1177/030089168106700602. [DOI] [PubMed] [Google Scholar]
- Zunino F., Savi G., Giuliani F., Gambetta R., Supino R., Tinelli S., Pezzoni G. Comparison of antitumor effects of daunorubicin covalently linked to poly-L-amino acid carriers. Eur J Cancer Clin Oncol. 1984 Mar;20(3):421–425. doi: 10.1016/0277-5379(84)90091-9. [DOI] [PubMed] [Google Scholar]



