Abstract
With the increasing patients and limited therapeutic options, diabetic nephropathy (DN) is a long-term complication of diabetic mellitus. The precise mechanism of DN is not yet fully understood and the effective blockade of the progression of nephropathy remains a therapeutic challenge. Application of traditional Chinese medicine (TCM) for diabetes and its related complications has received increasing attention due to its wide availability, low side effects, and proven therapeutic mechanisms and benefits. In the current review, we mainly focus on the recent laboratory studies of the TCM formulas including Wu-Ling-San (Poria Five Powder; Wǔ Líng Sǎn), Danggui-Buxue-Tang (Tangkuei and Astragalus Decoction; Dāng Guī Bǔ Xuè Tang), and Danggui-Shaoyao-San (Tangkuei and Paeonia Formula; Dāng Guī Sháo Yào Sǎn), conducted by the Committee on Chinese Medicine and Pharmacy at the Department of Health of Taiwan Government, in the amelioration of DN. These selected TCM formulas have anti-diabetic properties, with antihyperglycemic activity accompanied by amelioration of advanced glycation end product–mediated renal damage in streptozotocin-induced diabetic rats. However, the renoprotective effects of the selected TCM formulas did not correlate with suppressing renal renin–angiotensin system hyperactivity in diabetic rats. These TCM formulas also have the capacity to ameliorate the defective antioxidative defense system, leading to modulation of the oxidative stress, thereby resulting in downregulation of nuclear factor-kB as well as transforming growth factor-β1 and, consequently, attenuation of extracellular matrix components such as fibronectin or type IV collagen expression in diabetic renal cortex tissue. More detailed mechanistic researches and long-term clinical evaluations, as well as evaluation of safety of the selected TCM formulas are needed for their future applications in DN therapy.
Keywords: Danggui-Buxue-Tang, Danggui-Shaoyao-San, Diabetic nephropathy, Traditional Chinese medicine, Wu-Ling-San
INTRODUCTION
Diabetes mellitus (DM) represents one of the most important health problems worldwide and, according to recent estimations, it is likely to worsen to critical levels in the next decades, with the great concern that this disease is rising rapidly in younger population groups, including children and adolescents.[1] According to data from the International Diabetes Federation, the number of diabetics older than 20 years will rise from 285 million in 2010 to 439 million in 2030.[2] Therefore, target organ complications secondary to diabetes, especially micro and macrovascular complications, will be one of the most important medical concerns in the near future.
Among the diabetic complications, nephropathy is the most common cause of end-stage renal disease (ESRD) in developed countries and a major cause of morbidity and mortality in patients with diabetes.[3] It is characterized by structural abnormalities including hypertrophy of both glomerular and tubular elements, increase in the thickness of glomerular basement membranes, and progressive accumulation of extracellular matrix components.[4] It also results in functional alterations including the early increase in the glomerular filtration rate with intraglomerular hypertension, subsequent proteinuria, systemic hypertension, and eventual loss of renal function.[4] The development of irreversible renal change in DM, such as glomerulosclerosis and tubulointerstitial fibrosis, results ultimately in ESRD.[3] Measures to prevent the appearance and progression of diabetic nephropathy (DN) should therefore be instituted as early as possible. Clarification of the pathogenesis of DN and development of novel and effective therapeutic strategies are therefore high priorities.
Current therapies for DN
DN has several distinct phases of development and multiple mechanisms contribute to the development of the disease and its outcomes. Although adequate control of blood glucose levels may prevent the development of complications, it is difficult to achieve strict blood glucose control, leading to a year-by-year increase in the number of patients with diabetes.[5] Beyond glycemic control, other metabolic factors have been shown to be involved in the development of diabetic kidney disease, i.e. advanced glycation end products (AGEs).[6] Furthermore, an adequate control of high blood pressure and treatment of microalbuminuria are the major therapeutic targets.[7] To achieve adequate blood pressure control, a combination therapy with different classes of antihypertensive agents is often necessary, especially including angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs).[8] Besides hyperglycemia and high blood pressure, other risk factors have been identified in the development or progression of diabetic kidney disease, such as hyperlipidemia and obesity.[4] Increased lipid peroxidation in the kidney implies the level of susceptibility to diabetic oxidative stress, leading to diabetic complications. From this viewpoint, prevention of hyperlipidemia and/or lipid peroxidation resulting from oxidative stress is considered to play a crucial role in protection from disorders associated with diabetes.[9] The interventions in general clinical use are not capable of efficiently slowing or reversing the progression of nephropathy. Therefore, interventions that could optimally delay the development of DN are required.
RECENT FINDINGS FROM THE SELECTED TRADITIONAL CHINESE MEDICINAL FORMULAS FOR DN TREATMENT
Up to now, there have been many experiments focusing on the treatment of diabetes and its complications with traditional Chinese medicine (TCM) because of less toxicity and/or side effects, if the herbs or Chinese prescriptions are prescribed strictly according to the recommendation of the pharmacopoeia with attention to their origin, dose, way of preparation, and duration of intake. Following the most general principles of TCM, diabetes affects a patient's “qi (energy/life-force)” to cause weakness in circulatory system function. In TCM, the spleen is a source of vital energy and blood, and a controller of blood circulation. When spleen qi is weak, its blood controlling function is disturbed; further, the production of blood and “qi” is decreased, leading to ischemia and anoxia of kidney tissue and kidney capillary endothelial cells are damaged. Once the kidney endothelial cells are damaged, it will attract infiltration of inflammatory cells in the blood circulation and release the pathogenic inflammatory mediators. So, the process of the renal fibrosis is started.[3] Treatment of diabetes by the principles of TCM, therefore, emphasizes improvements of “qi” and circulatory function.[10] In prescribing formulas, spleen-strengthening formulas such as Wu-Ling-San (WLS; Poria Five Powder; Wǔ Líng Sǎn) could eliminate fluid in the lower and middle body, help dispel moisture, and restore blood circulation.[11,12]
It should be noted that the scientific basis for the therapeutic effects of TCM, which utilizes herbs for therapy under the guidance of traditional theory, has been established in the literature reports. Rehmannia Six Formula (RF; Liù Wèi Dì Huáng Wán) is a formula that is commonly used in TCM to treat patients with diabetes. There are six herbs that make up RF, namely Rehmannia glutinosa, Fructus corni, Dioscorea, Poria cocos, Alisma, and Paeonia suffruticosa. On the basis of the publications found in PubMed during the period 2000–2009, RF appears to have beneficial effects on blood glucose, neuropathy, and nephropathy. There is also evidence of anti-inflammatory and antioxidant effects.[13] Pharmacological studies have revealed that other TCM formulas also exert a positive influence on diabetes. To gather information regarding the use of traditional Chinese formulas in the treatment of DN, we performed a literature search in PubMed using specific search terms such as traditional Chinese formulas and DN. In the current review, we mainly focus on the recent laboratory studies of the TCM formulas including WLS, Danggui-Buxue-Tang (DBT; Dāng Guī Bǔ Xuè Tang), and Danggui-Shaoyao-San (DSS; Dāng Guī Sháo Yào Sǎn), conducted by the Committee on Chinese Medicine and Pharmacy at the Department of Health of Taiwan Government, in the amelioration of DN. The appearance and weight of each herb in formulas according to ancient medical literature are shown in Figure 1.
Figure 1.

The appearance and the weight of each herb in (a) Wu-Ling-San, (b) Danggui-Buxue-Tang, and (c) Danggui-Shaoyao-San
Wu-Ling-San (WLS; Poria Five Powder; Wǔ Líng Sǎn)
WLS, also called Hoelen Five Herb Formula, is a classic herbal combination for promoting water metabolism and is composed of four water-draining herbs including hoelen (Sclerotium Poriae Cocos; Fú Líng), alisma (Alismatis Rhizoma; Jí Xiè), polyporus sclerotium (Sclerotium polypori Umbrellati; Zhū Líng), and bighead atractylodes rhizome (Rhizoma Atractylodis Macrocephalae; Bái Zhú) plus a warm herb, cinnamon twig (Ramulus Cinnamomi Cassiae; Guì Zhī). WLS has been widely used to treat edema, such as scrotal edema and cardiac edema, urine retention or difficult urination, and has been applied for promoting blood circulation.[11,12] It has been reported that WLS suppresses the development of nephrocalcinosis induced by a high phosphorus diet in rats, inhibited the synthesis and expression of endothelin-1 in rats with anti-glomerular basement membrane nephritis, and reduced calcium oxalate crystallization in human urine.[14,15,16] Currently, it has been demonstrated that WLS possesses the therapeutic potential to ameliorate adriamycin-induced nephrotic syndrome in rats.[17] It provides an important pharmacological and therapeutic basis of WLS in the treatment of various kidney diseases.
Danggui-Buxue-Tang (DBT; Dāng Guī Bǔ Xuè tang)
DBT, also known Tangkuei liù wèi dì huángand Astragalus decoction, is widely employed in TCM because of the marked hematopoietic properties of this preparation.[18] DBT was formulated originally during the Jin dynasty with two main ingredients, angelica root (Radix Angelicae Sinensis; Dāng Guī Gēn) and astragalus root (Radix Astragali; Huáng Qí) in a ratio of 1:5.[19] It has been documented that higher amounts of angelica root–derived ferulic acid, and astragalus root–derived astragaloside IV, calycosin, and formononetin were found in DBT, with angelica root and astragalus root in a 1:5 ratio.[20] With this ratio of its ingredients, DBT has been found to reduce menopausal symptoms.[21] This formula treats consumptive fatigue and internal injury which causes deficient “qi” and blood, and “floating yang” appears externally. Recent findings indicate that DBT has the ability to promote hematopoietic function, stimulate cardiovascular circulation, prevent osteoporosis, and antagonize the activity of tumors.[19,22] Actually, DBT has an ability to lower higher plasma glucose in streptozotocin-induced diabetic rats (STZ-diabetic rats), the type 1 diabetes-like animal model.[23] DBT also displays the characteristic of rosiglitazone to ameliorate insulin resistance induced by a high-fructose diet in rats.[24] It seems that this traditional Chinese herbal preparation is valued in the glucose homeostasis and may therefore be utilized as adjuvant therapy for control of diabetes and its complications.
Danggui-Shaoyao-San (DSS; Dāng Guī Sháo Yào Sǎn)
DSS, also called Tangkuei and Paeonia Formula, comprising white peony root (Radix Paeoniae Alba), angelica root, chuanxiong rhizome (Rhizoma Chuanxiong), hoelen, bighead atractylodes rhizome and alisma, is a widely used formula of TCM derived from “Jingui Yaolue,” a medical classic written by Zhongjing Zhang in the Eastern Han Dynasty. Identification and determination of the major constituents in traditional Chinese medicinal prescriptions is constantly being carried out. Researchers have much information on several active fractions and components from DSS.[25] Monoterpene glycosides, phenolic compounds, and phthalides are the most representative components of DSS as far as both the contents and their biological activities are concerned. Monoterpene glycosides are responsible for the efficacy of white peony root. A case in point is albiflorin and paeoniflorin, which exhibits analgesia, spasmolysis, anti-inflammation, and anticoagulation activities.[26] Phenolic acids and phthalides in angelica root and chuanxiong rhizome also have vasodilatative, antithrombotic, antioxidative, anti-inflammatory, and muscle relaxant effects.[27] In addition, atractylenolides from bighead atractylodes rhizome showed gastrointestinal inhibitory, anti-inflammatory, and antioxidative activity.[28] Meanwhile, cytotoxic, anti-inflammatory, and antioxidant activities of triterpenes in alisma and hoelen have also been documented.[29,30] DSS has been used in China as a blood-activating and stasis-eliminating drug to treat gynecological disorders such as dysmenorrhea, amenorrhea, and infertility without observation of any side effects for thousands of years.[31,32] Recent studies show that it also possesses the capability of treating neural dysfunctions such as senile dementia, memory loss, and other cognitive disorders; therefore, the formula is used as a remedy for Alzheimer's disease in Japan.[33,34]
BENEFICIAL EFFECTS OF THE SELECTED TCM FORMULAS IN DN
DN is a progressive disease that causes glomerular fibrosis and impairment of renal function, with progression over time. Urinary albumin excretion has been demonstrated to be a good clinical predictor of renal lesions in DN.[35] In our previous study, the increase in urinary albumin concentration corresponding to hyperglycemia was more pronounced 8 weeks following the induction of diabetes by STZ. In addition, serum creatinine and blood urea nitrogen levels and creatinine clearance, generally considered as markers of renal function, were higher in STZ-diabetic rats than those of non-diabetic group, implying the presence of diabetic kidney disease with renal hyperfiltration.[23,36,37] Repeated treatment with DBT (3.6 g/kg/day), WLS (2.5 g/kg/day), or DSS (2.8 g/kg/day) for 8-12 weeks could attenuate albuminuria and ameliorate the loss of renal function and glomerular hyperfiltration in STZ-diabetic rats.[23,36,37] The selected traditional Chinese medicinal formulas be beneficial in the treatment of DN has been considered.
In addition to the increase in urinary albumin excretion, one of the most remarkable renal pathological findings in DN is mesangial expansion due to pathological accumulation of extracellular matrix (ECM) components, such as fibronectin and type IV collagen, in glomeruli.[38] In our STZ-induced type-1 diabetic rats, not only fibronectin but also type IV collagen accumulated in the mesangial area of glomeruli; on the contrary, the protein was expressed at lower levels in glomeruli of STZ-diabetic rats treated for 8-12 weeks with WLS (2.5 g/kg/day), DBT (3.6 g/kg/day), or DSS (2.8 g/kg/day). Furthermore, the increased kidney weight as well as accelerated mesangial expansion in glomeruli of STZ-diabetic rats were remissive by treatment with these prescriptions.[23,36,37] The results reflected these prescriptions have potential effects on the deterioration of renal fibrosis through reduction of renal ECM accumulation in STZ-diabetic rats, which may lead to ameliorate or delay the development of advanced diabetic renal injury.
POSSIBLE MECHANISMS OF ACTION OF THE SELECTED TCM FORMULAS TO AMELIORATE DN
Suppression of TGF-β expression by the selected TCM formulas to ameliorate DN
Members of the transforming growth factor (TGF)-β family of factors are established to modulate cell growth and differentiation.[39] TGF-β family members include TGF-β1, TGF-β2, and TGF-β3, each of which is encoded by a different gene on a different chromosome. TGF-β1 is the most predominant (~90%) and active TGF-β family member. TGF-β is mainly synthesized in blood-forming cells such as platelets, macrophages, and mononuclear cells. In the kidney, TGF-β is provided by infiltrating white cells (macrophages, T-cells, and B-cells) and activated renal cells (tubular cells, glomerular cells, and mesangial cells). Renal cells are also targets of TGF-β signaling. After interacting with the TGF-β receptor (TβR), TGF-β crosses the plasmalemma via the Smad protein to regulate nuclear gene transcription.[40]
Previous studies have revealed that kidney glomerular and tubular TGF-β1 mRNA and protein concentrations are elevated in STZ-diabetic animals, as well as in diabetes-prone BioBreeding rats, non-obese diabetic mice, and obese, hyperglycemic, insulin-resistant type-2 diabetic db/db mice.[41,42] Additionally, expression of TβR mRNA and protein in the kidney is increased in diabetic animals, and TGF-β1 synthesis by mononuclear cells in peripheral blood is elevated in diabetic patients.[43] TGF-β1 has been shown to induce collagen secretion from kidney cells and to inhibit collagenase activity, leading to accumulation of ECM.[44] TGF-β1 also transforms kidney epithelial cells into fibrocytes, thereby increasing the rates of ECM deposition and appearance of nephrosclerosis.[45] Moreover, TGF-β1 causes kidney glomerular and tubular cells to undergo hypertrophy, resulting in increased kidney size.[46] Thus, TGF-β1 has been considered as a therapeutic target in fibrotic disease such as DN and other chronic kidney diseases. It was worth noting that the overexpression of TGF-β1 in glomeruli of diabetic rats was lessened with WLS, DBT, or DSS treatment.[23,36,37] Thus, the nephropathy-protective effects of the selected TCM formulas were mediated by the downregulation of TGF-β1 expression.
The plasma glucose lowering effects of the selected TCM formulas are associated with the DN amelioration
Both hyperglycemia and hyperlipidemia have been linked to diabetic complications, especially renal damage.[47] In our previous study, significant increase in fasting blood glucose in STZ-diabetic was observed when compared to normal control group and this change was more marked at the 8th week following diabetes induction. Furthermore, the blood glucose lowering effect was obvious when STZ-diabetic rats were treated with DBT at the daily dosage of 3.6 g/kg for 8 weeks.[23] The plasma glucose lowering effect was marked when STZ-diabetic rats received WLS treatment at 2.5 g/kg/day for 10 weeks.[36] DSS also ameliorated the hyperglycemia in STZ-diabetic rats at the end of the 12-weeks treatment.[37] Neither DBT, nor WLS or DSS at any dosage made changes in the plasma levels of cholesterol and triglyceride in STZ-diabetic rats throughout the study period.[23,36,37] The lower blood glucose levels induced by treatment of STZ-diabetic rats with WLS, DBT, or DSS represents an obvious factor in the assessment of the mechanism (s) by which the selected TCM formulas prevent renal functional and structural changes that might be linked to reduce the hyperglycemic state in diabetic rats.
Inhibition of AGE–RAGE pathway by the selected TCM formulas to ameliorate DN
The hyperglycemia condition, a chronic metabolic disorder of glucose, results in irreversible tissue damage by the protein glycation reaction, which leads to the formation of glycosylated protein and AGEs.[4] It has been reported that AGEs trigger the activation of nuclear factor kappa B (NF-κB) by interaction with receptor for AGE (RAGE). NF-κB is present in the cytoplasm, as a complex with its inhibitory protein known as IκB. After activation by a number of physiological and nonphysiological stimuli, IκB dissociates from NF-κB within minutes and undergoes ubiquitination and degradation. Once NF-κB is released from the inhibitory unit IκB, the NF-κB is then translocated into the nucleus. Upon its nuclear translocation, NF-κB undergoes phosphorylation on serine 276 in its p65 subunit and associates with surrounding chromatin components. It subsequently binds with DNA and promotes the transcription of proinflammatory cytokines.[48] Studies have also demonstrated that NF-κB was involved in the induction of monocyte chemotactic protein-1 in mesangial cell cultured under high glucose condition and subsequently mediated macrophage accumulation.[49] Moreover, the AGE-RAGE interaction activates TGF-β1 signaling pathways and subsequently induces mesangial cell hypertrophy and glomerular sclerosis by ECM synthesis.[4] Therefore, AGEs’ accumulation in the kidney has been regarded as an index of progressive renal damage in DN.
Actually, not only the overexpression of AGEs and RAGE but also the higher levels of NF-kB and TGF-β1 in the kidney of STZ-diabetic rats were alleviated by a 10-week treatment with 2.5 g/kg/day WLS.[36] The higher renal levels of AGEs in STZ-diabetic rats were effectively lowered by 12 weeks of 2.8 g/kg/day DSS treatment.[36] Similarly, the elevated NF-κB and TGF-β1 protein expressions in the kidney of STZ-diabetic rats were reduced by 12 weeks of DSS treatment.[37] It seems that the selected traditional Chinese medicinal formulas influenced not only the AGE-RAGE signaling but also the NF-κB-TGF-β1-dependent pathway to some extent, thus leading to attenuate the renal damage caused by the protein glycation reaction.
Inhibition of oxidative stress by the selected TCM formulas to ameliorate DN
Individuals with diabetes and experimental animal models exhibit high oxidative stress due to persistent and chronic hyperglycemia.[50] The increased lipid peroxidation in the kidney implies the level of susceptibility to diabetic oxidative stress, leading to diabetic complications. From this viewpoint, prevention of hyperlipidemia and/or lipid peroxidation resulting from oxidative stress is considered to play a crucial role in protection from disorders associated with diabetes.[51] When considering AGEs from another viewpoint, Nε-(carboxymethyl) lysine (CML), pentosidine, and methylglyoxal derivatives are among some of the well-characterized compounds that commonly are used as AGE markers.[6] Particularly, CML is not only referred to as a glycoxidation product, but is also formed during the metal-catalyzed oxidation of polyunsaturated fatty acids in the presence of protein.[52] Therefore, CML could serve as a general bio-marker of oxidative stress resulting from carbohydrate and lipid oxidation reactions. We found that treatment of STZ-diabetic rats with 12 weeks of DSS (2.8 g/kg/day) not only lowered the renal CML level but also decreased the accumulation of lipid peroxidation products in the kidney.[37] Ten weeks of WLS administration also successfully reduced the renal levels of CML or thiobarbituric acid (TBA) reactive substance, an index of endogenous lipid peroxidation, at the dose of 2.5 g/kg/day.[36,53] The results indicated that the beneficial effect of selected TCM formulas on DN was linked to reduce the intensity of oxidative stress under diabetic state.
Among the antioxidative enzymes, superoxide dismutase (SOD) catalyzes dismutation of the superoxide anion into hydrogen peroxide, while glutathione peroxidase (GSH-Px) both detoxifies hydrogen peroxides and converts lipid hydroperoxides to non-toxic alcohols; thus, the antioxidant enzyme activities could reflect antioxidant defense status.[54] The reduced activities of SOD and GSH-Px in the kidney of STZ-diabetic rats could be elevated by DSS.[37] The selected TCM formulas protect the kidney of diabetic rats from oxidative damage by enhancing enzymatic antioxidative defense systems could be considerable.
The selected TCM formulas for treating DN act independently by blocking RAS
It has long been known that hypertension is an aggravating factor in increased intraglomerular pressure and may be the key hemodynamic determinant of diabetic renal injury as well; it is therefore well recognized that blood pressure control is important in diabetic patients.[7] Angiotensin (Ang) II is known to favor increased TGF-β1 production. ACEIs reduce both the generation of Ang II and the synthesis of TGF-β1.[55] In addition, Ang II is well recognized to be one of the major causes of mesangial matrix expansion in DN.[56] ACEI and ARBs have been shown to cause persistent normalization of blood pressure, retard the progression of renal failure, and markedly reduce the associated mortality.[57] In a previous study, the increase in Ang II concentrations in rats with DN was not affected by treatment with DBT, whereas the treatment resulted in reduced TGF-β1 expression in the kidney.[23] This finding demonstrated that DBT does not exert its renoprotective effects through inhibition of renin–angiotensin system (RAS). Furthermore, WLS and DSS were observed to have no influence on the blood pressure in STZ-diabetic rats.[36,37] These data have implications that the selected TCM formulas exhibit a renoprotective effect in an animal model of DN by inhibiting the expression of TGF-β1, which might be linked to its beneficial effect on the hyperglycemic state, but did not correlate with blocking intrarenal RAS. However, the other TCM formulas such as RF have the ability to suppress hyperactivity of RAS, leading to ameliorate the progressive renal failure in five out of six nephrectomized rats.[58] The pathology and mechanisms of DN are complicated, thus providing various formulas with different action mechanisms for prevention, amelioration, or treatment of DN. The renoprotective effects and the optimal application dose of the TCM formulas are worth to be evaluated further.
PERSPECTIVES AND FUTURE DIRECTIONS
Implication for practice
The findings from the selected TCM formulas are of merit in revealing that WLS, DBT, and DSS have anti-diabetic properties, with antihyperglycemic activity accompanied by an improvement in renal functions in STZ-diabetic rats. These selected TCM formulas also have the capacity to ameliorate the defective antioxidative defense system, leading to modulate the oxidative stress, thereby resulting in downregulation of NF-κB as well as TGF-β1 and, consequently, attenuation of ECM components such as fibronectin or type IV collagen expression in diabetic renal cortex tissue. The possible mechanisms of the selected TCM formulas on the amelioration of DN have been shown in Figure 2. The TCM formulas may be useful as adjuvant therapy in the treatment of diabetes and are also helpful to prevent and/or delay the onset of diabetes-induced renal injury. Until now, the therapeutic effects of the selected formulas including DBT, WLS, and DSS are limited to animal models. Using a metabolism coefficient of 6.25 to convert the effective daily oral dose of DBT (3.6 g/kg), WLS (2.5 g/kg), or DSS (2.8 g/kg) for rats into a clinical dose and assuming an average adult body weight of 60 kg,[59] we estimated the daily oral dose of DBT, WLS, or DSS applied in TCM to treat any diabetic patient in the future to be approximately 36, 24, and 27 g, respectively.
Figure 2.
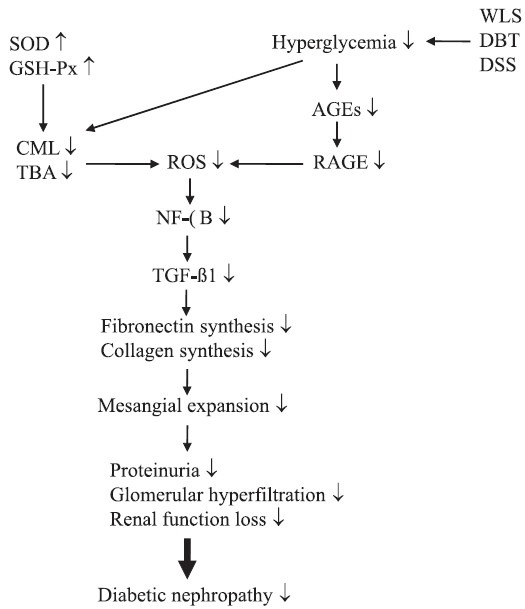
The possible mechanisms of action of Wu-Ling-San, Danggui-Buxue-Tang, or Danggui-Shaoyao-San on the amelioration of hyperglycemia-mediated renal injury in STZ-diabetic rats. The selected TCM formulas exhibit a renoprotective effect in an animal model of DN with an antihyperglycemic activity accompanied by suppression of AGE–RAGE signaling. These selected TCM formulas also protect the kidney of diabetic rats from oxidative damage by enhancing enzymatic antioxidative defense systems, thereby resulting in downregulation of NF-κB–TGF–β1-dependent pathway and, consequently, attenuation of ECM components such as fibronectin or type IV collagen expression in diabetic renal cortex tissue. ↓: decrease or reduction;↑: increase or enhance
Implications for research
Safety is a fundamental principle in the provision of herbal medicines and herbal products for health care, and a critical component of quality control. But there is a widespread misconception among most consumers and patients that “natural” always means “safe” and a common belief that remedies from natural origin are harmless and carry no risk. In fact, the health risks of herbal remedies or TCM formula include direct toxic effects, contaminations such as with heavy metals or unlabeled pharmaceutical agents, drug interactions, and the indirect risk that a herb without demonstrable efficacy may impair, delay, or replace conventional treatments.[60] Therefore, World Health Organization (WHO) published the WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems in 2004.[60] Thus, further studies should be contemplated to assess the long-term benefits and safety of the selected TCM formulas in the treatment/and or prevention of diabetes-induced renal injury and other related complications.
Most TCM remedies are formulated to contain different herbs in combination in order to enhance the curative efficacy and also reduce the side effects; it is relatively difficult to figure out which component from the formula is the main effective monomer for the therapy of DN. Further studies will be required to identify the ingredients and/or chemicals in the TCM formulas, which are responsible for the beneficial renal effects observed.
REFERENCES
- 1.Vivian EM. Type 2 diabetes in children and adolescents-the next epidemic? Curr Med Res Opin. 2006;22:297–306. doi: 10.1185/030079906X80495. [DOI] [PubMed] [Google Scholar]
- 2.Arredondo A. Diabetes: A global challenge with high economic burden for public health systems and society. Am J Public Health. 2013;103:e1–2. doi: 10.2105/AJPH.2012.301106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossing P. Diabetic nephropathy: Worldwide epidemic and effects of current treatment on natural history. Curr Diab Rep. 2006;6:479–83. doi: 10.1007/s11892-006-0083-y. [DOI] [PubMed] [Google Scholar]
- 4.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: Mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 5.LeRoith D, Rayfield EJ. The benefits of tight glycemic control in type 2 diabetes mellitus. Clin Cornerstone. 2007;8(Suppl 7):S19–29. doi: 10.1016/s1098-3597(07)80018-4. [DOI] [PubMed] [Google Scholar]
- 6.Yamagishi S, Matsui T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid Med Cell Longev. 2010;3:101–8. doi: 10.4161/oxim.3.2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glandt M, Bloomgarden ZT. Hypertension in diabetes: Treatment considerations. J Clin Hypertens (Greenwich) 2011;13:314–8. doi: 10.1111/j.1751-7176.2011.00442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolasinska-Malkowska K, Filipiak KJ, Gwizdala A, Tykarski A. Current possibilities of ACE inhibitor and ARB combination in arterial hypertension and its complications. Expert Rev Cardiovasc Ther. 2008;6:759–71. doi: 10.1586/14779072.6.5.759. [DOI] [PubMed] [Google Scholar]
- 9.Schrijvers BF, De Vriese AS. Novel insights in the treatment of diabetic nephropathy. Acta Clin Belg. 2007;62:278–90. doi: 10.1179/acb.2007.043. [DOI] [PubMed] [Google Scholar]
- 10.Cheng JT. Review: Drug therapy in Chinese traditional medicine. J Clin Pharmacol. 2000;40:445–50. doi: 10.1177/00912700022009198. [DOI] [PubMed] [Google Scholar]
- 11.Poon TY, Ong KL, Cheung BM. Review of the effects of the traditional Chinese medicine Rehmannia six formula on diabetes mellitus and its complications. J Diabetes. 2011;3:184–200. doi: 10.1111/j.1753-0407.2011.00130.x. [DOI] [PubMed] [Google Scholar]
- 12.Guangdong Medical Journal Editorial Department. Clinical observation of curative effects of Wu Ling San for treatment of glaucoma. Guangdong Yi Xue. 1982;3:40. [Google Scholar]
- 13.Chen DA. Treating 27 cases of edema in the lower limbs with Wu Ling San. Xinjiang Zhong Yi Yao. 1998;16:19–20. [Google Scholar]
- 14.Liu QL, Sato S, Kishikawa T, Matsuzaki H, Yamanaka N. Effectiveness of a traditional Chinese medicine, Wulingsan, in suppressing the development of nephrocalcinosis induced by a high phosphorus diet in young rats. Med Electron Microsc. 2001;34:103–14. doi: 10.1007/s007950170004. [DOI] [PubMed] [Google Scholar]
- 15.Hattori T, Fujitsuka N, Kurogi A, Shindo S. Sairei-to may inhibit the synthesis of endothelin-1 in nephritic glomeruli. Nippon Jinzo Gakkai Shi. 1997;39:121–8. [PubMed] [Google Scholar]
- 16.Yoshimura K, Miyake O, Okuyama A, Yoshioka T, Honda M, Yamaguchi S, et al. Effect of chorei-to and gorei-san on calcium oxalate crystallization in human urine. Hinyokika Kiyo. 1998;44:13–6. [PubMed] [Google Scholar]
- 17.He L, Rong X, Jiang JM, Liu PQ, Li Y. Amelioration of anti-cancer agent adriamycin-induced nephrotic syndrome in rats by Wulingsan (Gorei-San), a blended traditional Chinese herbal medicine. Food Chem Toxicol. 2008;46:1452–60. doi: 10.1016/j.fct.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Ning L, Chen CX, Jin RM, Wu YP, Zhang HG, Sun CL, et al. Effect of components of dang-gui-bu-xue decoction on hematopenia. Zhongguo Zhong Yao Za Zhi. 2002;27:50–3. [PubMed] [Google Scholar]
- 19.Song ZH, Ji ZN, Lo CK, Dong TT, Zhao KJ, Li OT, et al. Chemical and biological assessment of a traditional chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis: Orthogonal array design to optimize the extraction of chemical constituents. Planta Med. 2004;70:1222–7. doi: 10.1055/s-2004-835855. [DOI] [PubMed] [Google Scholar]
- 20.Dong TT, Zhao KJ, Gao QT, Ji ZN, Zhu TT, Li J, et al. Chemical and biological assessment of a chinese herbal decoction containing Radix Astragali and Radix Angelicae Sinensis: Determination of drug ratio in having optimized properties. J Agric Food Chem. 2006;54:2767–74. doi: 10.1021/jf053163l. [DOI] [PubMed] [Google Scholar]
- 21.Huntley AL, Ernst E. A systematic review of herbal medicinal products for the treatment of menopausal symptoms. Menopause. 2003;10:465–76. doi: 10.1097/01.GME.0000058147.24036.B0. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh CC, Lin WC, Lee MR, Hsu SL, Liu HS, Kao ST, et al. Dang-Gui-Bu-Xai-Tang modulated the immunity of tumor bearing mice. Immunopharmacol Immunotoxicol. 2003;25:259–71. doi: 10.1081/iph-120020474. [DOI] [PubMed] [Google Scholar]
- 23.Zhang YW, Xie D, Xia B, Zhen RT, Liu IM, Cheng JT. Suppression of transforming growth factor-beta1 gene expression by Danggui buxue tang, a traditional Chinese herbal preparation, in retarding the progress of renal damage in streptozotocin-induced diabetic rats. Horm Metab Res. 2006;38:82–8. doi: 10.1055/s-2006-925118. [DOI] [PubMed] [Google Scholar]
- 24.Liu IM, Tzeng TF, Liou SS. A Chinese herbal decoction, Dang Gui Bu Xue Tang, prepared from Radix Astragali and Radix Angelicae sinensis, ameliorates insulin resistance induced by a high-fructose diet in rats. Evid Based Complement Alternat Med. 2011;2011:248231. doi: 10.1093/ecam/nep004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen L, Qi J, Chang YX, Zhu D, Yu B. Identification and determination of the major constituents in Traditional Chinese Medicinal formula Danggui-Shaoyao-San by HPLC-DAD-ESI-MS/MS. J Pharm Biomed Anal. 2009;50:127–37. doi: 10.1016/j.jpba.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Tsai HY, Lin YT, Tsai CH, Chen YF. Effects of paeoniflorin on the formalin-induced nociceptive behaviour in mice. J Ethnopharmacol. 2001;75:267–71. doi: 10.1016/s0378-8741(00)00403-7. [DOI] [PubMed] [Google Scholar]
- 27.Huang WY, Sheu SJ. Separation and identification of the organic acids in Angelicae Radix and Ligustici Rhizoma by HPLC and CE. J Sep Sci. 2006;29:2616–24. doi: 10.1002/jssc.200600136. [DOI] [PubMed] [Google Scholar]
- 28.Dong H, He L, Huang M, Dong Y. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat Prod Res. 2008;22:1418–27. doi: 10.1080/14786410801931629. [DOI] [PubMed] [Google Scholar]
- 29.Huang YT, Huang DM, Chueh SC, Teng CM, Guh JH. Alisol B acetate, a triterpene from Alismatis rhizoma, induces Bax nuclear translocation and apoptosis in human hormone-resistant prostate cancer PC-3 cells. Cancer Lett. 2006;231:270–8. doi: 10.1016/j.canlet.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 30.Kikuchi T, Uchiyama E, Ukiya M, Tabata K, Kimura Y, Suzuki T, et al. Cytotoxic and apoptosis-inducing activities of triterpene acids from Poria cocos. J Nat Prod. 2011;74:137–44. doi: 10.1021/np100402b. [DOI] [PubMed] [Google Scholar]
- 31.Kotani N, Oyama T, Sakai I, Hashimoto H, Muraoka M, Ogawa Y, et al. Analgesic effect of a herbal medicine for treatment of primary dysmenorrhea-a double-blind study. Am J Chin Med. 1997;25:205–12. doi: 10.1142/S0192415X9700024X. [DOI] [PubMed] [Google Scholar]
- 32.Jiang H, Shen Y, Wang XG. Current progress of Chinese medicinal treatment of endometriosis. Chin J Integr Med. 2010;16:283–8. doi: 10.1007/s11655-010-0283-9. [DOI] [PubMed] [Google Scholar]
- 33.Kano Y, Takaguchi S, Nohno T, Hiragami F, Kawamura K, Iwama MK, et al. Chinese medicine induces neurite outgrowth in PC12 mutant cells incapable of differentiation. Am J Chin Med. 2002;30:287–95. doi: 10.1142/S0192415X02000260. [DOI] [PubMed] [Google Scholar]
- 34.Hatip-Al-Khatib I, Egashira N, Mishima K, Iwasaki K, Iwasaki K, Kurauchi K, et al. Determination of the effectiveness of components of the herbal medicine Toki-Shakuyaku-San and fractions of Angelica acutiloba in improving the scopolamine-induced impairment of rat's spatial cognition in eight-armed radial maze test. J Pharmacol Sci. 2004;96:33–41. doi: 10.1254/jphs.fpj04015x. [DOI] [PubMed] [Google Scholar]
- 35.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: A role for the glomerular endothelium? Diabetologia. 2008;51:714–25. doi: 10.1007/s00125-008-0961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu IM, Tzeng TF, Liou SS, Chang CJ. The amelioration of streptozotocin diabetes-induced renal damage by Wu-Ling-San (Hoelen Five Herb Formula), a traditional Chinese prescription. J Ethnopharmacol. 2009;124:211–8. doi: 10.1016/j.jep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 37.Liu IM, Tzeng TF, Liou SS, Chang CJ. Beneficial effect of traditional chinese medicinal formula danggui-shaoyao-san on advanced glycation end-product-mediated renal injury in streptozotocin-diabetic rats. Evid Based Complement Alternat Med. 2012;2012:140103. doi: 10.1155/2012/140103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Falk RJ, Scheinman JI, Mauer SM, Michael AF. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32:34–9. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- 39.Schnaper HW, Hayashida T, Hubchak SC, Poncelet AC. TGF-beta signal transduction and mesangial cell fibrogenesis. Am J Physiol Renal Physiol. 2003;284:243–52. doi: 10.1152/ajprenal.00300.2002. [DOI] [PubMed] [Google Scholar]
- 40.Miyazono K. TGF-beta signaling by Smad proteins. Cytokine Growth Factor Rev. 2000;11:15–22. doi: 10.1016/s1359-6101(99)00025-8. [DOI] [PubMed] [Google Scholar]
- 41.Cruzado JM, Lloberas N, Torras J, Riera M, Fillat C, Herrero-Fresneda I, et al. Regression of advanced diabetic nephropathy by hepatocyte growth factor gene therapy in rats. Diabetes. 2004;53:1119–27. doi: 10.2337/diabetes.53.4.1119. [DOI] [PubMed] [Google Scholar]
- 42.Hong SW, Isono M, Chen S, Iglesias-De La Cruz MC, Han DC, Ziyadeh FN. Increased glomerular and tubular expression of transforming growth factor-beta1, its type II receptor, and activation of the Smad signaling pathway in the db/db mouse. Am J Pathol. 2001;158:1653–63. doi: 10.1016/s0002-9440(10)64121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schrijvers BF, De Vriese AS, Flyvbjerg A. From hyperglycemia to diabetic kidney disease: The role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr Rev. 2004;25:971–1010. doi: 10.1210/er.2003-0018. [DOI] [PubMed] [Google Scholar]
- 44.Li JH, Huang XR, Zhu HJ, Johnson R, Lan HY. Role of TGF-beta signaling in extracellular matrix production under high glucose conditions. Kidney Int. 2003;63:2010–9. doi: 10.1046/j.1523-1755.2003.00016.x. [DOI] [PubMed] [Google Scholar]
- 45.Tian YC, Fraser D, Attisano L, Phillips AO. TGF-beta1-mediated alterations of renal proximal tubular epithelial cell phenotype. Am J Physiol Renal Physiol. 2003;285:130–42. doi: 10.1152/ajprenal.00408.2002. [DOI] [PubMed] [Google Scholar]
- 46.Monkawa T, Hiromura K, Wolf G, Shankland SJ. The hypertrophic effect of transforming growth factor-beta is reduced in the absence of cyclin-dependent kinase-inhibitors p21 and p27. J Am Soc Nephrol. 2002;13:1172–8. doi: 10.1097/01.asn.0000013162.29833.45. [DOI] [PubMed] [Google Scholar]
- 47.Kanwar YS, Wada J, Sun L, Xie P, Wallner EI, Chen S, et al. Diabetic nephropathy: Mechanisms of renal disease progression. Exp Biol Med (Maywood) 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 48.Lv ZM, Wang Q, Wan Q, Lin JG, Hu MS, Liu YX, et al. The role of the p38 MAPK signaling pathway in high glucose-induced epithelial-mesenchymal transition of cultured human renal tubular epithelial cells. PLoS One. 2011;6:e22806. doi: 10.1371/journal.pone.0022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okazaki T, Sakon S, Sasazuki T, Sakurai H, Doi T, Yagita H, et al. Phosphorylation of serine 276 is essential for p65 NF-kappaB subunit-dependent cellular responses. Biochem Biophys Res Commun. 2003;300:807–12. doi: 10.1016/s0006-291x(02)02932-7. [DOI] [PubMed] [Google Scholar]
- 50.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. J Am Soc Nephrol. 2003;14:S250–3. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 51.Dominguez JH, Tang N, Xu W, Evan AP, Siakotos AN, Agarwal R, et al. Studies of renal injury III: Lipid-induced nephropathy in type II diabetes. Kidney Int. 2000;57:92–104. doi: 10.1046/j.1523-1755.2000.00814.x. [DOI] [PubMed] [Google Scholar]
- 52.Fu MX, Requena JR, Jenkins AJ, Lyons TJ, Baynes JW, Thorpe SR. The advanced glycation end product, Nepsilon-(carboxymethyl) lysine, is a product of both lipid peroxidation and glycoxidation reactions. J Biol Chem. 1996;271:9982–6. doi: 10.1074/jbc.271.17.9982. [DOI] [PubMed] [Google Scholar]
- 53.Janero DR, Burghardt B. Analysis of cardiac membrane phospholipid peroxidation kinetics as malondialdehyde: Nonspecificity of thiobarbituric acid-reactivity. Lipids. 1988;23:452–8. doi: 10.1007/BF02535519. [DOI] [PubMed] [Google Scholar]
- 54.Seifried HE, Anderson DE, Fisher EI, Milner JA. A review of the interaction among dietary antioxidants and reactive oxygen species. J Nutr Biochem. 2007;18:567–79. doi: 10.1016/j.jnutbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 55.Benigni A, Zoja C, Corna D, Zatelli C, Conti S, Campana M, et al. Add-on anti-TGF-beta antibody to ACE inhibitor arrests progressive diabetic nephropathy in the rat. J Am Soc Nephrol. 2003;14:1816–24. doi: 10.1097/01.asn.0000074238.61967.b7. [DOI] [PubMed] [Google Scholar]
- 56.Mima A, Matsubara T, Arai H, Abe H, Nagai K, Kanamori H, et al. Angiotensin II-dependent Src and Smad1 signaling pathway is crucial for the development of diabetic nephropathy. Lab Invest. 2006;86:927–39. doi: 10.1038/labinvest.3700445. [DOI] [PubMed] [Google Scholar]
- 57.Janiak P, Bidouard JP, Cadrouvele C, Poirier B, Gouraud L, Grataloup Y, et al. Long-term blockade of angiotensin AT1 receptors increases survival of obese Zucker rats. Eur J Pharmacol. 2006;534:271–9. doi: 10.1016/j.ejphar.2006.01.032. [DOI] [PubMed] [Google Scholar]
- 58.Lee BC, Choi JB, Cho HJ, Kim YS. Rehmannia glutinosa ameliorates the progressive renal failure induced by 5/6 nephrectomy. J Ethnopharmacol. 2009;122:131–5. doi: 10.1016/j.jep.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 59.Hodge HC, Downs WL, Panner BS, Smith DW, Maynard EA. Oral toxicity and metabolism of diuron (N-(3,4-dichlorophenyl)-N’, N’- dimethylurea) in rats and dogs. Food Cosmet Toxicol. 1967;5:513–31. doi: 10.1016/s0015-6264(67)83153-5. [DOI] [PubMed] [Google Scholar]
- 60.De Angelis C, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical trial registration: A statement from the international committee of medical journal editors. N Z Med J. 2004;117:U1054. [PubMed] [Google Scholar]


