Abstract
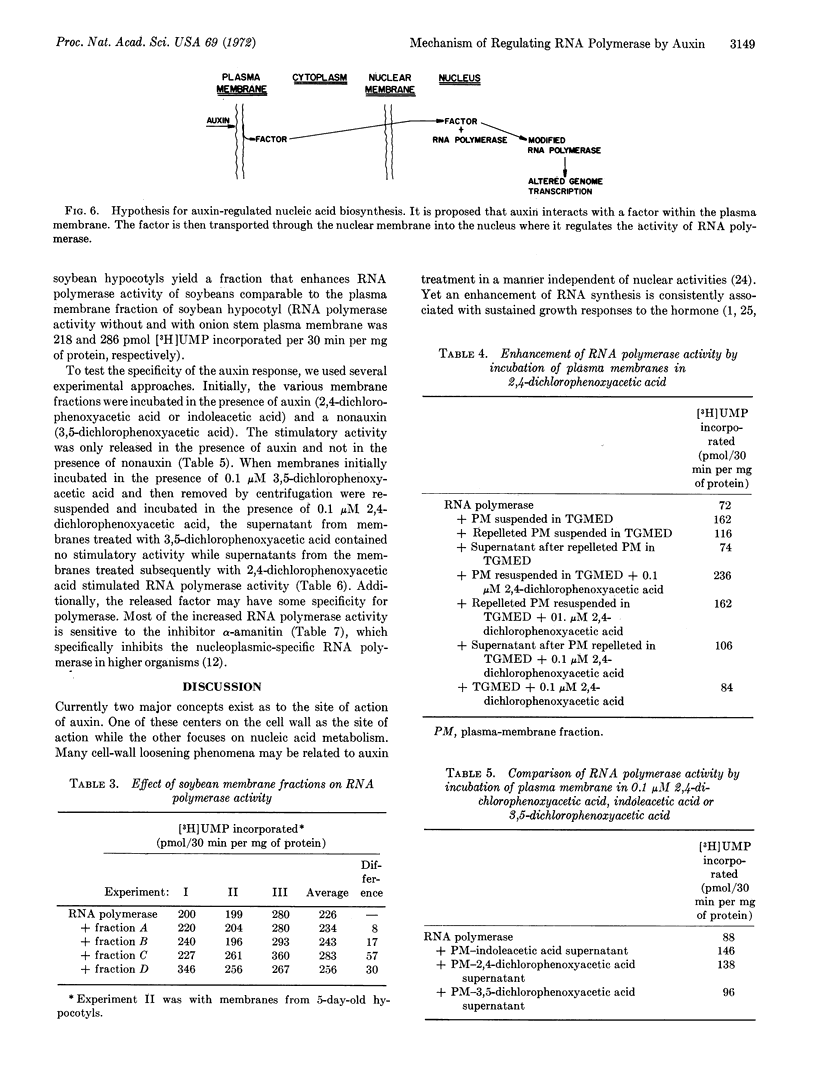
Using recently developed techniques for solubilization of RNA polymerase from soybean chromatin and isolation of plasma membrane fractions from plants we can show the presence of a transcriptional factor specifically released from the membranes by auxin, 2,4-dichlorophenoxyacetic acid. The nonauxin, 3,5-dichlorophenoxyacetic acid, does not release the factor, but subsequent exposure of the membranes to auxin results in its release. Factor activity could not be demonstrated in fractions devoid of plasma membranes. The presence of a regulatory factor for RNA polymerase associated with plant plasma membrane and specifically released by auxin provides a mechanism whereby both rapid growth responses and delayed nuclear changes could be derived from a common auxin receptor site associated with plasma membrane.
Keywords: soybean, transcription factor, chromatin
Full text
PDF
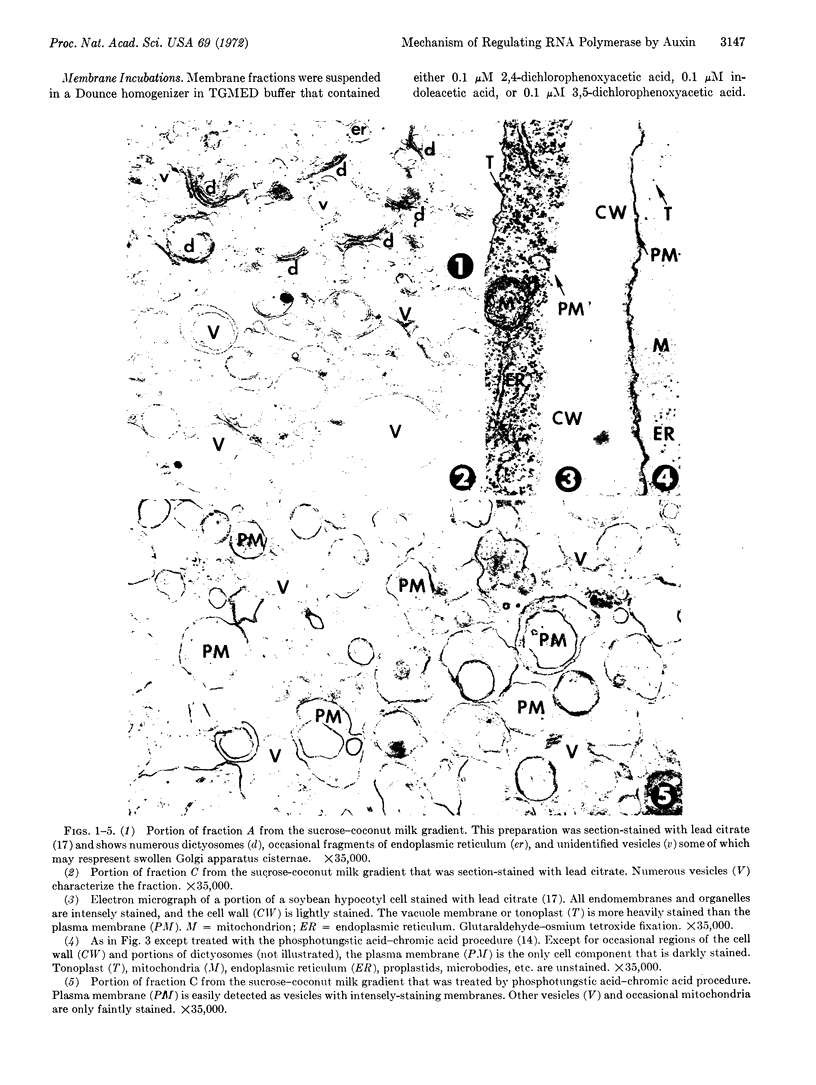

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P. Interaction of insulin with the cell membrane: the primary action of insulin. Proc Natl Acad Sci U S A. 1969 Jun;63(2):450–457. doi: 10.1073/pnas.63.2.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Isolation of the insulin receptor of liver and fat-cell membranes (detergent-solubilized-( 125 I)insulin-polyethylene glycol precipitation-sephadex). Proc Natl Acad Sci U S A. 1972 Feb;69(2):318–322. doi: 10.1073/pnas.69.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- HUANG R. C., BONNER J. Histone, a suppressor of chromosomal RNA synthesis. Proc Natl Acad Sci U S A. 1962 Jul 15;48:1216–1222. doi: 10.1073/pnas.48.7.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H. Solubilization and partial characterization of soybean chromatin-bound RNA polymerase. Biochem Biophys Res Commun. 1972 Jul 25;48(2):299–306. doi: 10.1016/s0006-291x(72)80050-0. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., O'Brien T. J., Cherry J. H. Stimulation of chromatin-bound RNA polymerase activity by a soluble factor. Biochim Biophys Acta. 1970 Dec 14;224(2):667–670. doi: 10.1016/0005-2787(70)90609-x. [DOI] [PubMed] [Google Scholar]
- Key J. L., Shannon J. C. Enhancement by Auxin of Ribonucleic Acid Synthesis in Excised Soybean Hypocotyl Tissue. Plant Physiol. 1964 May;39(3):360–364. doi: 10.1104/pp.39.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matthysse A. G., Phillips C. A protein intermediary in the interaction of a hormone with the genome. Proc Natl Acad Sci U S A. 1969 Jul;63(3):897–903. doi: 10.1073/pnas.63.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morré D. J. In vivo incorporation of radioactive metabolites by Golgi apparatus and other cell fractions of onion stem. Plant Physiol. 1970 Jun;45(6):791–799. doi: 10.1104/pp.45.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. J., Jarvis B. C., Cherry J. H., Hanson J. B. Enhancement by 2,4-dichlorophenoxyacetic acid of chromatin RNA polymerase in soybean hypocotyl tissue. Biochim Biophys Acta. 1968 Nov 20;169(1):35–43. doi: 10.1016/0005-2787(68)90006-3. [DOI] [PubMed] [Google Scholar]
- PENNINGTON R. J. Biochemistry of dystrophic muscle. Mitochondrial succinate-tetrazolium reductase and adenosine triphosphatase. Biochem J. 1961 Sep;80:649–654. doi: 10.1042/bj0800649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Shininger T. L., Ray M. M. ISOLATION OF beta-GLUCAN SYNTHETASE PARTICLES FROM PLANT CELLS AND IDENTIFICATION WITH GOLGI MEMBRANES. Proc Natl Acad Sci U S A. 1969 Oct;64(2):605–612. doi: 10.1073/pnas.64.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]
- Venis M. A. Stimulation of RNA transcription from pea and corn DNA by protein retained on sepharose coupled to 2,4-dichlorophenoxyacetic acid. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1824–1827. doi: 10.1073/pnas.68.8.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]



