Abstract
Execution of instrumental-activities of daily-living (IADL) requires the integration of its subcomponents namely, task-initiation, -planning and -performance. Research on their neuroanatomical correlates in Alzheimer's disease (AD) is sparse. Regional cerebral perfusion was measured using Single-Photon-Emission Computed Tomography in 13 bilateral regions-of-interest (ROI). The perfusion ratios obtained in 121 patients with AD were compared to that of 42 age-matched normal controls (NC). The perfusion correlates of IADL components, rated on the Disability Assessment in Dementia scale, were explored in the AD group. AD patients had lower perfusion in the posterior-cingulate and parietal regions bilaterally (p<0.01). Significant correlations were noted between IADL-initiation and bilateral fronto-striatal-anterior cingulate ROI (p<0.01), IADL-planning and right occipital ROI (p<0.05), and IADL-performance and right parietal ROI (p<0.05). Right lateral temporal perfusion was a common correlate of all three components (p<0.05). MMSE score and left anterior-cingulate perfusion explained 21% of the variance in IADL-initiation (R= 0.46; F=15.4; p<0.01). Perfusion correlates of various components of IADL differ in keeping with the heterogenous nature of cognitive processes involved in IADL.
Keywords: Alzheimer's disease, instrumental activities of daily living (IADL), cerebral perfusion
1. Introduction
Dementia in Alzheimer's Disease (AD) and other disorders is commonly defined by the presence of memory impairment and a decline in one other cognitive domain severe enough to impede an individual's functional ability in everyday life [2]. The progressive decline in activities of daily living during the course of the disease usually follows a hierarchical pattern of loss of functional routines. During the early stages of AD, patients have difficulty with complex instrumental activities of daily living (IADL), such as managing finances, going on an outing, shopping and preparing meals. This loss of IADL performance is eventually followed by a decline in self-care activities of daily living such as eating, dressing and bathing[13,27,37].
IADL is a multidimentional construct that reflects many different components. It has been suggested that breaking down these components of IADL into subcomponents at every step of an activity rather than the global task performance allows for more robust assessment of functional ability[3]. Functional assessments have been devised incorporating the motivational and cognitive aspects of every task such as ability to initiate an activity, ability to correctly plan the involved sequences and then effectively perform the sequences for an appropriate execution of the task[14]. Functional rating scales such as the Disability in Dementia scale have been devised to measure every step of task, which can be evaluated in conjunction with cortical brain changes that occur in AD to better understand underlying neuronal substrates of functional ability in AD [14].
Loss of functional autonomy can adversely affect patient's mood and behavior and add to caregiver stress. Functional status has also been shown to be an important determinant of the quality of life of an individual with AD [1]. Investigating functional performance in relation to clinical profiles can provide insight into functional ability in AD. Several studies have revealed a relationship between functional impairment and cognitive and behavioral symptoms in patients with AD that are thought to reflect frontal dysfunction[7,9,26,28,30,44,47]. Robust relationships have been reported between functional losses in AD and neuropathological hallmarks such as neuritic plaques and neurofibrillary tangle counts in orbitofrontal, medial temporal, occipital and anterior cingulate regions[5,31,42]. These findings together suggest that specific regions may be important mediators of functional impairment in AD. However, there is very little information on the associations between impairment in functional ability and in-vivo functional brain changes such as cerebral perfusion in AD.
Single photon emission computed tomography (SPECT) is a widely used clinical neuroimaging tool that provides a map of regional cerebral blood flow (rCBF), which can be used as a surrogate measure of neuroanatomical-function in AD. Typically, a radioactive tracer such as Tc-99m-ethyl-cysteinate-dimer (99mTc-ECD) is injected intravenously, which rapidly distributes through out the brain in accordance with rCBF. The tracer is believed to be taken up by neurons in accordance with blood flow, so that a regional uptake reflects the functional integrity of the specific region in the resting state. The distribution of the tracer provides a rCBF map. There is a limited amount of research on the relationship between cerebral perfusion parameters and functional ability in AD. Salmon et al. studied the relationship between cerebral metabolism using Positron Emission Tomography (PET) and overall IADL ability in mild to moderate AD[43]. They reported a significant correlation between total IADL score in their AD sample and regional glucose metabolism in the right inferior parietal cortex, right inferior temporal cortex and left superior occipital gyrus[43]. Ott et al. studied the relationship of driving ability to regional perfusion using SPECT in mild to moderate AD and found that driving score was associated with right temporoparietal, frontal and occipital perfusion as well as left temporoparietal perfusion [40]. However, the relationship between cerebral perfusion and the different sub-components of IADL in AD has not been previously explored. The objective of this study, therefore, was 1) to compare cerebral perfusion in specific brain regions in mild AD and healthy normal controls and 2) to explore the correlation between perfusion in these specific brain regions and individual components of IADL ability, namely initiation, planning and performance, in patients with mild AD.
2. Methods
2.1 Study participants
Perfusion and functional data of 121 patients with AD and 42 age-matched normal controls (NC) was drawn from the Sunnybrook Dementia Study, which is a prospective cohort of community-dwelling healthy elderly controls and patients with dementia recruited from a cognitive-neurology clinic at a university hospital. Patients between the ages of 60 and 90 years, who met the National Institute of Neurological and Communicative Disorders and Stroke-AD and Related Disorder Association (NINDS-ADRDA) diagnostic criteria for probable AD [33] and had a Mini Mental State Examination (MMSE)≥ 18, were included in this sub-study. Functional impairment due to unrelated disorders, such as arthritis, was exclusionary. The data for this study were obtained from the initial neuroimaging and functional assessment, which followed shortly after the patient's first assessment.
2.2 Regional cerebral perfusion determination
SPECT imaging was performed using a triple-head gamma camera (Prism 3000XP; Phillips Medical Systems Inc, Cleveland, Ohio) after injection of 20 mCi of Technetium-99m ethyl cysteinate dimer. Each view comprised a 128×128 pixel image with typically a 9.7 mm full-width at half-maximum reconstructed image resolution. Reconstructed images, performed using a ramp-filtered back-projection algorithm followed by a 3-dimensional restoration post-filter (Wiener filter, multiplier 1.0) and attenuation correction [10] were co-registered to an MRI-derived region-of-interest SPECT template in Talairach stereotaxic[29]. Mean perfusion ratios referenced to the cerebellum were derived in 40 brain regions of this template[29].
2.3 Regions of Interest
We targeted all limbic and association cortices of the brain known to have predominantly cognitive specialization. From the 40 SPECT template regions[29], we created 13 composite regions of interest (ROI) in each hemisphere by combining smaller regions. These regions were selected based on their putative roles in cognitive functioning and are summarized in Table 1[15,20,35,45].
Table 1.
Regions of interest (ROI), their corresponding topographical areas and volumes.
| ROI | Brodmann Area | ROI volume (cm3) | Cognitive specialization | |
|---|---|---|---|---|
| Right | Left | |||
| Frontal Pole | 10 | 13.2 | 11.3 | Comportment, Executive functions such as response inhibition, working memory |
| VLPFCa | 11/44/45 | 27.5 | 30.3 | |
| DLPFCb | 8/9/1946 | 39.6 | 35.9 | |
| Posterior cingulate | 23/31/7 | 22.5 | 21.7 | Memory, spatial-attention |
| Anterior cingulate | 24/25/32/33 | 12.3 | 11.65 | Vigilance, motivation |
| Temporal pole | 38 | 9.84 | 10.4 | Face and word recognition |
| Lateral temporal | 20/21/22/37/41/42 | 43.5 | 49.9 | Object recognition and language processing |
| Medial temporal | 27/28/34/35/36/37 | 30.4 | 26.6 | Learning and memory |
| Superior Parietal | 7 | 16.8 | 14 | Praxis, spatial attention, visuo-motor tracking, visuoperceptual processing |
| Inferior Parietal | 39/40 | 20.1 | 19.1 | |
| Basal Ganglia | n/a | 12.2 | 13.1 | Executive functions, Motivation and learning |
| Thalamus | n/a | 10.7 | 10.7 | |
| Occipital | 17/18/19 | 36.7 | 38.3 | Object processing, visual processing |
Ventrolateral prefrontal cortex
Dorsolateral prefrontal cortex
2.4 Functional assessment
IADL were assessed using the Disability in Dementia scale (DAD), a caregiver interview-based functional assessment targeting community-dwelling patients with dementia[14]. IADL ability on the DAD is captured on 27 items on tasks such as meal preparation, telephoning, transport, finances, medication administration and housework. Each task is subdivided into three components namely, initiation of the task, sequence planning and finally its actual performance. Each component is scored individually. Items of the questionnaire address each of these components separately and allow for dichotomous scoring of these subcomponents. The sum of affirmative responses for each component of each items in the questionnaire provides a total score for that component of IADL. This scale has been evaluated for its internal consistency, content validity and test-retest and interrater reliability in patients with AD[14].
2.5 Statistical analysis
Students t test were used for group comparisons and effect sizes were calculated. Independent variables were investigated for co-linearity and Pearson correlation and multiple linear regressions were used to explore the relationship of perfusion ratios to total IADL scores and each functional sub-components of IADL respectively. Statistical analyses were performed using SPSS version 12.0 (SPSS Inc, Chicago, IL).
3. Results
3.1 Baseline characteristics
No statistically significant differences were found between AD (n=121) and NC (n=42) in age (71 ± 9 vs 72 ± 7.1 years), gender (females: 52 % vs 49 %) or education (13.4 ± 4.2 vs 13.4 ± 4.2 years). Patients had a mean duration of symptoms of 3.3 ± 2.2 years with a mean MMSE of 24.3 ± 3.3 (MMSE in NC group was 28.9 ± 1.1, p<0.01) and mean IADL score of 19.4 ± 6.8.
3.2 Cerebral perfusion differences
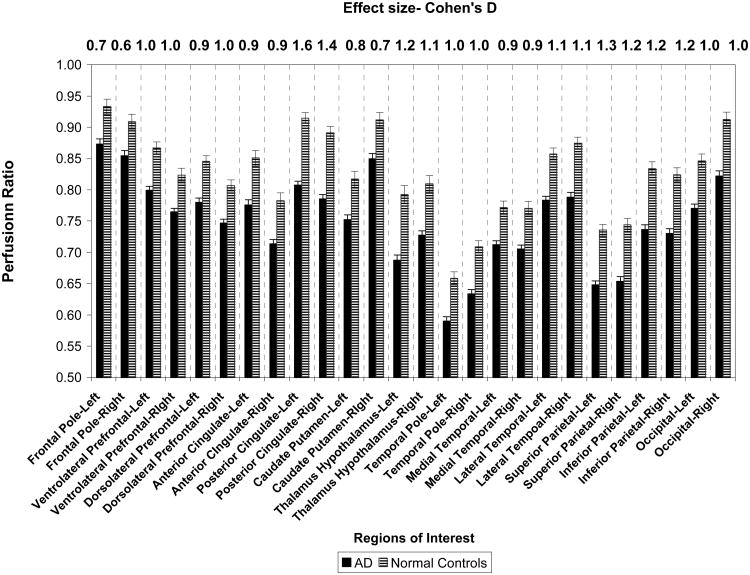
AD patients had significantly lower perfusion ratios in all 13 bilateral ROI compared to NC (p< 0.01) with effect sizes ranging from 0.6 to 1.2 (Figure 1). The relative pattern of perfusion in each brain region appeared to be similar in both groups with frontal and occipital regions showing higher perfusion ratios and temporal poles and superior parietal regions showing the lowest perfusion ratios. The areas that showed the greatest group differences were in bilateral posterior cingulate, superior parietal and inferior parietal regions.
Figure 1.
Differences in perfusion ratios between 121 patients with mild AD and 42 age-matched healthy normal controls (NC).
3.3 Perfusion ratios and IADL components
Total IADL score correlated significantly with perfusion ratios in right lateral temporal (r=0.2, p<0.05) and right superior parietal (r=0.21, p<0.01) ROI. Correlations between total IADL and perfusion in the right DLPFC (r=0.17, p=0.06), right inferior parietal (r=0.16, p=0.07) and right occipital (r=0.17, p=0.06) showed a trend towards significance.
Right lateral temporal ROI perfusion was significantly correlated with all three components of IADL (p<0.05). Additionally, IADL-initiation significantly correlated with ROI perfusion ratios in both frontal poles (r=0.2, p<0.05), DLPFC (r=0.3, p<0.01), VLPFC (r=0.3, p<0.01), anterior cingulate cortices (left: r= 0.3, p<0.01, right: r=0.2, p<0.05), basal ganglia (left: r=0.2, p<0.05, right: r=0.2, p<0.05) and thalami (r=0.2, p<0.05). IADL-planning correlated with right occipital perfusion (r=0.2, p<0.05) and IADL-performance correlated with right superior parietal perfusion (r=0.2, p<0.05). (Figure 2)
Figure 2.
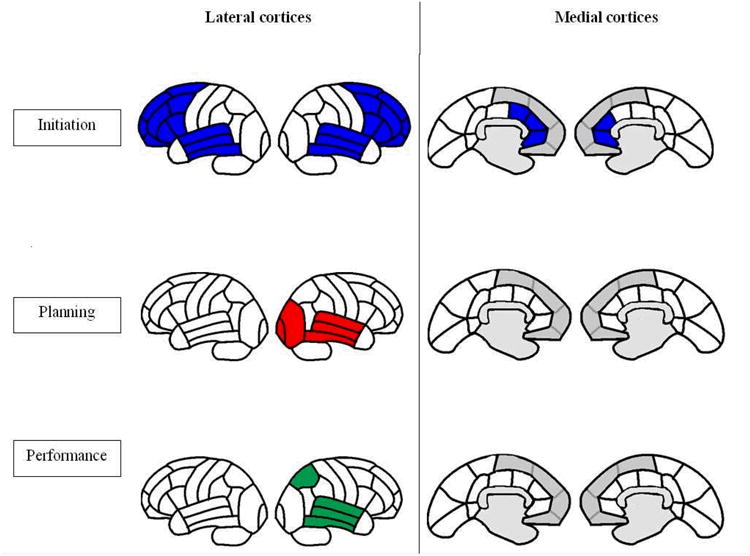
Perfusion correlates of the three components of IADL in patients with mild AD.
3.4 Regression analysis
Significant perfusion correlates were included as independent variables and the respective IADL sub-components served as dependent variables. Therefore, with initiation of IADL as the dependent variable, left anterior cingulate perfusion emerged the strongest correlate (R2 = 0.21; β=0.24; SE= 1.83; P=0.005) accounting for 5% of the variance beyond that of MMSE scores (R2= 0.15 β=0.25; SE= 0.05; P<0.001). For the planning and performance components of IADL no significant associations were seen on multiple regressions.
4. Discussion
In this study, we compared perfusion ratios across 13 ROI from each hemisphere in 121 patients with mild AD to that in 42 NC. We found that perfusion ratios across all 26 ROI were significantly lower in the mild AD group compared to healthy controls (Figure 1). The pattern of perfusion, however, was very similar in both groups such that within each group the perfusion ratios in frontal and occipital cortices were the highest and ratios in temporal and parietal cortices were the lowest indicating that the radiotracer uptake varies in different brain regions and underlies the importance of having normal controls for comparison. It is possible that certain brain regions may be more vulnerable to age-associated perfusion changes, such as temporal and parietal regions, with the effects of AD superadded to this change. The higher perfusion ratios in frontal and occipital regions in our AD sample is also in accordance with the relative lack of involvement of these regions in the early stages of the disease [11]. We also observed that the largest differences between groups were in posterior cingulate and parietal ROI consistent with other reports [23,46].
The literature on the selective correlations between perfusion in a given brain region and loss of IADL in mild AD is sparse. We sought to address this by correlating perfusion in twenty-six brain regions to overall IADL ability and with the individual components of IADL in our mild AD population. The significant relationship we found between overall IADL ability and right-hemisphere perfusion is similar to that reported in other studies[40],[43]. Right temporo-parietal perfusion is more strongly correlated with loss of ADL than the left hemisphere perfusion measures [39]. IADLs such as meal preparation, telephone use, transportation, going on an outing, written correspondence, leisure and household work are highly dependent on visuo-spatial and perceptual abilities such as spatial navigation, spatial awareness and visual processing and attention [16]. Spatial attention is recognized to be a right-hemisphere dominant function based on sound clinical and experimental evidence [20,25,35,36,38]. Specifically the right lateral temporal cortex mediates spatial awareness[24], processing of visual signals and perception of actions[18,24] and right parietal cortex plays a fundamental role in spatial attention, spatial working memory, visuomotor integration and motor planning [34]. In our study, perfusion in these regions was significantly correlated with overall IADL ability and perfusion in the right lateral temporal also emerged as a common correlate of all three IADL sub-components. Therefore, our finding that overall IADL ability, which is based on visuo-spatial-perceptual integration, was associated with right temporo-parietal perfusion in keeping with the known cognitive function of this region and with few previous studies on this topic [39,40,43].
When individual component correlates were studied, additional ROI perfusion correlates emerged that were possibly related to underlying cognitive requirements of the respective component process (Figure 2). Initiation of IADL showed additional bilateral frontal associations specifically the frontal poles, prefrontal cortex, anterior cingulate and as well as the striate regions in the frontal-subcortical circuits. In addition, multiple regression analyses, which accounted for MMSE scores as an index of severity, revealed that the left anterior cingulate was the main significant predictor of IADL-initiation. These results suggest that the anterior cingulate may play a key role in initiation of complex routine tasks. Lack of initiative, i.e., apathy, has been associated with greater neurofibrillary tangle burden in the anterior cingulate[32] and with hypoperfusion in the anterior cingulate [4]. The planning component of IADL in our study was correlated with right temporo-occipital ROI perfusion, presumably reflecting the cognitive specialization of this area for visual discrimination and object processing [15][19]. The actual performance component of IADL, however, correlated best with right superior parietal perfusion, which represents visual and spatial attention processing[15,22]. Recently functional neuroimaging studies and transcranial magnetic stimulation research have suggested that the parietal lobes are also critically involved in visuo-motor tracking and action planning[22]. Hence, the correlation we found between the performance component of IADL and right superior parietal perfusion underlies the importance of these cognitive processes in enacting instrumental functional activities of daily living.
Certain limitations need to be considered in understanding the results of this study. This study used a caregiver-rater approach as many patients with dementia often lose insight into their functional abilities early in the disease [41]. The strength of correlations between perfusion in various ROI and the dependent variables although statistically significant are not strong. However, they are in keeping with other brain-behavioral studies that have used SPECT, which may have to do with the nature of variables. The functional assessment variables were subjective based on caregiver's perception of patient's functional ability rather than direct patient performance measures, which may have reduced the strength of the correlation. Furthermore, we did not correct for multiple comparisons and had a relatively small sample size considering the number of models of comparisons because this was intended to be an exploratory analysis that could be hypothesis generating. Despite these limitations, the finding that different sub-components of IADL relate to different brain regions has not been previously explored to the best of our knowledge. Further research on these lines of elucidating neuronal substrates of components rather than as a composite functional ability may help us to develop our understanding of functional decline in AD in keeping with objective neuroimaging parameters.
In summary, this study points out that perfusion patterns in healthy elderly brains may be similar to those seen in early AD, but patients with AD have a significant diminution in perfusion across all brain regions but is more marked in the posterior cingulate and parietal regions (Figure 1). The threshold at which this diminution in perfusion correlates to decline with functional ability remains to be understood. Our results indicate that overall IADL ability correlates best with perfusion in right temporo-parietal regions, which may relate to the visuo-spatial nature of these activities. When individual aspects of IADL are examined, right lateral temporal perfusion emerges as a common correlate of all three components of IADL. In addition, perfusion in bilateral fronto-subcortico-thalamic regions correlates with IADL-initiation, right occipital perfusion correlates with IADL-planning and right superior parietal correlates with IADL-performance (Figure 2). These findings suggest that while IADL measures may show an association with brain areas sub-serving visuo-spatial function when measured as a composite function, different components of IADL appear to relate to regionally specific brain regions depending on the nature of the cognitive demands required for different components of functioning. It remains to be seen whether the relationships between other disease variables such as cognition and behavior show specific relationships for different sub-components of IADL, as compared to those studies that have shown associations between overall IADL ability and executive function[8,17], visuoperception[21], motor[6] and behavioural symptoms[12,41,44] in AD.
Acknowledgments
We acknowledge the assistance of B. Buck, P. Ebert, J. Foster, J. Bray, A Grigorovich, I. Lam, I. Guimont, E. Mawji, C. Lo, C. Szekely, A. Vance, M. Evans, C. Pond and R. Scheps with data collection and database entry. We thank Dr. Alex Kiss, PhD for assistance in statistical methodology and analysis.
Financial Support: This research was supported by the Canadian Institutes of Health Research (#MT13129), Ontario Mental Health Foundation, Alzheimer's Society of Canada and Alzheimer's Association U. S.
Footnotes
Disclosure Statement: There are no actual or potential conflicts of interest
References
- 1.Albert SM, Del Castillo-Castaneda C, Sano M, Jacobs DM, Marder K, Bell K, Bylsma F, Lafleche G, Brandt J, Albert M, Stern Y. Quality of life in patients with Alzheimer's disease as reported by patient proxies. J Am Geriatr Soc. 1996;44(11):1342–7. doi: 10.1111/j.1532-5415.1996.tb01405.x. [DOI] [PubMed] [Google Scholar]
- 2.APA. Diagnostic and Statistical Manual of Mental Disorders-DSM-IV. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 3.Beck CK, Frank LB. Assessing functioning and self-care abilities in Alzheimer disease research. Alzheimer Dis Assoc Disord. 1997;11(Suppl 6):73–80. [PubMed] [Google Scholar]
- 4.Benoit M, Koulibaly PM, Migneco O, Darcourt J, Pringuey DJ, Robert PH. Brain perfusion in Alzheimer's disease with and without apathy: a SPECT study with statistical parametric mapping analysis. Psychiatry Res. 2002;114(2):103–11. doi: 10.1016/s0925-4927(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 5.Bobinski M, Wegiel J, Tarnawski M, Reisberg B, de Leon MJ, Miller DC, Wisniewski HM. Relationships between regional neuronal loss and neurofibrillary changes in the hippocampal formation and duration and severity of Alzheimer disease. J Neuropathol Exp Neurol. 1997;56(4):414–20. doi: 10.1097/00005072-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Boyle PA, Cohen RA, Paul R, Moser D, Gordon N. Cognitive and motor impairments predict functional declines in patients with vascular dementia. Int J Geriatr Psychiatry. 2002;17(2):164–9. doi: 10.1002/gps.539. [DOI] [PubMed] [Google Scholar]
- 7.Boyle PA, Malloy PF, Salloway S, Cahn-Weiner DA, Cohen R, Cummings JL. Executive dysfunction and apathy predict functional impairment in Alzheimer disease. Am J Geriatr Psychiatry. 2003;11(2):214–21. [PubMed] [Google Scholar]
- 8.Cahn-Weiner DA, Boyle PA, Malloy PF. Tests of executive function predict instrumental activities of daily living in community-dwelling older individuals. Appl Neuropsychol. 2002;9(3):187–91. doi: 10.1207/S15324826AN0903_8. [DOI] [PubMed] [Google Scholar]
- 9.Cahn-Weiner DA, Ready RE, Malloy PF. Neuropsychological predictors of everyday memory and everyday functioning in patients with mild Alzheimer's disease. J Geriatr Psychiatry Neurol. 2003;16(2):84–9. doi: 10.1177/0891988703016002004. [DOI] [PubMed] [Google Scholar]
- 10.Chang LT. A method for attenuation correction in radionuclide computed tomography. IEEE Trans Nucl Sci. 1978;NS-25:638–43. [Google Scholar]
- 11.Delacourte A, David JP, Sergeant N, Buee L, Wattez A, Vermersch P, Ghozali F, Fallet-Bianco C, Pasquier F, Lebert F, Petit H, Di Menza C. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer's disease. Neurology. 1999;52(6):1158–65. doi: 10.1212/wnl.52.6.1158. [DOI] [PubMed] [Google Scholar]
- 12.Fitz AG, Teri L. Depression, cognition, and functional ability in patients with Alzheimer's disease. J Am Geriatr Soc. 1994;42(2):186–91. doi: 10.1111/j.1532-5415.1994.tb04950.x. [DOI] [PubMed] [Google Scholar]
- 13.Gauthier S, Gelinas I, Gauthier L. Functional disability in Alzheimer's disease. Int Psychogeriatr. 1997;9(Suppl 1):163–5. doi: 10.1017/s1041610297004857. [DOI] [PubMed] [Google Scholar]
- 14.Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer's disease: the disability assessment for dementia. Am J Occup Ther. 1999;53(5):471–81. doi: 10.5014/ajot.53.5.471. [DOI] [PubMed] [Google Scholar]
- 15.Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Kim YH, Meyer JR, Mesulam M. A large-scale distributed network for covert spatial attention: further anatomical delineation based on stringent behavioural and cognitive controls. Brain. 1999;122(Pt 6):1093–106. doi: 10.1093/brain/122.6.1093. [DOI] [PubMed] [Google Scholar]
- 16.Glosser G, Gallo J, Duda N, de Vries JJ, Clark CM, Grossman M. Visual perceptual functions predict instrumental activities of daily living in patients with dementia. Neuropsychiatry Neuropsychol Behav Neurol. 2002;15(3):198–206. [PubMed] [Google Scholar]
- 17.Grigsby J, Kaye K, Baxter J, Shetterly SM, Hamman RF. Executive cognitive abilities and functional status among community-dwelling older persons in the San Luis Valley Health and Aging Study. J Am Geriatr Soc. 1998;46(5):590–6. doi: 10.1111/j.1532-5415.1998.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 18.Han S, Jiang Y, Mao L. Right hemisphere dominance in perceiving coherence of visual events. Neurosci Lett. 2006;398(1-2):18–21. doi: 10.1016/j.neulet.2005.12.069. [DOI] [PubMed] [Google Scholar]
- 19.Haxby JV, Grady CL, Horwitz B, Ungerleider LG, Mishkin M, Carson RE, Herscovitch P, Schapiro MB, Rapoport SI. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. Proc Natl Acad Sci U S A. 1991;88(5):1621–5. doi: 10.1073/pnas.88.5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heilman KM, Van Den Abell T. Right hemisphere dominance for attention: the mechanism underlying hemispheric asymmetries of inattention (neglect) Neurology. 1980;30(3):327–30. doi: 10.1212/wnl.30.3.327. [DOI] [PubMed] [Google Scholar]
- 21.Hill RD, Backman L, Fratiglioni L. Determinants of functional abilities in dementia. J Am Geriatr Soc. 1995;43(10):1092–7. doi: 10.1111/j.1532-5415.1995.tb07006.x. [DOI] [PubMed] [Google Scholar]
- 22.Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44(13):2691–9. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 23.Johnson KA, Moran EK, Becker JA, Blacker D, Fischman AJ, Albert MS. Single photon emission computed tomography perfusion differences in mild cognitive impairment. J Neurol Neurosurg Psychiatry. 2007;78(3):240–7. doi: 10.1136/jnnp.2006.096800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karnath HO. New insights into the functions of the superior temporal cortex. Nat Rev Neurosci. 2001;2(8):568–76. doi: 10.1038/35086057. [DOI] [PubMed] [Google Scholar]
- 25.Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9(3):269–77. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- 26.Lechowski L, Dieudonne B, Tortrat D, Teillet L, Robert PH, Benoit M, Forette B, Vellas B. Role of behavioural disturbance in the loss of autonomy for activities of daily living in Alzheimer patients. Int J Geriatr Psychiatry. 2003;18(11):977–82. doi: 10.1002/gps.999. [DOI] [PubMed] [Google Scholar]
- 27.Lehfeld H, Erzigkeit E. Functional Aspects of Dementia. In: Gauthier S, Cummings JL, editors. Alzheimer's Disease and Related Disorders Annual. London, UK: Martin-Dunitz Ltd; 2000. pp. 155–78. [Google Scholar]
- 28.Levy ML, Cummings JL, Fairbanks LA, Bravi D, Calvani M, Carta A. Longitudinal assessment of symptoms of depression, agitation, and psychosis in 181 patients with Alzheimer's disease. Am J Psychiatry. 1996;153(11):1438–43. doi: 10.1176/ajp.153.11.1438. [DOI] [PubMed] [Google Scholar]
- 29.Lobaugh NJ, Caldwell CB, Black SE, Leibovitch FS, Swartz RH. Three brain SPECT region-of-interest templates in elderly people: normative values, hemispheric asymmetries, and a comparison of single- and multihead cameras. J Nucl Med. 2000;41(1):45–56. [PubMed] [Google Scholar]
- 30.Lopez OL, Brenner RP, Becker JT, Ulrich RF, Boller F, DeKosky ST. EEG spectral abnormalities and psychosis as predictors of cognitive and functional decline in probable Alzheimer's disease. Neurology. 1997;48(6):1521–5. doi: 10.1212/wnl.48.6.1521. [DOI] [PubMed] [Google Scholar]
- 31.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of activities of daily living in Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(1):56–9. doi: 10.1097/01.wad.0000201852.60330.16. [DOI] [PubMed] [Google Scholar]
- 32.Marshall GA, Fairbanks LA, Tekin S, Vinters HV, Cummings JL. Neuropathologic correlates of apathy in Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21(3):144–7. doi: 10.1159/000090674. [DOI] [PubMed] [Google Scholar]
- 33.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 34.Mesulam M. Attentional Networks Confusional States Neglect Syndromes. In: Mesulam M, editor. Principles of Behavioral and Cognitive Neurology. 2nd. New York: Oxford University Press Inc.; 2000. pp. 174–239. [Google Scholar]
- 35.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10(4):309–25. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 36.Mesulam MM. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354(1387):1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K. The rate of decline in function in Alzheimer's disease and other dementias. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 1999;54(2):M65–9. doi: 10.1093/gerona/54.2.m65. [DOI] [PubMed] [Google Scholar]
- 38.Moscovitch C, Kapur S, Kohler S, Houle S. Distinct neural correlates of visual long-term memory for spatial location and object identity: a positron emission tomography study in humans. Proc Natl Acad Sci U S A. 1995;92(9):3721–5. doi: 10.1073/pnas.92.9.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nobili F, Copello F, Buffoni F, Vitali P, Girtler N, Bordoni C, Safaie-Semnani E, Mariani G, Rodriguez G. Regional cerebral blood flow and prognostic evaluation in Alzheimer's disease. Dement Geriatr Cogn Disord. 2001;12(2):89–97. doi: 10.1159/000051241. [DOI] [PubMed] [Google Scholar]
- 40.Ott BR, Heindel WC, Whelihan WM, Caron MD, Piatt AL, Noto RB. A single-photon emission computed tomography imaging study of driving impairment in patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 2000;11(3):153–60. doi: 10.1159/000017229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pearson JL, Teri L, Reifler BV, Raskind MA. Functional status and cognitive impairment in Alzheimer's patients with and without depression. J Am Geriatr Soc. 1989;37(12):1117–21. doi: 10.1111/j.1532-5415.1989.tb06674.x. [DOI] [PubMed] [Google Scholar]
- 42.Roth M, Tomlinson BE, Blessed G. Correlation between scores for dementia and counts of ‘senile plaques’ in cerebral grey matter of elderly subjects. Nature. 1966;209(18):109–10. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- 43.Salmon DP, Thal LJ, Butters N, Heindel WC. Longitudinal evaluation of dementia of the Alzheimer type: a comparison of 3 standardized mental status examinations. Neurology. 1990;40(8):1225–30. doi: 10.1212/wnl.40.8.1225. [DOI] [PubMed] [Google Scholar]
- 44.Senanarong V, Poungvarin N, Jamjumras P, Sriboonroung A, Danchaivijit C, Udomphanthuruk S, Cummings JL. Neuropsychiatric symptoms, functional impairment and executive ability in Thai patients with Alzheimer's disease. Int Psychogeriatr. 2005;17(1):81–90. doi: 10.1017/s1041610205000980. [DOI] [PubMed] [Google Scholar]
- 45.Stuss DT, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–33. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- 46.Talbot PR, Lloyd JJ, Snowden JS, Neary D, Testa HJ. A clinical role for 99mTc-HMPAO SPECT in the investigation of dementia? J Neurol Neurosurg Psychiatry. 1998;64(3):306–13. doi: 10.1136/jnnp.64.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tekin S, Fairbanks LA, O'Connor S, Rosenberg S, Cummings JL. Activities of daily living in Alzheimer's disease: neuropsychiatric, cognitive, and medical illness influences. Am J Geriatr Psychiatry. 2001;9(1):81–6. [PubMed] [Google Scholar]