Abstract
Apathy is associated with impaired neuropsychological functioning in individuals with HIV. While cognitive reserve (CR) delays neurocognitive decline, CR's relationship with apathy has never been studied. We examined CR's association with apathy in 116 HIV-positive individuals recruited from an urban AIDS center and assessed whether this relationship is moderated by age and/or disease severity. Participants completed the Wechsler Test of Adult Reading and Apathy Evaluation Scale. A CR-composite, combining years of education and word-reading ability, significantly predicted apathy (t = −2.37, p = .02). CR's relationship with apathy was not moderated by age, but participants with nadir CD4 levels ≤200 demonstrated a stronger association (t = −3.25, p = .002) than those with nadir CD4 levels > 200 (t = −0.61, p = .55). These findings suggest a protective effect of CR against apathy in HIV-infected individuals across the age span, particularly after a certain threshold of disease severity.
Keywords: Cognitive reserve, Apathy, HIV, Aging, Neuropsychology
Introduction
Human immunodeficiency virus (HIV) enters the central nervous system (CNS) early in the course of the disease (Navia, Cho, Petito, & Price, 1986) and can be detected in the brain within 2 weeks of infection (Paul, Cohen, Navia, & Tashima, 2002). After entering the nervous system, the virus aggregates in the subcortical and frontal brain regions, notably the basal ganglia, globus pallidus, caudate nucleus, and prefrontal white matter (Brew, Rosenblum, & Price, 1988; Paul et al., 2002). Neuroimaging studies have demonstrated HIV-associated atrophy in both cortical and subcortical regions of the brain (Hall et al., 1996; Jernigan et al., 1993; Paul et al., 2002; Raininko et al., 1992), resulting in declines in neuropsychological performance (Bornstein et al., 1993; Castellon et al., 2006; Ferrando et al., 1998; Heaton et al., 1995, 2011; Van Gorp, Miller, Satz, & Visscher, 1989). In fact, 50% of individuals with HIV infection experience neurocognitive complications (Cysique & Brew, 2009; Heaton et al., 1995), which can range from subtle deficits on neuropsychological tests to severe dementia syndromes that profoundly disrupt an individual's functioning and activities of daily living.
Another neuropsychiatric symptom that results from CNS viral damage is apathy, defined as lack of motivation not attributable to the diminished level of consciousness, intellectual impairment, or emotional distress (Marin, 1991). Apathy is especially prominent in disorders affecting the frontal lobe and subcortical structures (Cummings, 1993; Marin, 1991; Marin, Firinciogullari, & Biedrzycki, 1993). Previous research has demonstrated that apathy is significantly associated with cognitive impairment in individuals with HIV, Parkinson's disease, Alzheimer's disease, and stroke (Castellon, Hinkin, Wood, & Yarema, 1998; Cole et al., 2007; Hinkin, Castellon, & Hardy, 2000; Paul, Brickman, et al., 2005; Paul, Flanigan, et al., 2005; Starkstein, Bolduc, Preziosi, & Robinson, 1989; Starkstein, Jorge, Mizrahi, & Robinson, 2006). Furthermore, neuroimaging studies have linked apathy to the lower volume of the nucleus accumbens and poor white matter integrity in the prefrontal cortex (Hoare et al., 2011; Paul et al., 2002; Paul, Brickman, et al., 2005), leading many researchers to conclude that apathy's simultaneous decrease in the behavioral, cognitive, and emotional aspects of goal-directed behavior results from prefrontal brain damage (Cummings, 1993; Hoare et al., 2011; Tekin & Cummings, 2002).
Given the hypothesized association between apathy and frontal-subcortical brain circuitry, it is not surprising that apathy is prominent among individuals with HIV, with an estimated prevalence rate of 26% (Castellon et al., 2006; Paul, Flanigan, et al., 2005; Tate et al., 2003). Among individuals with HIV, one study (Kamat et al., 2012) found higher levels of self-reported apathy to be associated with greater impairment in functioning, and two studies have reported a relationship between apathy and poor medication adherence (Rabkin et al., 2000). Additionally, three previous studies have reported significant correlations between apathy and cognitive dysfunction in individuals with HIV (Castellon et al., 1998; Castellon, Hinkin, & Myers, 2000; Paul, Flanigan, et al., 2005). Castellon and colleagues (1998, 2000) reported increased apathy to be strongly correlated with poor performance on measures of working memory, divided attention, and response inhibition. More recently, Paul, Flanigan, and colleagues (2005) found that apathy was significantly related to performance on measures of learning efficiency and cognitive flexibility.
While apathy is associated with poor neuropsychological functioning (Castellon et al., 1998; Kuzis et al., 1999; Starkstein & Brockman, 2011), cerebral reserve has been shown to delay cognitive decline in the face of CNS insult in HIV and other brain diseases, such as Alzheimer's disease, Parkinson's disease, stroke, and various dementia syndromes (Koerts, Tucha, Lange, & Tucha, 2012; Ojala-Oksala et al., 2012; Pillai et al., 2011). Cerebral reserve refers to the ability to maintain cognitive functioning despite damage sustained to the brain (Stern, 2002). This phenomenon can be explained by passive differences in brain structure, such as number and/or density of neurons and synapses, commonly referred to as “brain reserve” (Stern, 2002; Scarmeas & Stern, 2003), or active differences in the functionality of brain processes, such as the efficiency of neural processing and the ability to recruite alternative brain netwoks to compensate for the effects of pathology, commonly referred to as “cognitive reserve (CR)” (Richards & Deary, 2005; Stern, 2002).
Previous literature has demonstrated CR's capacity to protect against cognitive decline in individuals with HIV (Basso & Bornstein, 2000b; Foley et al., 2012; Satz et al., 1993; Stern, Silva, Chaisson, & Evans, 1996). For example, Stern and colleagues (1996) demonstrated that HIV-positive patients with lower CR exhibited more deficits on measures of attention, memory, executive function, and visuospatial abilities than higher CR counterparts. Further studies on CR and HIV confirm that HIV-infected individuals with higher levels of CR display less neuropsychological impairment than those with lower CR (Basso & Bornstein, 2000b; Foley et al., 2012; Satz et al., 1993). Additionally, individuals above the age of 50 and those with advanced stages of HIV continue to benefit from CR's protective effects (Basso & Bornstein, 2000a, 2000b; Foley et al., 2012). Thus, it appears that CR delays neuropsychological manifestations of HIV-related CNS damage, even among older age groups and at late stages of HIV disease progression.
Several variables have been hypothesized to maintain or enhance CR, including premorbid intelligence as well as lifetime experiences of mental stimulation, such as education, occupational attainment, and participation in leisure activities (Cosentino & Stern, 2012; Stern, 2002, 2009). Word-reading ability is often used as a proxy for premorbid intelligence (Franzen, Burgess, & Smith-Seemiller, 1997), because it can withstand the effects of many types of brain damage and is more reliant on existing knowledge than current cognitive functioning (Willshire, Kinsella, & Prior, 1991). Recent research (Brickman et al., 2011) recommends examining a composite of years of education and simple word-reading ability as proxies for both psychosocial experience and innate intelligence, respectively. Thus, the present study employs a sample-based composite score which places equal weight on highest level of educational attainment and performance on the Wechsler Test of Adult Reading (WTAR; Wechsler, 2001) in an effort to investigate the relationship between CR and apathy in patients with HIV.
While previous evidence reveals that CR is protective against neuropsychological decline in individuals with HIV (Basso & Bornstein, 2000b; Foley et al., 2012; Satz et al., 1993; Stern et al., 1996), the relationship between CR and apathy has yet to be studied in this population. As individuals with HIV live longer due to efficacious treatments (CDC, 2005), apathy's impact on quality of life is of increasing clinical importance and further clarification of its resilience and risk factors is clearly warranted. We examined CR's relationship with apathy in HIV-positive patients from across the lifespan. Since brain reserve was not directly measurable in the current study, CR was utilized as a proxy measure. We hypothesized that consistent with the active model of cerebral reserve, higher levels of CR will be associated with lower levels of apathy in individuals with HIV due to better compensatory functioning, thus diminishing the experience of apathy despite frontal dysfunction. We also explored the moderating role of age and disease severity on this relationship to ascertain whether CR's impact decreases with age and/or at increasingly compromised HIV disease stages. Consistent with preliminary findings on CR in older patients with HIV (Foley et al., 2012), we predicted that CR's protective quality would remain stable across age groups. Additionally, we predicted that CR's association with apathy would remain stable throughout the course of HIV disease progression, as has been found by previous studies on CR and HIV (Basso & Bornstein, 2000a, 2000b; Foley et al., 2012).
Methods
Participants
This study examined data from 116 HIV-positive individuals recruited from the Center for Positive Living/I.D. Clinic at the Montefiore AIDS Center, Bronx, NY, between November 2011 and April 2012. Participants were recruited to provide representation in 10-year age bins from age 20 to 70 and above. Eligibility requirements included age 18 and older and diagnosis of HIV. Diagnosis was confirmed by review of medical records, HIV-related blood investigations, and medication records, by two clinical research assistants who were blinded to performance on the study predictor and outcome measures. Individuals with a known history of head injury, neurologic disorder, or developmental disability per self-report or medical record were excluded. Patients with a Mini-Mental State Examination (MMSE) score below 25, which is the recommended cutoff for normal cognition (Crum et al., 1993), as well as those with self-reported current use of cocaine, heroin, and/or other intravenous drugs, were not included.
Materials and Procedure
Each participant provided written, informed consent following study protocols approved by the local institutional review board. Eligible participants completed demographic questionnaires and self-report measures of apathy and mood. A trained research assistant administered the WTAR to assess premorbid intelligence (Wechsler, 2001), as part of a larger neuropsychological test battery.
Apathy
Apathy was measured with Marin's Apathy Evaluation Scale-Self (AES; Marin, 1991). The psychometric properties of this scale have been well established with internal consistency reliability ranging from 0.86 to 0.94, and test–retest reliability ranging from 0.76 to 0.94 (Marin, Biedrzycki, & Firinciogullari, 1991; Marin et al., 1993; Marin, Firinciogullari, & Biedrzycki, 1994). The AES is a brief self-report measure of apathy consisting of 18 items related to motivation, self-initiation, and drive over the past 4 weeks. It has been shown to be psychometrically robust for assessing apathy across a wide array of disease populations (Clarke et al., 2011) and has been previously used with HIV-positive individuals (Castellon et al., 2000; Paul, Brickman, et al., 2005; Paul, Flanigan, et al., 2005; Rabkin et al., 2000). The mean AES score in a normative healthy sample is 24.4 (4.5), with a suggested cutoff criterion of 34 (Kant et al., 1998). Participants responded to their degree of agreement with each item using a 4-point Likert scale, where 1 = not at all and 4 = extremely. Scores range from 18 to 72, with higher scores reflecting greater apathy. The dependent measure in the current study was total AES score.
Mood
Mood was assessed with the Beck Depression Inventory-II (BDI-II), a self-report rating scale that queries the presence and prominence of cognitive, affective, and somatic symptoms of depression over the past 2 weeks (Beck, 1987). Scores on this 21-item inventory range from 0 (symptom absent) to 3 (presence of symptom is pronounced), yielding a possible total range from 0 to 63. BDI-II cognitive-affective and somatic subscores were calculated according to the manual (Beck, 1987), because positively endorsed somatic items may be related to physical illness in medically ill populations. This method is commonly used by other HIV investigators (Castellon et al., 1998, 2006; Law et al., 1994, 1995).
Wechsler Test of Adult Reading
The WTAR (Wechsler, 2001), a reading test comprised of a list of 50 words that have atypical grapheme to phoneme translations, was administered to provide an estimation of pre-morbid intellectual and memory abilities. Word-reading tests are commonly used to estimate premorbid intelligence in a variety of clinical populations (Franzen et al., 1997), based on the assumptions that reading is highly correlated with intelligence in the general population, is relatively resistant to the effects of many types of brain damage, and is more reliant on existing knowledge than current cognitive functioning (Willshire et al., 1991). Test–retest reliability of the WTAR is high, with coefficients in the 0.90 range (Green et al., 2008; Mathias, Bowden, & Barrett-Woodbridge, 2007; Whitney, Shepard, Mariner, Mossbarger, & Herman, 2010). The number of words pronounced correctly served as the dependent measure.
The Stroop Color-Word Interference Test
The Stroop Color-Word Interference Test (Stroop, 1935; Golden, 1978) measures the relative speeds of word reading, color naming, and naming colors used to print an incongruous color name. It assesses multiple functions but is primarily used as a measure of response inhibition and cognitive flexibility (Spreen & Strauss, 1998). Poor performance is associated with frontal lobe damage and frontal systems dysfunction (Spreen & Strauss, 1998). Test–retest reliabilities are high and have been found to range from 0.83 to 0.91 in healthy individuals (Spreen & Strauss, 1998; Uttl & Graf, 1997). Participants' raw score on the color-word condition of this test was used in the current study to control for their response inhibition and cognitive flexibility, which have been previously associated with apathy level (Castellon et al., 2000; Paul, Flanigan, et al., 2005; Posada et al., 2010). The number of correct items completed in 45 s served as the measure of interest.
CR composite
A CR composite score was calculated as an average of the sample-based z-scores for years of education and word-reading ability on the WTAR (Wechsler, 2001). By using a single CR composite score that places equal weight on education and word reading, we reduced Type I error and increased the vigor of the measurement. Combining years of education and simple word-reading ability is a widely used and reliable method (Brickman, Siedlecki, & Stern, 2010; Rentz et al., 2010), as it incorporates psychosocial exposure and intrinsic intelligence, which are both critical to the assessment of CR (Stern, 2009).
HIV-related medical measures
Two independent researchers trained and certified in the hospital's electronic records system extracted and confirmed the following measures from participants' medical records: Date of HIV diagnosis, presence of AIDS diagnosis, current CD4 cell count, CD4 nadir, current plasma RNA level, and highest recorded plasma RNA level. Highest recorded plasma RNA level was utilized as opposed to the current viral level because of the waxing and waning nature of the viral load in patients undergoing treatment (Thompson et al., 2005). Recent neuroimaging research has shown that HIV-related cortical atrophy is likely a result of cumulative disease history as opposed to current disease status (Kallianpur et al., 2012; Thompson et al., 2005). Furthermore, neuronal damage occurs even after the successful suppression of viral plasma, due to reservoirs of HIV-infected monocytes that accumulate in perivascular spaces in the brain and initiate inflammatory processes (Kallianpur et al., 2012). Therefore, the highest recorded level of the viral load is likely a more useful indicator of disease severity, especially in a population currently receiving viral suppressive treatment (90.5% of our sample was on HAART). Disease duration, measured as months since HIV diagnosis, was also calculated for each participant.
Statistical Analysis
The Statistical Package for the Social Sciences (SPSS), Version 17.0 (2009), was used for all data analyses. Distributions were inspected for kurtosis and skew, and square root or log10 transformations were performed as indicated. HIV RNA plasma levels were expressed in log10 units because of the large range, from less than 400 copies (“undetectable”) to several million. Pearson and point-biserial correlations were used to examine the associations between the following variables: Age, gender, AES score, BDI-II cognitive-affective subscore, CR composite, WTAR score, years of education, disease duration, CD4 nadir, and log10 highest HIV plasma level. Because of the multiple measures and comparisons entailed, we used p < .01 to denote statistical significance, with p = .01 to p < .05 to indicate trend significance. We utilized hierarchical regression analysis to determine the association between CR and apathy, using the CR composite as the predictor and AES score as the dependent variable. Age, gender, BDI-II cognitive-affective subscore, comorbid hepatitis C virus (HCV), disease duration, CD4 nadir, log10 highest HIV plasma level, and performance on the Stroop Color Word Interference Test (Golden, 1978; Stroop, 1935) were entered as covariates in Step 1 to control for their potential confounding effects on apathy. Specifically, previous research has shown apathy to be associated with male gender, older age, HCV, and impaired performance on tests of response inhibition and cognitive flexibility (Brodaty, Altendorf, Withall, & Sachdev, 2010; Castellon et al., 2000; Paul, Flanigan, et al., 2005; Posada et al., 2010). To assess whether age or disease severity moderated the impact of CR on apathy, we entered the following two-way product terms at Step 2: CR × age, CR × log10 highest plasma level, CR × CD4 nadir, and CR × disease duration. Simple slopes tests (Aiken & West, 1991) were utilized to interpret the significant interactions. To further assess the role of nadir CD4 cell count on the relationship between CR and apathy, participants were stratified by nadir CD4 cell count (later disease stage, ≤200; earlier disease stage, >200). Independent sample t-tests were utilized to examine differences in AES score, CR composite score, and Stroop color-word performance between the two groups. Multiple linear regression analysis was conducted, using the CR composite as the predictor and AES score as the dependent variable, for participants with earlier and later disease stages. Age, gender, BDI-II cognitive-affective subscore, comorbid HCV, and performance on the Stroop Color-Word Interference Test (Golden, 1978; Stroop, 1935) were entered as covariates to control for their potential confounding effects on apathy.
Results
Demographic and medical characteristics for the study participants are presented in Table 1. Participants' ages ranged from 22 to 79, and 63.8% (n = 74) of participants were 50 years or older. Participants' education level ranged from 5 to 19 years, and 54% (n = 63) attained at least a high-school diploma. Approximately 23.3% (n = 27) of participants reported a history of intravenous drug use, and 15.5% (n = 18) had a diagnosis of comorbid HCV according to medical records. Women comprised 66.4% of the sample. Regarding racial composition, 55.2% of participants identified themselves as Black, 32.8% as White, and 12% as other. In terms of ethnicity, 40.5% of the sample self-identified as being Hispanic. Approximately 51.7% (n = 60) of participants had a diagnosis of AIDS at the time of assessment, and 90.5% were on antiretroviral therapy. Mean disease duration, measured as years since initial diagnosis, was 12.2 (1.3) years. The mean total BDI-II score in the current sample was 14.6 (10.6), demonstrating mild levels of depression for the group, overall. The mean cognitive-affective and somatic subscales were 7.9 (7.4) and 6.8 (4.4), respectively. The mean AES score in the current sample was 30.1 (7.76), and 31% (n = 36) of participants reported clinically significant apathy according to the recommended cutoff. Mean WTAR standard score was 78.9 (13.2) in the current sample, which is considered to be in the borderline intellectual range. Correlations for study variables of interest are presented in Table 2. WTAR score and years of education were significantly correlated in our sample (r = .287, p < .001), indicating limited consistency between participants' reading level and years of education.
Table 1.
Demographic and medical characteristics of sample
| Characteristic | Total sample (n = 116) |
|
|---|---|---|
| Mean | SD | |
| Age (years) | 51.9 | 14.48 |
| Education (years) | 11.7 | 2.36 |
| BDI | ||
| Total score | 14.55 | 10.56 |
| Cognitive-affective subscore | 7.93 | 7.35 |
| Somatic subscore | 6.78 | 4.42 |
| Apathy Evaluation Scale | 30.1 | 7.76 |
| MMSE | 27.57 | 1.54 |
| WTAR (SS) | 78.90 | 13.21 |
| CD4 cell count, most recent | 558.47 | 409.25 |
| CD4 cell count, nadir | 246.58 | 228.23 |
| log10HIV plasma level, most recent | 1.18 | 1.72 |
| log10HIV plasma level, highest | 4.10 | 1.68 |
Notes: WTAR = Wechsler Test of Adult Reading; MMSE = Mini-Mental State Examination; BDI-II = Beck Depression Inventory-II; HIV = human immunodeficiency virus.
Table 2.
Bivariate correlations for demographic, disease, apathy, and cognitive reserve variables
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | ||||||||||
| 2. Gender | −.06 | |||||||||
| 3. MMSE score | −.39a | .07 | ||||||||
| 4. Apathy | −.07 | .08 | −.26a | |||||||
| 5. BDI-II, cog-affective subscore | −.16b | .14b | −.05 | .64a | ||||||
| 6. Disease duration | .13b | .08 | .12 | .05 | .04 | |||||
| 7. log10 highest plasma RNA level | −.02 | .03 | −.03 | .23a | .11 | −.14b | ||||
| 8. Nadir CD4 cell count | −.08 | .00 | −.03 | −.06 | −.02 | −.11 | −.14b | |||
| 9. WTAR score | .01 | −.08 | .32a | −.21a | −.13b | .08 | .07 | .13b | ||
| 10. Years of education | −.07 | .00 | .23a | −.23a | −.13b | −.01 | −.14b | .16b | .29a | |
| 11. Cognitive reserve composite score | .00 | −.05 | .32a | −.26a | −.16b | .04 | −.05 | .17a | .80a | .80a |
Notes: Gender: 0 = men, 1 = women. WTAR = Wechsler Test of Adult Reading; MMSE = Mini-Mental State Examination; BDI-II = Beck Depression Inventory-II.
aCorrelation significant at the .01 level (two-tailed).
bCorrelation significant at the .05 level (two-tailed).
CR and Apathy
Results of the regression model are presented in Table 3 and revealed that CR significantly predicted AES score (t = −2.37, p = .02), while controlling for age, gender, HCV status, BDI-II cognitive-affective subscore, Stroop color-word performance, disease duration, nadir CD4 cell count, and log10 highest HIV plasma level.
Table 3.
Hierarchical regression results for predicting apathy
| Predictor | B | SE | t | sig. | Δr2 |
|---|---|---|---|---|---|
| Step 1 | |||||
| Age | 0.05 | 0.00 | 0.02 | .98 | .00 |
| Gender | 1.33 | 0.02 | 0.23 | .82 | .00 |
| BDI- II, cog-affective subscore | 0.09 | 0.53 | 6.38 | .00 | .25 |
| log10 highest plasma RNA level | 0.00 | 0.17 | 2.05 | .04 | .03 |
| Nadir CD4 cell count | 0.00 | 0.06 | 0.73 | .47 | .00 |
| Disease duration (years) | 0.09 | 0.02 | 0.26 | .79 | .00 |
| Comorbid hepatitis C virus | 1.92 | 0.13 | 1.54 | .13 | .01 |
| Stroop color-word performance | 0.08 | −0.02 | −0.18 | .86 | .00 |
| CR composite score | 0.80 | −0.20 | −2.37 | .02 | .03 |
| Step 2 | |||||
| CR × log10 highest plasma RNA | −0.16 | 0.00 | −1.10 | .27 | .01 |
| CR × nadir CD4 cell count | 0.31 | 0.00 | 2.27 | .03 | .03 |
| CR × disease duration | 0.00 | 0.11 | −0.02 | .98 | .00 |
| CR × age | 0.29 | 0.05 | 1.03 | .31 | .01 |
Notes: CR = cognitive reserve; BDI-II = Beck Depression Inventory-II. Gender: 0 = men, 1 = women. Comorbid hepatitis C virus: 0 = no, 1 = yes. Δr2 = change in r2 for adding this predictor to the model last. Step 1: R2 = .43, F = 7.8, p < .001. Step 2: R2 = .48, F = 6.5, p < .001.
Age and Disease Severity Moderators
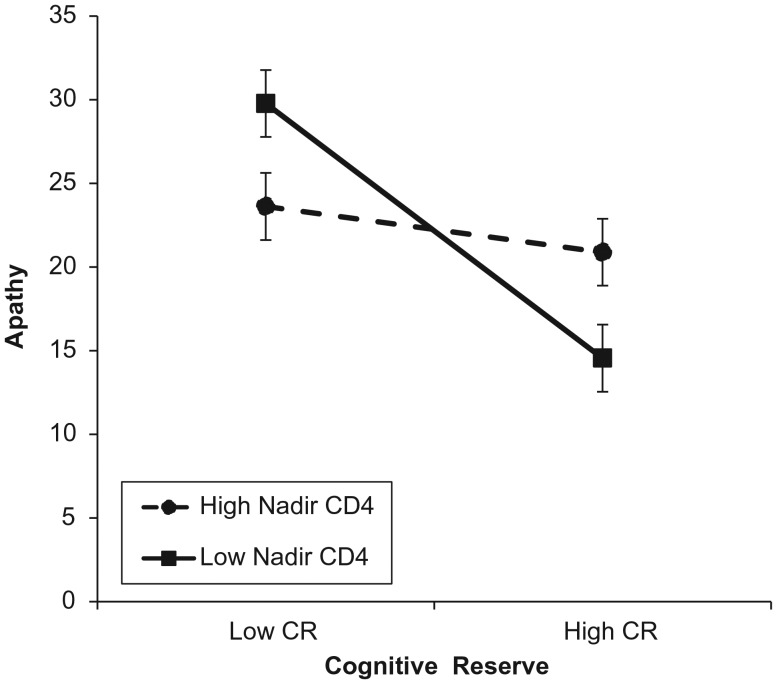
Results of hierarchical regression analysis are presented in Table 3 and indicated that age did not significantly moderate the relationship between CR and AES score (t = 1.03, p = .31). Disease duration and log10 highest HIV plasma level also did not significantly moderate the relationship between CR and apathy; however, there was a significant interaction between CR and nadir CD4 cell count (t = 2.27, p = .03). This interaction is visually depicted in Fig. 1, with high/low values of nadir CD4 cell count as ±1 SD, respectively, from the sample mean (Aiken & West, 1991; Cohen, Cohen, West, & Aiken, 2003). Examination of simple slopes revealed that the relationship between CR and apathy was stronger for participants with lower nadir CD4 cell counts (t = −4.56, p < .001) and was not significant for those with higher nadir CD4 cell counts (t = −0.30, p = .76). When stratified by nadir CD4 cell count, 57 participants had nadir CD4 cell counts of 200 and lower, indicating a later stage of HIV infection, and 59 had nadir CD4 counts of above 200. Independent sample t-tests revealed that there were no significant group differences in level of apathy (t = 1.63, p = .10) or Stroop color-word performance (t = −1.87, p = .06). However, there was a significant group difference in CR composite score (t = −2.76, p = .01), such that participants with later stages of HIV had significantly lower levels of CR. Multiple regression results are presented in Table 4 and indicated that among participants with advanced HIV (nadir CD4 ≤ 200), CR significantly predicted AES score (t = −3.25, p = .002), while controlling for age, gender, HCV status, BDI-II cognitive-affective subscore, and Stroop color-word performance. However, among participants with earlier disease stages (nadir CD4 > 200), CR did not significantly predict AES score (t = −0.61, p = .55).
Fig. 1.
Significant interaction between CR and nadir CD4 count in predicting apathy. High and low values of nadir CD4 count represented as 1 SD above and below the mean of the current sample. CR represents the minimum and maximum values in the current sample. Error bars represent 95% confidence limit. Examination of simple slopes revealed that the relationship between CR and apathy was stronger for those with lower nadir CD4 cell counts (t = −4.56, p < .001) and was not significant for those with higher nadir CD4 cell counts (t = −0.30, p = .76).
Table 4.
Multiple regression results for predicting apathy when sample stratified by nadir CD4 cell count
| Predictor | Nadir CD4 ≤ 200 (n = 57) |
Nadir CD4 > 200 (n = 59) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B | SE | t | sig. | Δr2 | B | SE | t | sig. | Δr2 | |
| Age | −0.37 | 0.07 | −2.73 | .01 | .06 | 0.28 | 0.08 | 1.80 | .08 | .05 |
| Gender | −0.06 | 1.47 | −0.66 | .52 | .00 | 0.06 | 2.25 | 0.44 | .66 | .00 |
| Comorbid hepatitis C virus | 0.20 | 2.00 | 1.92 | .06 | .03 | 0.02 | 3.32 | 0.15 | .88 | .00 |
| Stroop color-word performance | −0.14 | 0.09 | −1.06 | .29 | .01 | −0.01 | 0.13 | −0.09 | .93 | .00 |
| BDI-II, cog-affective subscore | 0.54 | 0.10 | 5.31 | .00 | .23 | 0.49 | 0.14 | 3.94 | .00 | .22 |
| Cognitive reserve composite score | −0.31 | 0.90 | −3.25 | .00 | .09 | −0.08 | 1.34 | −0.61 | .55 | .01 |
Notes: BDI-II = Beck Depression Inventory-II. Gender: 0 = men, 1 = women. Comorbid hepatitis C virus: 0 = no, 1 = yes. Δr2 = change in r2 for adding this predictor to the model last. Nadir CD4 ≤ 200: R2 = .63, F = 12.94, p < .001. Nadir CD4 > 200: R2 = .35, F = 4.10, p = .002.
Discussion
The current study demonstrates that CR is associated with lower levels of apathy in HIV-positive individuals. While previous literature has demonstrated that CR protects against neuropsychological decline in individuals with HIV, this is the first study, to our knowledge, to specifically evaluate the relationship between CR and apathy in an HIV-positive population. Additionally, consistent with previous HIV literature (Foley et al., 2012), we found that CR's association with apathy was not moderated by age and continues to have a protective effect at advanced disease stages (Basso & Bornstein, 2000a, 2000b; Foley et al., 2012). In fact, despite displaying lower levels of CR, participants with nadir CD4 cell counts of ≤200 demonstrated a stronger relationship between CR and apathy, suggesting that CR's protective effect may actually increase at later disease stages. HIV-positive individuals at advanced disease stages with lower levels of CR appear to be at greater risk for apathy, suggesting that apathy may serve as a bio-behavioral marker for CNS pathology. Taken together, these findings support the theory that HIV-positive individuals with higher CR can withstand more viral damage to the CNS before exhibiting apathy, particularly in individuals with advanced disease.
Numerous studies have revealed that higher levels of intelligence and education (i.e., CR) are good predictors of individuals' ability to sustain brain damage without exhibiting cognitive deficits (Brickman et al., 2011; Katzman, 1993; Satz et al., 1993; Scarmeas & Stern, 2003; Stern, 2002, 2009, 2011). However, findings regarding the protective quality of CR on neuropsychiatric symptoms have been equivocal (Bornstein, Miller, & van Schoor, 1989; Deb, Lyons, Koutzoukis, Ali, & McCarthy, 1999; Gomez-Hernandez, Max, Kosier, Paradiso, & Robinson, 1997; Groom, Shaw, O'Connor, Howard, & Pickens, 1998; Hibbard et al., 2004; Holsinger et al., 2002). Studies examining education level and neuropsychiatric symptoms in individuals with traumatic brain injuries and neurodegenerative disorders failed to find any association (Gomez-Hernandez et al., 1997; Groom et al., 1998; Hibbard et al., 2004; Holsinger et al., 2002). More recently, though, premorbid intelligence was found to protect against depression in traumatic brain injury survivors (Salmond, Menon, Chatfield, Pickard, & Sahakian, 2006). Among individuals with HIV, we found that CR significantly predicted apathy level while controlling for depression. The fact that the association between CR and apathy was found to be independent of depression indicates that, consistent with our hypothesis, there is a unique relationship between these two variables. To our knowledge, the current study is the first to demonstrate CR as a resilience factor against apathy in HIV.
We hypothesize that the observed relationship between CR and apathy in our study population is due to a protective effect of CR against apathy via more efficient neural processing and more effective compensation. Perhaps, individuals with higher cognitive skills have a greater ability to optimize emotional functioning through the use of alternative cognitive strategies or more efficient use of brain networks. Support for the active model of CR in individuals with HIV has been demonstrated by Ernst (2002) who found that HIV-infected individuals exhibited greater activation of the lateral prefrontal cortex on fMRI compared with healthy controls, suggesting that individuals with HIV may draw on additional or accessory brain pathways to compensate for underlying brain pathology (Morgan et al., 2012). Future neuroimaging studies should examine the recruitment of more efficient neural pathways in HIV-infected individuals with CR in order to further investigate this hypothesis. It is also possible that, in accordance with the brain reserve model, increased frontal subcortical resilience, as measured by higher density of synapses, dendritic branching, or brain volume, may passively protect individuals from exhibiting symptoms of apathy. However, the current study did not assess for brain reserve and future studies are necessary to evaluate this possible link.
There are several other possible explanations for our novel finding that should be explored in future research. For instance, it is possible that CR is merely a marker for a mediating factor that protects against apathy. Although our model adjusted for many confounders, such as gender, age, comorbid HCV, and disease severity, it is likely that additional unmeasured confounders exist. Perhaps HIV-positive individuals with higher CR engage in healthier behaviors or in more frequent and/or higher quality social interactions that act to reduce apathy. Although it is often presumed that health behaviors translate directly into HIV disease severity, which was accounted for in the current study, there remains the possibility that a non-HI-related health marker protects against apathy. Future studies on CR and apathy should examine a wider range of health behaviors and social interactions in order to assess whether these are mediating factors.
It is also important to emphasize that correlation does not demonstrate causation, and thus the exact nature of the relationship between CR and apathy is unclear. It is possible that both CR and apathy are impaired by a common pathological process of HIV infection. We must note our lack of an HIV negative control group as a limitation to the current study. Without examining the relationship between CR and apathy in an otherwise healthy matched sample, we cannot conclude whether this relationship is exclusive to those with HIV or occurs independent of the disease. Further examination of the CR-apathy relationship among healthy individuals as well as patients with other CNS diseases will help elucidate whether specific HIV-related neuropathology underlies this relationship or whether a universal CR-apathy association exists. Lastly, due to the cross-sectional nature of the current study, we cannot rule out the reverse causality hypothesis, in which CR is actually reduced by apathy as opposed to protecting against it. It is possible that individuals with higher baseline levels of apathy demonstrate lower levels of educational attainment or do not engage in cognitively stimulating activities that would promote intelligence and word-reading ability. Therefore, longitudinal studies, as well as the use of pre- and post-injury/disease apathy scales, such as the Frontal Behavior Systems Checklist, are necessary to clarify the nature and direction of the relationship between apathy and CR.
While CR has been associated with delayed neuropsychological and functional decline in numerous neurodegenerative disorders (Basso & Bornstein, 2000b; Satz et al., 1993; Scarmeas et al., 2003; Stern, 2009, 2011; Stern et al., 1996; Morgan et al., 2012), the current study is the first to demonstrate an association between CR and apathy, a common neuropsychiatric symptom in CNS diseases (Cummings, 1993; Marin, 1991; Marin et al., 1993). Consistent with previous HIV literature, we found that this relationship was stable across all age groups and that CR continued to be protective against apathy at advanced disease stages. Interestingly, the association between CR and apathy was stronger among participants with nadir CD4 cell counts of 200 and lower, indicating that CR may be particularly beneficial among HIV-infected individuals with advanced stages of the disease. As this is the first study to examine the relationship between CR and apathy, additional research, particularly longitudinal studies, is necessary to clarify the demonstrated relationship. Further research examining the moderating role of nadir CD4 cell count is necessary, as this may have important apathy treatment implications. Research on CR and apathy in healthy controls and individuals with other neurodegenerative disorders is also necessary to ascertain whether this relationship is unique to HIV neuropathology or occurs independent of HIV.
Funding
This work was supported by funding from the National Institute of Allergy and Infectious Diseases (P30AI051519-09s).
Conflict of Interest
None declared.
Acknowledgements
The authors thank the research participants who enrolled in this study, all of the research staff who helped with data collection (especially Eugene Dolce), and the community HIV care providers who referred many of their patients to our study.
References
- Aiken L. S., West S. G. Multiple regression: Testing and interpreting interactions. Thousand Oaks, CA USA: Sage Publications; 1991. [Google Scholar]
- Basso M. R., Bornstein R. A. Effects of immunosuppression and disease severity upon neuropsychological functioning in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2000a;22(1):104–114. doi: 10.1076/1380-3395(200002)22:1;1-8;FT104. [DOI] [PubMed] [Google Scholar]
- Basso M. R., Bornstein R. A. Estimated premorbid intelligence mediates neurobehavioral change in individuals infected with HIV across 12 months. Journal of Clinical and Experimental Neuropsychology. 2000b;22(2):208–218. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- Beck A. T. Beck Depression Inventory manual. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Bornstein R. A., Miller H. B., van Schoor J. T. Neuropsychological deficit and emotional disturbance in head-injured patients. Journal of Neurosurgery. 1989;70(4):509–513. doi: 10.3171/jns.1989.70.4.0509. [DOI] [PubMed] [Google Scholar]
- Bornstein R. A., Nasrallah H. A., Para M. F., Whitacre C. C., Rosenberger P., Fass R. J. Neuropsychological performance in symptomatic and asymptomatic HIV infection. AIDS. 1993;7:519–524. doi: 10.1097/00002030-199304000-00011. [DOI] [PubMed] [Google Scholar]
- Brew B. J., Rosenblum M., Price R. W. AIDS dementia complex and primary HIV brain infection. Journal of Neuroimmunology. 1988;20:133–140. doi: 10.1016/0165-5728(88)90144-0. [DOI] [PubMed] [Google Scholar]
- Brickman A. M., Siedlecki K. L., Muraskin J., Manly J. J., Luchsinger J. A., Yeung L. K., et al. White matter hyperintensities and cognition: Testing the reserve hypothesis. Neurobiology and Aging. 2011;32(9):1588–1598. doi: 10.1016/j.neurobiolaging.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickman A. M., Siedlecki K. L., Stern Y. Cognitive and brain reserve. In: Depp C. A., Jeste D. V., editors. Successful cognitive and emotional aging. Arlington, VA, USA: American Psychiatric Publishing; 2010. pp. 157–172. [Google Scholar]
- Brodaty H., Altendorf A., Withall A., Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. International Psychogeriatrics. 2010;22(3):426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- Castellon S. A., Hardy D. J., Hinkin C. H., Satz P., Stenquist P. K., van Gorp W. G., et al. Components of depression in HIV-1 infection: Their differential relationship to neurocognitive performance. Journal of Clinical and Experimental Neuropsychology. 2006;28(3):420–437. doi: 10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon S. A., Hinkin C. H., Myers H. F. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. Journal of the International Neuropsychological Society. 2000;6(3):336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- Castellon S. A., Hinkin C. H., Wood S., Yarema K. T. Apathy, depression, and cognitive performance in HIV-1 infection. Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(3):320–329. doi: 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- CDC. HIV/AIDS Surveillance Report. Atlanta: CDC; 2005. [Google Scholar]
- Clarke D. E., Ko J. Y., Kuhl E. A., van Reekum R., Salvador R., Marin R. S. Are the available apathy measures reliable and valid? A review of the psychometric evidence. Journal of Psychosomatic Research. 2011;70(1):73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J., Cohen P., West S. G., Aiken L. S. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Mahwah, NJ, USA: Lawrence Erlbaum Associates Publishers; 2003. [Google Scholar]
- Cole M. A., Castellon S. A., Perkins A. C., Ureno O. S., Robinet M. B., Reinhard M. J., et al. Relationship between psychiatric status and frontal-subcortical systems in HIV infected individuals. Journal of the International Neuropsychological Society. 2007;13(3):549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S., Stern Y. Consideration of Cognitive Reserve. In: Ravdin L. D., Katzen H. L., editors. Handbook on the Neuropsychology of Aging and Dementia. New York, Heidelberg, Dordrecht, London: Springer Science+Business Media; 2012. pp. 11–23. [Google Scholar]
- Crum R. M., Anthony J. C., Bassett S. S., Folstein M. F. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Cummings J. L. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cysique L. A., Brew B. J. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: A review. Neuropsychology Review. 2009;19:169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Deb S., Lyons I., Koutzoukis C., Ali I., McCarthy G. Rate of psychiatric illness 1 year after traumatic brain injury. American Journal of Psychiatry. 1999;156(3):374–378. doi: 10.1176/ajp.156.3.374. [DOI] [PubMed] [Google Scholar]
- Ernst T., Chang L., Jovicich J., Ames N., Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59(9):1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Ferrando S., van Gorp W., McElhiney M., Goggin K., Sewell M., Rabkin J. Highly active antiretroviral treatment in HIV infection: Benefits for neuropsychological function. AIDS. 1998;12:F65. doi: 10.1097/00002030-199808000-00002. [DOI] [PubMed] [Google Scholar]
- Foley J. M., Ettenhofer M. L., Kim M. S., Behdin N., Castellon S. A., Hinkin C. H. Cognitive reserve as a protective factor in older HIV-positive patients at risk for cognitive decline. Applied Neuropsychology. 2012;19(1):16–25. doi: 10.1080/09084282.2011.595601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzen M. D., Burgess E. J., Smith-Seemiller L. Methods of estimating premorbid functioning. Archives of Clinical Neuropsychology. 1997;12(8):711–738. [PubMed] [Google Scholar]
- Golden J. C. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- Gomez-Hernandez R., Max J. E., Kosier T., Paradiso S., Robinson R. G. Social impairment and depression after traumatic brain injury. Archives of Physical and Medical Rehabilitation. 1997;78(12):1321–1326. doi: 10.1016/s0003-9993(97)90304-x. [DOI] [PubMed] [Google Scholar]
- Green R. E. A., Melo B., Christensen B., Ngo L.-A., Monette G., Bradbury C. Measuring premorbid IQ in traumatic brain injury: An examination of the validity of the Wechsler Test of Adult Reading (WTAR) Journal of Clinical and Experimental Neuropsychology. 2008;30(2):1–10. doi: 10.1080/13803390701300524. [DOI] [PubMed] [Google Scholar]
- Groom K. N., Shaw T. G., O'Connor M. E., Howard N. I., Pickens A. Neurobehavioral symptoms and family functioning in traumatically brain-injured adults. Archives of Clinical Neuropsychology. 1998;13(8):695–711. [PubMed] [Google Scholar]
- Hall M., Whaley R., Robertson K., Hamby S., Wilkins J., Hall C. The correlation between neuropsychological and neuroanatomic changes over time in asymptomatic and symptomatic HIV-1-infected individuals. Neurology. 1996;46:1697–1702. doi: 10.1212/wnl.46.6.1697. [DOI] [PubMed] [Google Scholar]
- Heaton R. K., Franklin D. R., Ellis R. J., McCutchan J. A., Letendre S. L., Leblanc S., et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton R. K., Grant I., Butter N., White D. A., Kirson D., Atkinson J. H., et al. The HNRC 500- neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Hibbard M. R., Ashman T. A., Spielman L. A., Chun D., Charatz H. J., Melvin S. Relationship between depression and psychosocial functioning after traumatic brain injury. Archives of Physical and Medical Rehabilitation. 2004;85(4 Suppl. 2):S43–S53. doi: 10.1016/j.apmr.2003.08.116. [DOI] [PubMed] [Google Scholar]
- Hinkin C. H., Castellon S. A., Hardy D. J. Dual task performance in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 2000;22(1):16–24. doi: 10.1076/1380-3395(200002)22:1;1-8;FT016. [DOI] [PubMed] [Google Scholar]
- Hoare J., Fouche J. P., Spottiswoode B., Sorsdahl K., Combrinck M., Stein D. J., et al. White-matter damage in Clade C HIV-positive subjects: A diffusion tensor imaging study. Journal of Neuropsychiatry and Clinical Neuroscience. 2011;23(3):308–315. doi: 10.1176/jnp.23.3.jnp308. [DOI] [PubMed] [Google Scholar]
- Holsinger T., Steffens D. C., Phillips C., Helms M. J., Havlik R. J., Breitner J. C., et al. Head injury in early adulthood and the lifetime risk of depression. Archives of General Psychiatry. 2002;59(1):17–22. doi: 10.1001/archpsyc.59.1.17. [DOI] [PubMed] [Google Scholar]
- Jernigan T., Archibald S., Hesselink J., Atkinson J., Velin R., McCutchan J. A., et al. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. Archives of Neurology. 1993;50:250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Kallianpur K. J., Kirk G. R., Sailasuta N., Valcour V., Shiramizu B., Nakamoto B. K., et al. Regional cortical thinning associated with detectable levels of HIV DNA. Cereb Cortex. 2012;22(9):2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R., Woods S. P., Marcotte T. D., Ellis R. J., Grant I. HNRP Group. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Archives of Clinical Neuropsychology. 2012;27(5):520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R., Duffy J. D., Pivovarnik A. Prevalence of apathy following head injury. Brain Inj. 1998;12:87–92. doi: 10.1080/026990598122908. [DOI] [PubMed] [Google Scholar]
- Katzman R. Education and the prevalence of dementia and Alzheimer's disease. Neurology. 1993;43(1):13–20. doi: 10.1212/wnl.43.1_part_1.13. [DOI] [PubMed] [Google Scholar]
- Koerts J., Tucha L., Lange K. W., Tucha O. The influence of cognitive reserve on cognition in Parkinson's disease. Journal of Neural Transmission. 2012;120(4):593–596. doi: 10.1007/s00702-012-0916-6. [DOI] [PubMed] [Google Scholar]
- Kuzis G., Sabe L., Tiberti C., Merello M., Leiguarda R., Starkstein S. E. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52:1403–1407. doi: 10.1212/wnl.52.7.1403. [DOI] [PubMed] [Google Scholar]
- Law W. A., Mapou R. L., Roller T. L., Martin A., Nannis E. D., Temoshok L. R. Reaction time slowing in HIV-1-infected individuals: Role of the preparatory interval. Journal of Clinical and Experimental Neuropsychology. 1995;17(1):122–133. doi: 10.1080/13803399508406587. [DOI] [PubMed] [Google Scholar]
- Law W. A., Martin A., Mapou R. L., Roller T. L., Salazar A. M., Temoshok L. R., et al. Working memory in individuals with HIV infection. Journal of Clinical and Experimental Neuropsychology. 1994;16(2):173–182. doi: 10.1080/01688639408402628. [DOI] [PubMed] [Google Scholar]
- Marin R. S. Apathy: A neuropsychiatric syndrome. Journal of Neuropsychiatry and Clinical Neurosciences. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Marin R. S., Biedrzycki R. C., Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marin R. S., Firinciogullari S., Biedrzycki R. C. The sources of convergence between measures of apathy and depression. Journal of Affective Disorders. 1993;28(2):117–124. doi: 10.1016/0165-0327(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Marin R. S., Firinciogullari S., Biedrzycki R. C. Group differences in the relationship between apathy and depression. Journal of Nervous and Mental Disease. 1994;182:235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Mathias J. L., Bowden S. C., Barrett-Woodbridge M. Accuracy of the Wechsler Test of Adult Reading (WTAR) and National Adult Reading Test (NART) when estimating IQ in a healthy Australian sample. Australian Psychologist. 2007;42(1):49–56. [Google Scholar]
- Morgan E. E., Woods S. P., Smith C., Weber E., Scott J. C., Grant I., et al. Lower cognitive reserve among individuals with syndromic HIV-Associated Neurocognitive Disorders (HAND) AIDS Behavior. 2012;16(8):2279–2285. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navia B. A., Cho E. S., Petito C. K., Price R. W. The AIDS dementia complex: II. Neuropathology. Annals of Neurology. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Ojala-Oksala J., Jokinen H., Kopsi V., Lehtonen K., Luukkonen L., Paukkunen A., et al. Educational history is an independent predictor of cognitive deficits and long-term survival in postacute patients with mild to moderate ischemic stroke. Stroke. 2012;43(11):2931–2935. doi: 10.1161/STROKEAHA.112.667618. [DOI] [PubMed] [Google Scholar]
- Paul R. H., Brickman A. M., Navia B., Hinkin C., Malloy P. F., Jefferson A. L., et al. Apathy is associated with volume of the nucleus accumbens in patients infected with HIV. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(2):167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R., Cohen R., Navia B., Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and Biobehavioral Review. 2002;26:353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Paul R., Flanigan T. P., Tashima K., Cohen R., Lawrence J., Alt E., et al. Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:114–118. doi: 10.1176/jnp.17.1.114. [DOI] [PubMed] [Google Scholar]
- Pillai J. A., Hall C. B., Dickson D. W., Buschke H., Lipton R. B., Verghese J. Association of crossword puzzle participation with memory decline in persons who develop dementia. Journal of the International Neuropsychological Society. 2011;17(6):1006–1013. doi: 10.1017/S1355617711001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posada C., Moore D. J., Woods S. P., Vigil O., Ake C., Perry W., et al. Implications of hepatitis C virus infection for behavioral symptoms and activities of daily living. Journal of Clinical and Experimental Neuropsychology. 2010;32(6):637–644. doi: 10.1080/13803390903418900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin J. G., Ferrando S. J., van Gorp W., Ricppi R., McElhiney M., Sewell M. Relationship among apathy, depression, and cognitive impairment in HIV/AIDS. Journal of Neuropsychiatry and Clinical Neuroscience. 2000;12:451–457. doi: 10.1176/jnp.12.4.451. [DOI] [PubMed] [Google Scholar]
- Raininko R., Elovaara I., Virta A., Valanne L., Haltia M., Valle S. L. Radiological study of the brain at various stages of human immunodeficiency virus infection: Early development of brain atrophy. Neuroradiology. 1992;34:190–196. doi: 10.1007/BF00596333. [DOI] [PubMed] [Google Scholar]
- Rentz D. M., Locascio J. J., Becker J. A., Moran E. K., Eng E., Buckner R. L., et al. Cognition, reserve, and amyloid deposition in normal aging. Annals of Neurology. 2010;67(3):353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., Deary I. J. A life course approach to cognitive reserve: A model for cognitive aging and development? Annals of Neurology. 2005;58(4):617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- Salmond C. H., Menon D. K., Chatfield D. A., Pickard J. D., Sahakian B. J. Cognitive reserve as a resilience factor against depression after moderate/severe head injury. Journal of Neurotrauma. 2006;23(7):1049–1058. doi: 10.1089/neu.2006.23.1049. [DOI] [PubMed] [Google Scholar]
- Satz P., Morgenstern H., Miller E. N., Selnes O. A., McArthur J. C., Cohen B. A., et al. Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the multicenter AIDS Cohort Study (MACS) Journal of Acquired Immune Deficiency Syndrome. 1993;6(5):503–511. [PubMed] [Google Scholar]
- Scarmeas N., Stern Y. Cognitive reserve and lifestyle. Journal of Clinical and Experimental Neuropsychology. 2003;25(5):625–633. doi: 10.1076/jcen.25.5.625.14576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmeas N., Zarahn E., Anderson K. E., Hilton J., Flynn J., Van Heertum R. L., et al. Cognitive reserve modulates functional brain responses during memory tasks: A PET study in healthy young and elderly subjects. Neuroimage. 2003;19(3):1215–1227. doi: 10.1016/s1053-8119(03)00074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O., Strauss E. A compendium of neuropsychological tests. 2nd ed. New York: Oxford University Press; 1998. [Google Scholar]
- Starkstein S. E., Bolduc P. L., Preziosi T. J., Robinson R. G. Cognitive impairments in different stages of Parkinson's disease. Journal of Neuropsychiatry and Clinical Neurosciences. 1989;1(3):243–248. doi: 10.1176/jnp.1.3.243. [DOI] [PubMed] [Google Scholar]
- Starkstein S. E., Brockman S. Apathy and Parkinson's disease. Current Treatment Options in Neurology. 2011;13(3):267–273. doi: 10.1007/s11940-011-0118-9. [DOI] [PubMed] [Google Scholar]
- Starkstein S. E., Jorge R., Mizrahi R., Robinson R. G. A prospective longitudinal study of apathy in Alzheimer's disease. Journal of Neurology, Neurosurgery and Psychiatry. 2006;77(1):8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society. 2002;8(3):448–460. [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. Neuropsychologia. 2009;47(10):2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve. In: Raskin S. A., editor. Neuroplasticity and rehabilitation. New York: Guilford Press; 2011. pp. 89–102. [Google Scholar]
- Stern R. A., Silva S. G., Chaisson N., Evans D. L. Influence of cognitive reserve on neuropsychological functioning in asymptomatic human immunodeficiency virus-1 infection. Archives of Neurology. 1996;53(2):148–153. doi: 10.1001/archneur.1996.00550020052015. [DOI] [PubMed] [Google Scholar]
- Stroop J. R. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tate D., Paul R. H., Flanigan T. P., Tashima K., Nash J., Adair C., et al. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care and STDs. 2003;17(3):115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Tekin S., Cummings J. L. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thompson P. M., Dutton R. A., Hayashi K. M., Toga A. W., Lopez O. L., Aizenstein H. J., et al. Thinning of the cerebral cortex visualized in HIV/AI DS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uttl B., Graf P. Color-Word Stroop test performance across the adult life span. Journal of Clinical and Experimental Neuropsychology. 1997;19(3):405–420. doi: 10.1080/01688639708403869. [DOI] [PubMed] [Google Scholar]
- Van Gorp W., Miller E., Satz P., Visscher B. Neuropsychological performance in HIV -1 immunocompromised patients: A preliminary report. Journal of Clinical and Experimental Neuropsychology. 1989;11:763–773. doi: 10.1080/01688638908400930. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Test of Adult Reading. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Whitney K. A., Shepard P. H., Mariner J., Mossbarger B., Herman S. M. Validity of the Wechsler Test of Adult Reading (WTAR): Effort considered in a clinical sample of U.S. military veterans. Applied Neuropsychology. 2010;17(3):196–204. doi: 10.1080/09084282.2010.499787. [DOI] [PubMed] [Google Scholar]
- Willshire D., Kinsella G., Prior M. Estimating WAIS-R IQ from the National Adult Reading Test: A cross-validation. Journal of Clinical and Experimental Neuropsychology. 1991;13(2):204–216. doi: 10.1080/01688639108401038. [DOI] [PubMed] [Google Scholar]