Abstract
Objectives
To describe the pattern of incident illness in children after initiation of antiretroviral therapy (ART) in a large public health sector in Lusaka, Zambia.
Methods
A systematic review was performed to extract data from medical records of children (i.e., under 16 years) initiating ART in the Lusaka, Zambia HIV care and treatment program. Incident conditions were listed separately and then grouped according to broad categories. Predictors for incident diagnoses were determined using univariate and multivariable analysis.
Results
Between May 2004 and July 2006, 1,940 HIV-infected children initiated ART. Of these, 1,391 (71.1%) had their medical records reviewed. Median age at ART initiation was 77 months and 631 (45.4%) were females. 859 (62%) children had an incident condition during this period, with a median time of occurrence of 63 days from ART initiation. 28 different incident conditions were documented. When categorized, the most common were mucocutaneous conditions (incidence rate [IR]: 101.1 per 100 child-years, 95%CI: 92.3-110.5) and upper respiratory tract infection (IR: 100.6 per 100 child-years; 95% CI 91.9-110.0). Children with severe immunosuppression (i.e., CD4 < 10%) were more likely to develop lower respiratory tract infection (15.4% vs. 8.4%; p = 0.0002), mucocutaneous conditions (41.3% vs. 29.5%; p < 0.0001) and gastrointestinal conditions (19.8% vs. 14.5%; p = 0.02), when compared to those with CD4 ≥ 10%.
Conclusion
There is a high incidence of new illness following ART initiation, emphasizing the importance of close monitoring during this period.
Introduction
At the start of 2008, an estimated two million children were living with HIV worldwide (Joint United Nations Programme on HIV/AIDS, 2008) In North America and Europe, where antiretroviral therapy (ART) has been available for decades, significant improvements in survival have been achieved (Walker et al., 2002, Viani et al., 2004). Where services for HIV care and treatment are accessible, similar trends have been observed in parts of sub-Saharan Africa (KIDS-ART-LINC Collaboration, 2008). In Lusaka, Zambia, for example, scale-up of a large public sector HIV treatment program has resulted in early but significant improvements in CD4 cell counts, weight-for-age, and hemoglobin concentrations among children on ART (Bolton-Moore et al., 2007). In South Africa, investigators observed rates of high survival at 24 months of follow-up among children on ART (Jaspan et al., 2008). Similar health improvements have been noted elsewhere in Cote d'Ivoire, Uganda, and Kenya (Adje-Toure et al., 2008, Kekitiinwa et al., 2008, Wamalwa et al., 2007). These clinical outcomes likely do not derive solely from provision of antiretroviral drugs, but also from adjunct interventions such as cotrimoxazole prophylaxis (Mulenga et al., 2007, Chintu et al., 2004) and improvements in healthcare access and delivery.
Despite the benefits of ART, HIV-infected children remain at risk for many common illnesses. In a study of 67 Kenyan children with advanced HIV disease (median CD4%: 5.8%), 58% were hospitalized within the first six months of ART initiation due to causes such as pneumonia, pulmonary tuberculosis, diarrhea, and failure to thrive (Wamalwa et al., 2007). Among 192 Thailand children with comparable disease status (mean CD4%: 5.2%), approximately 30% were hospitalized within the first 24 weeks of ART initiation, most commonly for pneumonia and bacterial infections (Puthanakit et al., 2007). The high frequency of these events in the first 6 months – followed by considerably lower rates following that window – suggests that immune reconstitution inflammatory syndrome contributes to some degree, likely manifesting in a number of mild or serious conditions (Nacher et al., 2007, Walters et al., 2008, Zampoli et al., 2007). We performed a medical record review of HIV-infected children enrolled into long-term HIV care and treatment to better understand causes of morbidity following ART initiation in Lusaka, Zambia.
Patients and Methods
This study was conducted across 13 public sector clinics providing HIV care and treatment in Lusaka, supported by the Zambian Ministry of Health and the U.S. Centers for Disease Control and Prevention. Details of clinical care and follow up have been described elsewhere (Bolton-Moore et al., 2007). Briefly, we enrolled children with known or suspected HIV infection into long-term care. Initial evaluation included a World Health Organization (WHO) clinical staging and laboratory determination of CD4+ cell count and percentage, hemoglobin, and aspartate aminotransferase or alanine aminotransferase. Children started ART based on the severity of disease as determined by the WHO clinical stage and the CD4+ cell percentage (World Health Organization, 2006). First-line ART comprised a nucleoside reverse transcriptase inhibitor backbone of zidovudine or stavudine with lamivudine, and a non-nucleoside reverse transcriptase inhibitor (nevirapine or efavirenz). Antiretroviral drugs were provided free-of-charge by the Zambian government.
Children included in this analysis cohort were under 16 years of age at time of enrollment and initiated ART between May 13, 2004 to June 5, 2006. A team of trained health workers performed a systematic chart review using a standardized data collection tool. Data on new conditions were documented throughout each patient's clinical course following ART initation; where a definitive diagnosis was not made, signs or symptoms were recorded. Data from the chart extraction were linked to an electronic medical record used for programmatic monitoring (Fusco et al., 2005), allowing us to describe basic demographic and medical characteristics of the cohort and determine incident diagnoses. Most diagnoses were based on clinical suspicion alone. Nearly all patient care was provided by clinical officers (equivalent to physician assistants in the United States and Europe), which is typical in our setting. Task-shifting in this manner has facilitated the provision of HIV care despite an extreme shortage of health workers (Morris et al., 2009).
For each identified condition, we calculated an incidence rate per 100 child-years with 95% exact Poisson confidence intervals (CI). Children contributed person-time until first diagnosis, at which point they were censored for that specific condition. The same child, however, could continue to contribute person-time for other conditions. Diagnoses were listed separately and then grouped according to broader categories. To better understand the disease burden among different age ranges, we stratified the analysis based on three groups: < 18 months, 18 to 59 months, and ≥ 60 months of age. We performed univariate analysis to determine predictors for any incident diagnosis, including immunosuppression at ART enrollment. Categorical values were compared using Pearson's Chi-squared test; continuous variables were compared using Student's t-test and Wilcoxon Rank Sum test. We also performed multivariable analysis using logistic regression, adjusting for potential confounders such as disease status. Analysis was performed on SAS version 9.1 (Cary, NC, USA). Use of this programmatic data was approved by the University of Zambia Research Ethics Committee (Lusaka, Zambia) and the University of Alabama at Birmingham Institutional Review Board (Birmingham, AL, USA).
Results
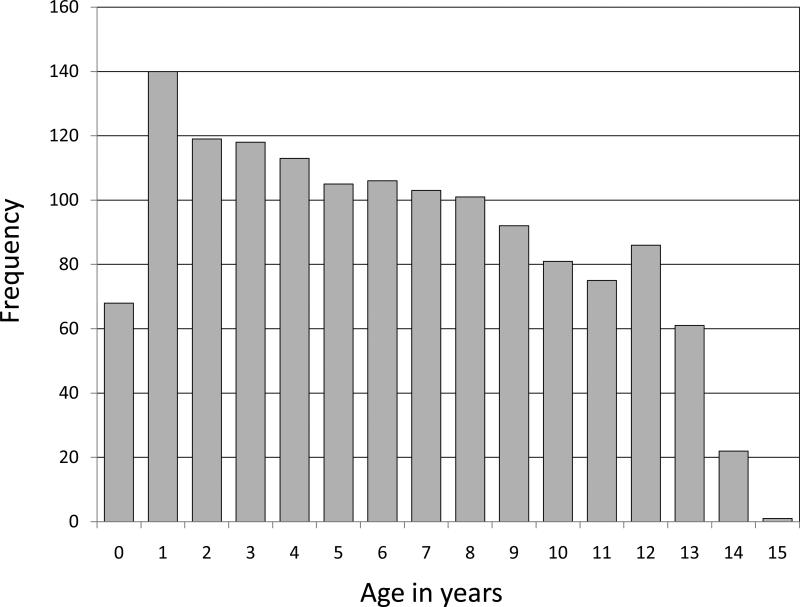
From May 13, 2004 to June 5, 2006, 1,940 HIV-infected children (i.e., under 16 years) were enrolled and initiated ART across the 13 targeted facilities. Of these, 1,391 (71.1%) had their charts reviewed and extracted by the study team. The median age at ART initiation was 77 months (interquartile range [IQR]: 40-118): 113 (8%) were less than 18 months, 425 (31%) were 18 to 59 months, and 853 (61%) were 60 months or older. Age distribution of the analysis cohort is shown in Figure 1. The characteristics of the cohort – by age group and overall – are shown in Table 1.
Figure 1.
Age in years at time of antiretroviral therapy initiation among children included in this systematic chart review (May 2004-June 2006)
Table 1.
Baseline demographic and medical characteristics of 1,391 children starting antiretroviral therapy in Lusaka, Zambia (May 2004 to June 2006)
| 0 to < 18 months | 18 to < 60 months | ≥ 60 months | Overall | |||||
|---|---|---|---|---|---|---|---|---|
| N=113 | N=425 | N=853 | N=1391 | |||||
| n | Value | n | Value | n | Value | n | Value | |
| Sex, n (%) | ||||||||
| Female | 47 | 41.6% | 183 | 43.1% | 401 | 47.0% | 631 | 45.4% |
| Male | 66 | 58.4% | 242 | 56.9% | 452 | 53.0% | 760 | 54.6% |
| Weight-for-age z score, mean (sd)** | 103 | −2.3(2.3) | 412 | −2.1(1.7) | 804 | −2.3(1.6) | 1319 | −2.2(1.7) |
| > −1 | 26 | 25.2% | 106 | 25.7% | 159 | 19.8% | 291 | 22.1% |
| > –2 through –1 | 15 | 14.6% | 79 | 19.2% | 199 | 24.8% | 293 | 22.2% |
| > –3 through –2 | 22 | 21.4% | 86 | 20.9% | 191 | 23.8% | 299 | 22.7% |
| ≤−3 | 40 | 38.8% | 141 | 34.2% | 255 | 31.7% | 436 | 33.1% |
| CD4+ cell percentage at ART initiation, mean (sd) | 95 | 15.9(8.4) | 392 | 14.0(8.2) | 744 | 12.2(8.1) | 1231 | 13.0(8.2) |
| ≥20.0 | 24 | 25.3% | 79 | 20.2% | 102 | 13.7% | 205 | 16.6% |
| 10.0% - 19.9% | 50 | 52.6% | 175 | 44.6% | 327 | 44.0% | 552 | 44.8% |
| < 10.0% | 21 | 22.1% | 138 | 35.2% | 315 | 42.3% | 474 | 38.5% |
| Hemoglobin, mean (sd), g/dL | 95 | 9.4(2.0) | 396 | 9.6(2.3) | 770 | 10.3(2.3) | 1261 | 10.0(2.3) |
| ≥ 8.0 g/dL | 79 | 83.2% | 326 | 82.3% | 669 | 86.9% | 1074 | 85.2% |
| < 8.0 g/dL | 16 | 16.8% | 70 | 17.7% | 101 | 13.1% | 187 | 14.8% |
| WHO stage at ART initiation, n (%) | ||||||||
| I or II | 14 | 12.4% | 96 | 22.6% | 248 | 29.2% | 358 | 25.8% |
| III or IV | 99 | 87.6% | 328 | 77.4% | 601 | 70.8% | 1028 | 74.2% |
The 1,391 children included in this analysis contributed a total of 2423 child-years. 859 (62%) developed an incident diagnosis and were subsequently censored at a median time of 63 (IQR: 29, 152) days from time of ART initiation. In univariate analysis, few characteristics appeared predictive of a documented incident condition. Children in the two older age categories (i.e. 18 months to < 60 months; ≥ 60 months) with an incident diagnosis were more likely to have lower CD4+ cell percentages at time of enrollment. Under 18 months, CD4% did not predict incident diagnoses and there was an overall greater percentage of incident diagnoses in this age group in CD4%>10% as compared to older children. Children in the 18 to < 60 months age range with incident diagnoses tended to have more advanced clinical disease when compared to those without such conditions (WHO stage III or IV, 80.5% vs. 72.6%; p = 0.06). Similar associations were not observed in the other age categories. In a multivariable model, lower CD4% and higher WHO stage at baseline were associated with higher risk for developing an incident condition. Low weight-for-age Z-scores appeared protective against developing an incident diagnosis. No associations were observed with age, sex, or enrollment hemoglobin (Table 3).
Table 3.
Predictors for developing an incident diagnosis following initiation of antiretroviral therapv
| Adjusted RR (95% CI)* | ||
|---|---|---|
| Age, months | 0 to < 18 | 1.0 |
| 18 to < 60 | 1.0 (0.6 - 1.7) | |
| ≥60 | 1.1 (0.7 - 1.8) | |
| Sex | Female | 1.0 |
| Male | 1.0 (0.8 - 1.3) | |
| Weight for age, z score | > −1 | 1.0 |
| −1 to > −2 | 0.7 (0.5 - 1.1) | |
| −2 to > −3 | 0.6 (0.4 - 0.9) | |
| ≤−3 | 0.6 (0.4 - 0.9) | |
| CD4+ percentage | > 20% | 1.0 |
| 10 - 20% | 1.5 (1.0 - 2.1) | |
| < 10% | 2.2 (1.5 - 3.2) | |
| WHO Stage | I or II | 1.0 |
| III or IV | 1.6 (1.2 - 2.1) | |
| Hemoglobin | ≥8 g/dL | 1.0 |
| <8 g/dL | 1.0 (0.7 - 1.4) |
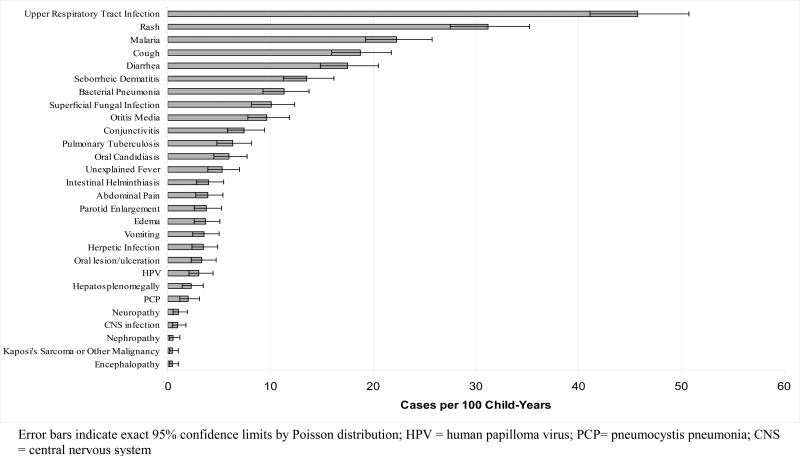
Overall, 28 different diagnoses or conditions were documented in the medical records (Figure 2). We categorized these into six categories: upper respiratory tract infection (upper respiratory infection, cough, otitis media), lower respiratory tract infection (bacterial pneumonia, pulmonary tuberculosis, pneumocystis pneumonia), mucocutaneous conditions (rash, seborrheic dermatitis, superficial fungal infection, conjunctivitis, oral candidiasis, herpetic lesion, oral lesion/ulceration, human papilloma virus), malaria, gastrointestinal conditions (diarrhea, vomiting, abdominal pain, intestinal helminthiasis), and other (unexplained fever, edema, parotid enlargement, hepatosplenomegaly, neuropathy, central nervous system infection, nephropathy, malignancy [including Kaposi's sarcoma], encephalopathy). Due to the limitations inherent to our data, we were unable to further categorize these conditions according to suspected etiology.
Figure 2.
Overall rates for all listed incident diagnoses and conditions among children included in this systematic chart review (May 2004-June 2006)
Of these categories, most common were mucocutaneous conditions (incidence rate [IR]: 101.1 per 100 child-years, 95%CI: 92.3-110.5) and upper respiratory tract infection (IR: 100.6 per 100 child-years; 95%CI: 91.9-110.0). This was followed by gastrointestinal conditions, lower respiratory tract infections, and malaria. Median time from ART initiation to incident diagnosis for each category was between 74 to 125 days (Table 3). Children with severe immunosuppression (i.e., CD4 <10%) were more likely to develop lower respiratory tract infection (15.4% vs. 8.4%; p = 0.0002), mucocutaneous conditions (41.3% vs. 29.5%; p < 0.0001) and gastrointestinal conditions (19.8% vs. 14.5%; p = 0.02), when compared to those with CD4 ≥ 10%. Incidence of upper respiratory tract infection, malaria and other conditions did not differ significantly by immune status (data not shown). In addition, incidence rates for each category differed by age. Malaria and mucocutaneous conditions were more common in older children, for example, while gastrointestinal conditions were more common among those under 18 months of age. The median time from ART initation to development of incident condition is shown in Table 4.
Table 4.
Incidence rates per 100 patient years by age category
| Condition | 0 to < 18 months N = 113 | 18 - 59 months N = 425 | ≥60 Months N = 853 | Overall N = 1391 | Days to diagnosis, median (IQR) |
|---|---|---|---|---|---|
| Upper respiratory tract infection | 94.8 (66.7 - 130.6) | 114.9 (97.1 - 135.0) | 95.5 (85.1 - 106.9) | 100.6 (91.9 - 110.0) | 114 (48, 242) |
| Lower respiratory tract iinfection | 32.4 (18.5 - 52.6) | 19.7 (13.9 - 27.0) | 24.7 (20.3 - 29.9) | 23.9 (20.3 - 27.9) | 74 (32, 161) |
| Mucocutaneous conditions | 77.4 (52.6 -109.8) | 98.6 (83.0 - 116.2) | 105.5 (94.2 - 117.8) | 101.1 (92.3 - 110.5) | 101 (41, 208) |
| Malaria | 17.6 (9.1 - 30.7) | 24.3 (18.5 - 31.3) | 21.9 (18.1 - 26.2) | 22.2 (19.2 - 25.6) | 94 (85, 255) |
| Gastrointestinal conditions | 71.6 (48.7 - 101.7) | 52.1 (41.8 - 64.3) | 27.3 (22.6 - 32.7) | 36.8 (32.2 - 41.8) | 107 (43, 210) |
| Other | 25.5 (13.6 - 43.6) | 19.4 (13.7 - 26.8) | 22.0 (17.9 - 26.8) | 21.6 (18.2 - 25.3) | 125 (44, 230) |
Discussion
Overall, 62% of children included in this analysis developed one of 28 incident conditions over the observation period. The severity and type of incident diagnoses were quite variable. Among children who were ≥ 18 months, lower CD4% was associated with development of an incident condition. Children under the age of 18 months were at risk for incident conditions across all CD4% strata.
Strengths of this analysis include its large patient population and its focus on the primary care setting, where most children in Zambia access health services. We recognize, however, that conditions may have been under-reported. A common practice locally is for very ill children to be taken directly to the University Teaching Hospital, a tertiary care facility that was not included among our targeted facilities. If events were not accurately reported to clinic staff, then they would not be considered in this analysis. Diagnostic facilities in the local health center may have also limited our ability to identify certain illnesses, particularly rare and/or sub-clinical presentations. Many of our listed conditions were thus diagnosed empirically according to algorithms from the WHO's Integrated Management of Childhood Illness or were recorded as symptoms or signs (e.g., rash, cough, fever, abdominal pain, edema, vomiting). Finally, since we censored patients at the time of their first incident diagnosis following ART initiation, we were unable to characterize subsequent diagnoses or recurrent conditions which many times are emblematic of a weakened immune state.
While numerous studies have described the positive impact of ART on incident opportunistic infections among children (Puthanakit et al., 2007, Ylitalo et al., 2006, Viani et al., 2004), only a few have attempted to estimate incidence rates for specific illnesses. Analysis of the PACTG 219C, for example, determined incidence of common illnesses among HIV-infected children in the United States during the “HAART era.” The most common illness diagnosed between 2001 to 2004 was bacterial pneumonia at a rate of 2.15 cases per 100 child-years (Gona et al., 2006), a finding less than one-fifth of our estimate in Lusaka. That these results differ greatly from ours is not surprising; in fact, it was the motivation for this study. To our knowledge, ours is the first to attempt a similar type of analysis in sub-Saharan Africa.
These data are a valid representation of the conditions being reported in the field and provide insight into the diagnostic and treatment challenges experienced in this setting. We provide valuable information to program managers for purposes of resource prioritization and allocation. Optimization of diagnostic and treatment algorithms could improve the management of specific conditions, particularly where task-shifting is heavily utilized (Morris et al., 2009). Targeted investments in diagnostic capacity could improve discernability between non-specific clinical presentations and allow for prompt treatment. Introduction of cheap, non-antiretroviral medications such as oral antihistamines and acetaminophen could also provide a great deal of symptomatic relief with relatively low costs. Such changes could greatly improve the clinical management of malaria, for example, which was diagnosed at a rate over 20 per 100 person-years. Improved diagnostic modalities such as rapid tests would improve the clinical certainty of this frequently diagnosed condition among HIV-infected children (Bebell et al., 2007). When negative, clinicians could further pursue other causes of undifferentiated fever, also common in this population.
We found that children starting ART were most likely to develop dermatological conditions, particularly among those with demonstrated immunosuppression. Epidemiologic studies have generally shown that the introduction of ART reduces the incidence of mucocuteneous diagnoses such as herpes zoster (Levin et al., 2009, Seoane Reula et al., 2005); unfortunately, we did not have for comparison analogous clinical data from children not on ART. Upper respiratory illnesses were also frequently diagnosed among our study population, at a rate over four times higher than that of lower tract infections (e.g. bacterial pneumonia, pulmonary tuberculosis, pneumocystis pneumonia). These latter conditions are noteworthy because they contribute significantly to the mortality in this population, as demonstrated by a necropsy series of 264 children in Lusaka (Chintu et al., 2002). Across all categories, a high proportion of events occurred early in the course of ART, with the median time to event well under 6 months. Immune reconstitution inflammatory syndrome may be a contributing factor to these illnesses, an area that requires further investigation in developing world settings.
Similar to others (Ylitalo et al., 2006), we found that incident conditions were associated with lower CD4 percentages at time of ART initation in our overall population. When we stratified by age group, the association was strongest among those children ≥ 18 months of age. Baseline CD4 percentage was not associated with increased risk for an incident diagonsis among those under 18 months. This finding could result from the relatively small sample size in this under 18 month category (n = 95). It could also be explained, however, by the high risk for comorbidities associated with this age range, regardless of the baseline CD4 percentage. Initiation of ART at higher CD4 percentages – including the WHO's recommendation to treat all HIV-infected infants under 12 months (World Health Organization, 2008) – should help to reduce comorbidities within the first 12 to 18 months of life, including immune reconstitution inflammatory syndrome. In the CHER study, for example, HIV-infected children with CD4 ≥ 25% who immediately initiated ART had a significant reduction in mortality when compared to those who deferred treatment until CD4 dropped below 20% (4% vs. 16%; p = 0.0002) (Violari et al., 2008).
In our analysis, we were surprised to find that children with severe malnutrition (i.e. Z-score ≤ −2) appeared to have fewer incident diagnoses. This finding could be explained by survivor bias, since low Z score has been associated with up to a four-fold risk for mortality in our population (Bolton-Moore et al., 2007). It is possible that children who were severely underweight died before an incident condition could be diagnosed. Another explanation may be the poor ascertainment of incident diagnoses among severely malnourished, HIV-infected children, a phenomenon we have observed locally. Children in this condition may present with few non-specific signs and symptoms despite severe illnesses.
In summary, this descriptive analysis emphasizes the importance of close monitoring following ART initiation among children, particularly for infectious co-morbidities. The heavy reliance of clinicians on signs and symptoms points to the need for intensified clinical mentoring in primary care settings and greater support for diagnostic capacity. Initiation of antimicrobial prophylaxis such as co-trimoxazole and early treatment with ART should be implemented at the field level, given their potential to improve survival and long-term quality of life.
Table 2.
Factors associated with having an incident diagnosis among pediatric patients with a detailed chart review in Lusaka, Zambia broken down by age group (May 2004 to June 2006)
| 0 to < 18 months | 18 to < 60 months | ≥ 60 months | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| With Dx | Without Dx | With Dx | Without Dx | With Dx | Without Dx | ||||||||||
| n | Value | n | Value | p | n | Value | n | Value | p | n | Value | n | Value | p | |
| Sex, n (%) | |||||||||||||||
| Female | 29 | 44.6% | 30 | 62.5% | 0.45+ | 105 | 41.0% | 78 | 46.2% | 0.30+ | 252 | 46.8% | 149 | 47.3% | 0.90+ |
| Male | 36 | 55.4% | 18 | 37.5% | 151 | 59.0% | 91 | 53.8% | 286 | 53.2% | 166 | 52.7% | |||
| Weight-for-age z score, mean (sd)** | 61 | −2.4 (2.0) | 42 | −2.3 (2.6) | 0.76^ | 251 | −2.0 (1.8) | 161 | −2.2 (1.5) | 0.20^ | 509 | −2.3 (1.6) | 295 | −2.3 (1.5) | 0.89^ |
| > −1 | 14 | 22.9% | 12 | 28.6% | 0.36+ | 73 | 29.1% | 33 | 20.5% | 0.28+ | 105 | 20.6% | 54 | 18.3% | 0.74+ |
| > –2 through –1 | 12 | 19.7% | 3 | 7.1% | 46 | 18.3% | 33 | 20.5% | 124 | 24.4% | 75 | 25.4% | |||
| > –3 through –2 | 12 | 19.7% | 10 | 23.8% | 49 | 19.5% | 37 | 23.0% | 116 | 22.8% | 75 | 25.4% | |||
| ≤−3 | 23 | 37.7% | 17 | 40.5% | 83 | 33.1% | 58 | 36.0% | 164 | 32.2% | 91 | 30.9% | |||
| CD4+ cell percentage* at ART initiation, mean (sd) | 53 | 15.8 (7.2) | 42 | 15.9 (9.7) | 0.94^ | 233 | 13.0 (7.6) | 159 | 15.5 (8.9) | 0.004^ | 453 | 11.5 (8.2) | 291 | 13.1 (7.8) | 0.009^ |
| ≥20 | 14 | 26.4% | 10 | 23.8% | 0.71+ | 37 | 15.9% | 42 | 26.4% | 0.04+ | 53 | 11.7% | 49 | 16.8% | 0.002+ |
| 10 -< 20 | 26 | 49.1% | 24 | 57.1% | 108 | 46.4% | 67 | 42.1% | 186 | 41.1% | 141 | 48.5% | |||
| < 10 | 13 | 24.5% | 8 | 19.1% | 88 | 37.8% | 50 | 31.5% | 214 | 47.2% | 101 | 34.7% | |||
| Hemoglobin, mean (sd), g/dL | 54 | 9.3 (1.7) | 41 | 9.6 (2.4) | 0.53^ | 161 | 9.5 (2.4) | 235 | 9.8 (2.1) | 0.30+ | 482 | 10.2 (2.3) | 288 | 10.3 (2.3) | 0.55^ |
| ≥8 g/dL | 45 | 83.3% | 34 | 17.1% | 0.96+ | 189 | 80.4% | 137 | 85.1% | 0.23+ | 421 | 87.3% | 248 | 86.1% | 0.62+ |
| <8 g/dL | 9 | 16.7% | 7 | 82.9% | 46 | 19.6% | 24 | 14.9% | 61 | 12.7% | 40 | 13.9% | |||
| WHO stage at ART initiation, n (%) | |||||||||||||||
| I or II | 8 | 12.3% | 6 | 12.5% | 0.98+ | 50 | 19.5% | 46 | 27.4% | 0.06+ | 146 | 27.3% | 102 | 32.5% | 0.11+ |
| III or IV | 57 | 87.7% | 42 | 87.5% | 206 | 80.5% | 122 | 72.6% | 389 | 72.2% | 212 | 67.5% | |||
Percentage of total lymphocytes
Calculated from standardized growth charts (see “Methods”)
Student's t-test
Chi-square
~Wilcoxon Rank-Sum, Dx = diagnosis
Acknowledgements
The authors acknowledge the Zambian Ministry of Health for consistent and high-level support of operations research in the context of HIV program expansion. Investigator salary or trainee support is provided by the National Institutes of Health (R24-TW007988, D43-TW001035, K01-TW06670; P30-AI027767), Oak Ridge Insitute for Science and Education (Department of Enegery Proposal 1236-1236-06), and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061).
References
- Adje-Toure C, Hanson DL, Talla-Nzussouo N, Borget MY, Kouadio LY, Tossou O, Fassinou P, Bissagnene E, Kadio A, Nolan ML, Nkengasong JN. Virologic and immunologic response to antiretroviral therapy and predictors of HIV type 1 drug resistance in children receiving treatment in Abidjan, Cote d'Ivoire. AIDS Res Hum Retroviruses. 2008;24:911–7. doi: 10.1089/aid.2007.0264. [DOI] [PubMed] [Google Scholar]
- Bebell LM, Gasasira A, Kiggundu M, Dokomajilar C, Kamya MR, Charlebois ED, Havlir D, Rosenthal PJ, Dorsey G. HIV-1 infection in patients referred for malaria blood smears at government health clinics in Uganda. J Acquir Immune Defic Syndr. 2007;46:624–30. doi: 10.1097/QAI.0b013e31815b2dc5. [DOI] [PubMed] [Google Scholar]
- Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, Chintu N, Stringer EM, Chi BH, Sinkala M, Kankasa C, Wilson CM, Wilfert CM, Mwango A, Levy J, Abrams EJ, Bulterys M, Stringer JS. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA. 2007;298:1888–99. doi: 10.1001/jama.298.16.1888. [DOI] [PubMed] [Google Scholar]
- Chintu C, Bhat GJ, Walker AS, Mulenga V, Sinyinza F, Lishimpi K, Farrelly L, Kaganson N, Zumla A, Gillespie SH, Nunn AJ, Gibb DM. Cotrimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet. 2004;364:1865–71. doi: 10.1016/S0140-6736(04)17442-4. [DOI] [PubMed] [Google Scholar]
- Chintu C, Mudenda V, Lucas S, Nunn A, Lishimpi K, Maswahu D, Kasolo F, Mwaba P, Bhat G, Terunuma H, Zumla A. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet. 2002;360:985–90. doi: 10.1016/S0140-6736(02)11082-8. [DOI] [PubMed] [Google Scholar]
- Fusco H, Hubschman T, Mbweeta V, et al. Electronic Patient Tracking Supports Rapid Expansion of HIV Care and Treatment in Resource-Constrained settings. IAS Conf HIV Pathog Treat 3rd. 2005 [Google Scholar]
- Gona P, Van Dyke RB, Williams PL, Dankner WM, Chernoff MC, Nachman SA, Seage GR., 3rd Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- Jaspan HB, Berrisford AE, Boulle AM. Two-year outcomes of children on non nucleoside reverse transcriptase inhibitor and protease inhibitor regimens in a South African pediatric antiretroviral program. Pediatr Infect Dis J. 2008;27:993–8. doi: 10.1097/INF.0b013e31817acf7b. [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on Hiv/Aids (Unaids) Report on the global AIDS epidemic. World Health Organization; Geneva: 2008. [Google Scholar]
- Kekitiinwa A, Lee KJ, Walker AS, Maganda A, Doerholt K, Kitaka SB, Asiimwe A, Judd A, Musoke P, Gibb DM. Differences in Factors Associated With Initial Growth, CD4, and Viral Load Responses to ART in HIV-Infected Children in Kampala, Uganda, and the United Kingdom/Ireland. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31818cdef5. [DOI] [PubMed] [Google Scholar]
- Kids-Art-Linc Collaboration Low Risk of Death, but Substantial Program Attrition, in Pediatric HIV Treatment Cohorts in Sub-Saharan Africa. J Acquir Immune Defic Syndr. 2008 doi: 10.1097/QAI.0b013e31818aadce. [DOI] [PubMed] [Google Scholar]
- Levin MJ, Anderson JP, Seage GR, 3rd, Williams PL. Short-term and long-term effects of highly active antiretroviral therapy on the incidence of herpes zoster in HIV-infected children. J Acquir Immune Defic Syndr. 2009;50:182–91. doi: 10.1097/QAI.0b013e31819550a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris MB, Chapula BT, Chi BH, Mwango A, Chi HF, Mwanza J, Manda H, Bolton C, Pankratz DS, Stringer JS, Reid SE. Use of task-shifting to rapidly scale-up HIV treatment services: experiences from Lusaka, Zambia. BMC Health Serv Res. 2009;9:5. doi: 10.1186/1472-6963-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga V, Ford D, Walker AS, Mwenya D, Mwansa J, Sinyinza F, Lishimpi K, Nunn A, Gillespie S, Zumla A, Chintu C, Gibb DM. Effect of cotrimoxazole on causes of death, hospital admissions and antibiotic use in HIV-infected children. AIDS. 2007;21:77–84. doi: 10.1097/QAD.0b013e3280114ed7. [DOI] [PubMed] [Google Scholar]
- Nacher M, Vantilcke V, Mahamat A, El Guedj M, Vaz T, Randrianjohany A, Clyti E, Aznar C, Carme B, Couppie P. Increased incidence of cutaneous mycoses after HAART initiation: a benign form of immune reconstitution disease? AIDS. 2007;21:2248–50. doi: 10.1097/QAD.0b013e3282887ea7. [DOI] [PubMed] [Google Scholar]
- Puthanakit T, Aurpibul L, Oberdorfer P, Akarathum N, Kanjananit S, Wannarit P, Sirisanthana T, Sirisanthana V. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seoane Reula E, Bellon JM, Gurbindo D, Munoz-Fernandez MA. Role of antiretroviral therapies in mucocutaneous manifestations in HIV-infected children over a period of two decades. Br J Dermatol. 2005;153:382–9. doi: 10.1111/j.1365-2133.2005.06758.x. [DOI] [PubMed] [Google Scholar]
- Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–31. doi: 10.1086/423178. [DOI] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, Mcintyre JA. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Schwartlander B, Bryce J. Meeting international goals in child survival and HIV/AIDS. Lancet. 2002;360:284–9. doi: 10.1016/S0140-6736(02)09550-8. [DOI] [PubMed] [Google Scholar]
- Walters E, Cotton MF, Rabie H, Schaaf HS, Walters LO, Marais BJ. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr. 2008;8:1. doi: 10.1186/1471-2431-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamalwa DC, Farquhar C, Obimbo EM, Selig S, Mbori-Ngacha DA, Richardson BA, Overbaugh J, Emery S, Wariua G, Gichuhi C, Bosire R, John-Stewart G. Early response to highly active antiretroviral therapy in HIV-1-infected Kenyan children. J Acquir Immune Defic Syndr. 2007;45:311–7. doi: 10.1097/QAI.0b013e318042d613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization Integrated management of childhood illnesses (IMCI) [January 26, 2009]; http://www.who.int/child_adolescent_health/topics/prevention_care/child/imci/en/.
- [January 26, 2009];World Health Organization Report of the WHO technical reference group, pediatric HIV/ART care guidlines group meeting. 2008 Apr 10-11; http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf.
- World Health Organization . Antiretroviral therapy of HIV infection in infants and children in resource-limited settings: towards universal access. Geneva: 2006. [Google Scholar]
- Ylitalo N, Brogly S, Hughes MD, Nachman S, Dankner W, Van Dyke R, Seage GR., 3rd Risk factors for opportunistic illnesses in children with human immunodeficiency virus in the era of highly active antiretroviral therapy. Arch Pediatr Adolesc Med. 2006;160:778–87. doi: 10.1001/archpedi.160.8.778. [DOI] [PubMed] [Google Scholar]
- Zampoli M, Kilborn T, Eley B. Tuberculosis during early antiretroviral-induced immune reconstitution in HIV-infected children. Int J Tuberc Lung Dis. 2007;11:417–23. [PubMed] [Google Scholar]